Introduction
Ventricular septal defects (VSDs) can be either congenital or acquired. The acquired form, referred to as ventricular septal rupture, occurs spontaneously and is associated with severe ischemic or inflammatory conditions. Congenital VSDs are the most common cardiac anomaly in children and are the second most common congenital abnormality in adults, following the bicuspid aortic valve.[1]
The primary pathophysiological mechanism involves abnormal communication between the right and left ventricles and, in rare cases, between the left ventricle and right atrium, leading to shunt formation and subsequent hemodynamic compromise. While many cases experience spontaneous closure, persistent large defects can lead to detrimental complications, including pulmonary arterial hypertension (PAH), Eisenmenger syndrome, ventricular dysfunction, and an increased risk of arrhythmias.[2][3][4] VSDs were first identified by Dalrymple in 1847.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
VSDs result from developmental abnormalities or disruptions in the formation of the interventricular septum during the complex process of embryological heart morphogenesis. Although VSDs often occur in isolation, they can be associated with other congenital heart defects, including atrial septal defects, patent ductus arteriosus, right aortic arch, and pulmonic stenosis. Additionally, VSDs may coexist with conditions such as aortic coarctation, subaortic stenosis, tetralogy of Fallot, and transposition of the great arteries, adding to the complexities of congenital heart disease.
Recent epidemiological studies have indicated that conditions such as maternal epilepsy (especially when treated with carbamazepine, as shown in animal studies), migraines, chronic hypertension, and paroxysmal supraventricular tachycardia may be associated with an elevated risk of VSDs. This association is supported by a study involving over 3000 live-born cases of congenital heart disease, which compared them to neonates born without congenital disabilities or with other isolated congenital abnormalities.[6][7][8]
Maternal infections (such as rubella, influenza, and febrile illness), maternal diabetes, and phenylketonuria have been associated with an increased risk of VSDs. Exposure to toxins such as alcohol, marijuana, cocaine, and certain medications, including metronidazole and ibuprofen, is also linked to VSDs. Williams et al found that moderate-to-high levels of alcohol consumption in expectant mothers were associated with isolated VSDs. Furthermore, mothers who used marijuana exhibited a 2-fold increase in the risk of isolated simple VSD. Recently, a stressful maternal lifestyle has also been correlated with a higher incidence of VSDs.[9][10][11][12]
A notable Italian study conducted in 2011 by Fesslova et al examined the recurrence of congenital heart disease in pregnant women with a familial predisposition. Among the 65 infants born with congenital heart disease, which represented 21.5% of the total cohort, 14 were diagnosed with VSD, indicating a significant genetic component.[13]
In women with congenital heart disease, the complications observed in their offspring are closely linked to the mother's cardiac function, particularly in cases of maternal cyanosis or reduced cardiac output. The recurrence rate of heart defects in the children of mothers with congenital heart disease is approximately 6%, with a similar 6% risk of VSD recurrence among these children.[14][15]
Several genetic factors contribute to VSDs, including chromosomal abnormalities, single-gene mutations, and polygenic inheritance. A recently discovered TBX5 mutation has been associated with septal defects in patients with Holt-Oram syndrome. Additionally, non-inherited risk factors also play a role in the development of VSDs.[16][17][18]
Epidemiology
Isolated VSD accounts for 37% of all congenital heart diseases in children, with an incidence of approximately 0.3% in newborns. The prevalence decreases significantly in adults due to spontaneous closure in up to 90% of cases. While VSDs show no gender predilection, some studies suggest that females are more likely to present with milder forms of the condition.[19][20]
Pathophysiology
The interventricular septum is an asymmetric, curved wall shaped by pressure differences between the ventricles. The septum consists of 2 distinct parts—the membranous and muscular portions. The membranous portion includes the atrioventricular segment, while the muscular portion comprises the trabecular, infundibular, and inlet segments.[21][22]
VSD arises from the incomplete development or fusion of intraventricular septal components during embryonic cardiac morphogenesis. The different anatomical locations and histological variations of VSDs have led to several classification and nomenclature systems. A unified classification system has been introduced to simplify VSD descriptions and eliminate confusion from multiple synonyms. This system organizes VSDs into 4 significant groups, as mentioned below.[23]
Infundibular (Outlet) Ventricular Septal Defect
- Location: This is positioned beneath the semilunar valves (aortic and pulmonary) within the outlet septum of the right ventricle, above the crista supraventricularis.
- Characteristics: Infundibular VSD, also referred to as supracristal, is a rare VSD type that accounts for only 6% of all cases but occurs in about 30% of the Asian population. Aortic valve prolapse and regurgitation frequently develop due to inadequate support for the right and noncoronary cusps of the aortic valve. Spontaneous closure of these defects is rare.
Perimembranous Ventricular Septal Defect
- Location: This is located in the membranous septum, below the crista supraventricularis.
- Characteristics: Perimembranous VSD is the most common type, accounting for 80% of all VSDs. Perimembranous VSD may involve the muscular septum and can interchangeably be called membranous VSD. The septal leaflet of the tricuspid valve can form a pouch, which may reduce shunting, resulting in spontaneous closure.
Inlet or Atrioventricular Canal Ventricular Septal Defect
- Location: This is located just inferior to the inlet valves (tricuspid and mitral) within the inlet portion of the right ventricular septum.
- Characteristics: Inlet or atrioventricular canal VSD represents about 8% of all VSDs and is more frequently observed in patients with Trisomy 21.
Muscular (Trabecular) Ventricular Septal Defect
- Location: This is located in the muscular septum, usually in the apical, central, and outlet regions of the interventricular septum.
- Characteristics: Muscular VSD can occur as multiple defects, resembling a "Swiss cheese" appearance, and account for up to 20% of VSDs in infants. The incidence is lower in adults due to a tendency for spontaneous closure.
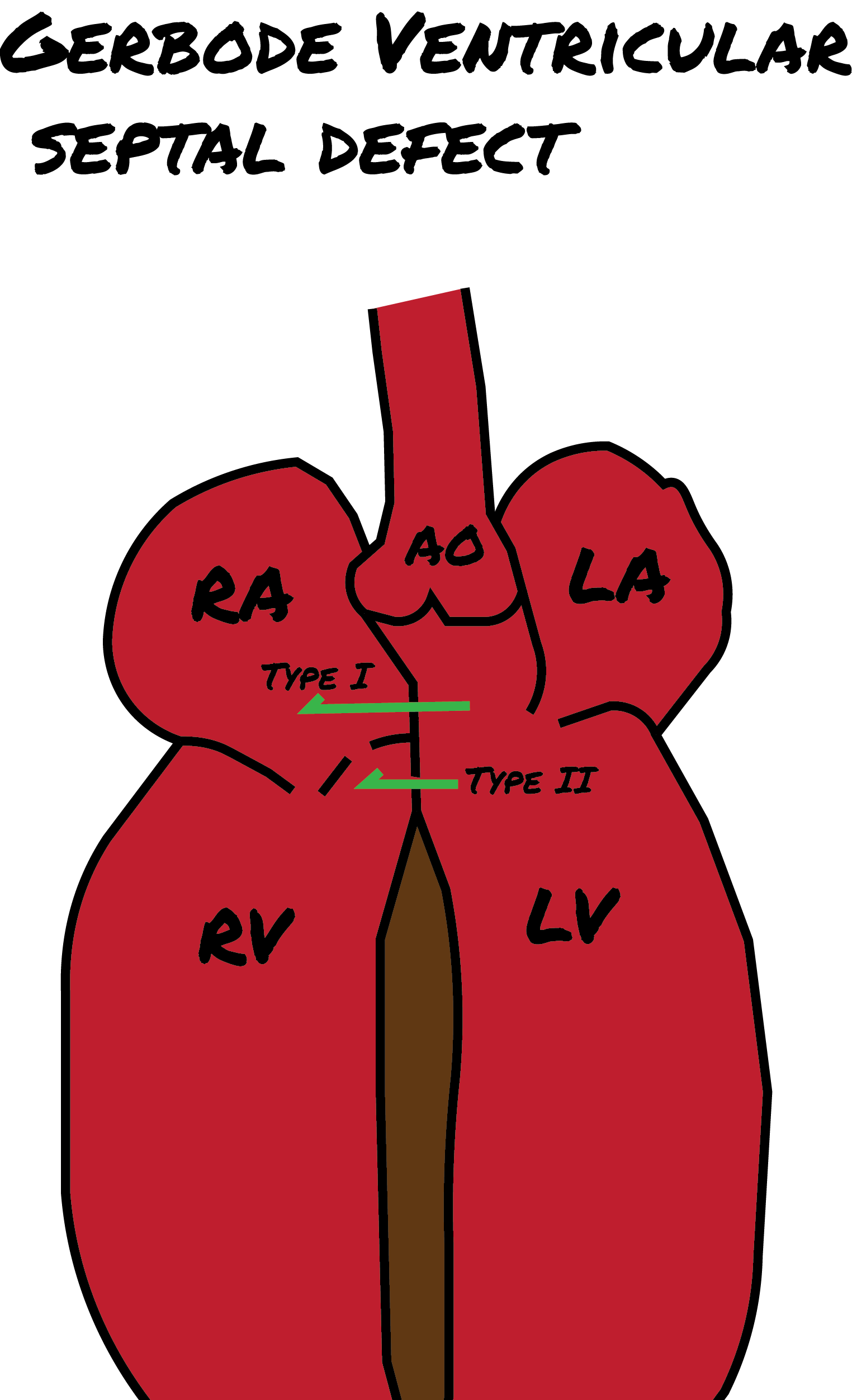
Gerbode Defects
In addition to the above 4 types of VSDs, the Gerbode defect is a distinct cardiac anomaly named after Frank Gerbode and colleagues from Stanford University School of Medicine, who first described it in 1958. This defect involves a direct connection between the left ventricle and the right atrium and is classified into 3 forms, as mentioned below (see Image. Gerbode Defects).
- Type 1: True Gerbode or supravalvular type is the rarest type, originating from a congenital deficiency in the atrioventricular component of the membranous septum.
- Type 2: Subvalvular type involves ventriculo-atrial shunting caused by a central VSD along with a deficiency in the septal leaflet of the tricuspid valve.
- Type 3: This represents a combination of the characteristics observed in both types 1 and 2.[24][25][26]
The primary pathophysiological mechanism of VSD involves the creation of a shunt between the right and left ventricles. The hemodynamic significance of the VSD depends on the volume of blood shunted and the direction of that shunting. These factors are influenced by the size and location of the VSD, as well as the pulmonary vascular resistance.
In addition to classifying VSDs by location, they can also be categorized by size, which is determined in relation to the diameter of the aortic annulus. VSDs are considered small if their dimensions are equal to or less than 25% of the aortic annulus diameter, medium if they measure more than 25% but less than 75%, and large if they exceed 75% of the aortic annulus diameter.
In prolonged, large left-to-right shunts, the pulmonary vascular endothelium undergoes irreversible changes, resulting in persistent PAH. When the pressure within the pulmonary circulation exceeds that of the systemic circulation, the shunt direction reverses, leading to a right-to-left shunt. This condition is known as Eisenmenger syndrome and is observed in approximately 10% to 15% of individuals with VSD.
Conventional teaching has long stated that corrective surgery is contraindicated in established cases of Eisenmenger syndrome. However, advancements in surgical techniques and a better understanding of the syndrome's pathophysiology indicate that surgical intervention may now be a viable option in select cases.[27]
History and Physical
The clinical presentation of unrepaired VSDs primarily depends on the presence of a hemodynamically significant shunt, which directly correlates with the size of the defect. VSDs can be categorized as follows:
- Small VSDs: These defects cause minimal left-to-right shunting without leading to left ventricular fluid overload or PAH. Consequently, individuals with small VSDs are often asymptomatic, and the condition is frequently discovered incidentally during physical examinations.
- Medium VSDs: Individuals with medium-sized VSDs experience moderate left ventricular volume overload and may exhibit no or mild PAH. Symptoms typically present later in childhood and may be accompanied by mild congestive heart failure (CHF).
- Large VSDs: Individuals with significant defects often develop CHF early in childhood due to severe left ventricular overload and PAH.
The murmur associated with a VSD is typically pansystolic and best heard at the left lower sternal border. In small defects, the murmur is harsh and loud, whereas in larger defects, it tends to be softer and less intense. Handgrip exercises, which increase afterload, can intensify the murmur. Murmurs associated with infundibular defects are best heard in the pulmonic area. A diastolic decrescendo murmur, along with a wide pulse pressure, may indicate aortic regurgitation. Increased left ventricular flow can lead to a mid-diastolic rumble at the lower left sternal border. Additionally, a septal aneurysm may occasionally produce a systolic click, particularly in membranous defects.
In cases of Eisenmenger syndrome, clinical manifestations include cyanosis, desaturation, dyspnea, syncope, secondary erythrocytosis, and clubbing. The typical murmur associated with VSD may be absent, and an accentuated pulmonic component of the second heart sound may be present.[28]
Evaluation
Color Doppler transthoracic echocardiography (TTE) is the most valuable diagnostic tool for VSDs due to its high sensitivity, detecting up to 95% of cases, particularly for nonapical lesions larger than 5 mm. This imaging technique provides crucial morphological information, including the size, location, and number of defects, along with hemodynamic data, such as jet size, severity, and estimates of pulmonary artery pressure.
Additionally, TTE is useful for identifying associated conditions, such as aortic insufficiency and other congenital heart defects, while also assessing right and left ventricular chamber size and function. However, TTE has limitations, including operator dependence and difficulties posed by poor acoustic windows. When conventional TTE results are inconclusive, transesophageal echocardiography is recommended for further evaluation.[29][30]
Electrocardiography (ECG) results are normal in about half of patients with VSD. The ECG may reveal left ventricular hypertrophy in individuals with large shunts when abnormalities are present. In patients with PAH, the ECG may demonstrate right bundle branch block, right axis deviation, and signs of right ventricular hypertrophy and strain.
Chest radiography usually appears normal in individuals with minor defects. However, larger defects may show an enlarged cardiac silhouette, along with increased left ventricular size. In patients with PAH, signs of right ventricular enlargement and increased pulmonary artery diameter may also be evident.
Cardiac magnetic resonance imaging (MRI) and computed tomography (CT) are beneficial for evaluating complex anatomy, such as VSDs associated with other congenital heart anomalies or defects located in unusual areas that are difficult to visualize with conventional TTE.
Cardiac catheterization is a vital diagnostic tool that provides accurate hemodynamic information, particularly regarding pulmonary vascular resistance and the response to vasodilators. This information is valuable for individuals being evaluated for surgical closure of VSDs. Furthermore, cardiac catheterization offers detailed insights into coexisting conditions, such as aortic regurgitation, and is beneficial in cases involving multiple VSDs or when coronary artery disease is suspected.
Treatment / Management
Approximately 85% to 90% of small isolated VSDs undergo spontaneous closure within the first year of life. Patients with small, asymptomatic VSDs, in the absence of PAH, typically have an excellent prognosis without intervention. However, if intervention becomes necessary, the management approach involves VSD closure.[31]
Management of Eisenmenger syndrome is typically performed at specialized centers due to the complexity of these cases. Historically, surgical intervention was the primary option, with heart-lung transplantation considered the ultimate solution. However, recent advancements in interventional techniques have demonstrated promising results.
Routine antibiotic prophylaxis for infective endocarditis in patients with unrepaired VSDs is no longer recommended.[32] Endocarditis prophylaxis is primarily indicated for individuals with cyanotic congenital heart disease, a history of previous endocarditis episodes, those with prosthetic heart valves, or individuals who have undergone repair with prosthetic material.
VSD closure is generally indicated for medium-to-large defects causing significant hemodynamic compromise, especially in symptomatic cases with left ventricular dysfunction. Intervention may also be warranted in instances of progressive aortic insufficiency or following an episode of endocarditis. The 2008 guidelines from the American College of Cardiology and the American Heart Association outline the following indications for surgical closure of VSDs:
- History of endocarditis.
- Pulmonary to systemic blood flow (Qp:Qs) ratio of greater than or equal to 2 with clinical evidence of left ventricular fluid overload.
Surgical intervention may also be considered for milder shunts (Qp:Qs >1.5) if there is evidence of left ventricular systolic or diastolic dysfunction. Additionally, surgical closure is recommended when the pulmonary artery pressure and pulmonary vascular resistance are less than two-thirds of systemic pressure and systemic vascular resistance, respectively.
Surgical Repair
Surgical repair of VSDs offers several benefits, including a reduced risk of endocarditis, potential improvement in PAH, and enhanced overall survival. In the absence of PAH, the operative mortality rate is approximately 1%. However, surgical intervention may present potential complications.
Complications of VSD surgical repair include:
- Residual or recurrent VSD.
- Valvular incompetence: This includes tricuspid regurgitation and aortic insufficiency.
- Arrhythmias: Common postoperative arrhythmias may include atrial fibrillation, complete heart block, and ventricular tachycardia.
- Left ventricular dysfunction.
- Progression of PAH.
The primary contraindication for surgical VSD closure is the presence of irreversible PAH, as this significantly increases the risk of perioperative mortality and complications. In these cases, alternative management strategies are explored to address the underlying condition while minimizing risk.[33](A1)
Percutaneous Closure
Indications for transcatheter closure: Transcatheter closure is increasingly recognized as an effective intervention for various types of VSDs, with specific indications guiding its use based on the defect's characteristics and the patient's clinical condition.
- Muscular VSDs: Transcatheter closure is primarily recommended for muscular VSDs, particularly those located in the mid-muscular or apical regions, as these defects are often difficult to access surgically.
- Perimembranous VSDs: Selected cases of perimembranous VSDs may be appropriate for transcatheter closure, depending on factors such as the defect's size and location, its proximity to the aortic valve, and the associated risk of complications such as heart block.
- Hemodynamically significant VSDs: Patients exhibiting significant left-to-right shunts that result in left heart volume overload, heart failure symptoms, or growth failure despite optimal medical management may be considered for intervention.
- Residual VSDs postoperative: Residual defects following surgical repair of a VSD may be appropriate for transcatheter closure, particularly if they are hemodynamically significant or symptomatic.
Contraindications: Certain clinical scenarios may preclude the use of transcatheter closure for VSDs.
- Large defects: VSDs that are too large to be effectively closed with commercially available devices.
- Proximity to vital structures: VSDs located near vital structures, such as the aortic valve, tricuspid valve, or conduction tissue (eg, the Bundle of His), where there is a high risk of damaging the valves or causing heart block.
- Infective endocarditis: Active infection or a history of infective endocarditis associated with the defect.
- Pulmonary hypertension with shunt reversal (Eisenmenger syndrome): Patients with irreversible pulmonary vascular disease are not candidates for closure.
Closure of Gerbode Defects
This intervention is typically recommended to prevent the progression of tricuspid regurgitation and the potential development of infective endocarditis. Although spontaneous closure of Gerbode defects is rare, effective treatment options are available.
- Surgical closure is the most common and widely used treatment for Gerbode defects, offering well-established and favorable outcomes.
- Transcatheter device closure serves as an effective alternative treatment. Although less common, successful cases of transcatheter closure have been reported for both congenital and acquired Gerbode defects. Key considerations during the procedure include achieving proper device alignment, mitigating potential conduction disturbances, and minimizing the risk of exacerbating tricuspid regurgitation.
In summary, VSD is the most common congenital anomaly identified at birth. While small defects often close spontaneously within the first year of life, larger defects can lead to significant complications. Surgical closure and transcatheter device closure are the primary interventions for managing larger defects effectively.[19][34][35](B3)
Differential Diagnosis
Although VSD is a distinct condition, several cardiac and non-cardiac disorders can present with similar symptoms. The differential diagnosis for VSD includes:
- Atrioventricular septal defect
- Atrial septal defect
- Patent ductus arteriosus
- Pulmonary stenosis
- Tetralogy of Fallot
- Mitral valve prolapse
- Tricuspid regurgitation
- Eisenmenger syndrome
- Infective endocarditis
- Myocarditis
Prognosis
Patients who have undergone VSD repair generally have a favorable prognosis. However, they have an elevated long-term risk of arrhythmia, endocarditis, and heart failure compared to the general population.[36]
Complications
Complications of VSD may include:
- Eisenmenger syndrome
- Aortic insufficiency from aortic valve leaflet prolapse
- Endocarditis
- Embolization
- Heart failure
- Pulmonary hypertension
- Arrythmias [37]
Consultations
Evaluating and managing VSD may require consultations with the following specialists:
- Pediatric and adult cardiologists
- Cardiothoracic surgeons
- Cardiac electrophysiologists
Deterrence and Patient Education
Parents of children with small VSDs should be informed that medical or surgical intervention is often unnecessary, as these defects commonly close on their own. Prophylactic antibiotics for endocarditis are generally no longer required, but maintaining good oral hygiene is crucial to minimize the risk of endocarditis.[32] Adherence to prescribed medications and regular follow-ups with specialists are essential for all patients with VSDs.
Pearls and Other Issues
Key facts to keep in mind about VSDs include:
- This is the most common congenital heart defect in children.
- Small VSDs often require no intervention, with spontaneous closure being common.
- The pansystolic murmur of VSD can be mistaken for that of mitral regurgitation; however, the VSD murmur intensifies near the sternum, while the murmur of mitral regurgitation is louder away from the sternum.
- Prophylactic antibiotics to prevent endocarditis are no longer routinely recommended.
- Larger or unrepaired VSDs can lead to complications such as arrhythmias, heart failure, and endocarditis.
- Although antibiotics may not be necessary, good oral hygiene is crucial to reduce the risk of endocarditis.
Enhancing Healthcare Team Outcomes
Optimal management of VSD involves a collaborative approach among the interprofessional healthcare team, including a pediatrician, cardiologist, cardiac surgeon, nurses (intensive care and cardiac), physical therapist, and social worker. Educating patients (and their parents) is essential to ensure adherence to follow-up care.
Some individuals with perimembranous VSD may develop aortic valve prolapse, requiring surgical intervention. Unrepaired VSDs can elevate pulmonary vascular resistance, leading to Eisenmenger syndrome. Unfortunately, treatment options for Eisenmenger syndrome are limited, with heart and lung transplantation being the primary alternatives. However, due to organ shortages, many patients with Eisenmenger syndrome experience progressive right heart failure and cyanosis, often resulting in unfavorable outcomes.[38][39]
Young, asymptomatic children with small VSDs generally have favorable outcomes. However, symptoms can arise if complications such as anemia, infection, or endocarditis develop. In contrast, individuals with large, unrepaired VSDs face poorer outcomes due to left-to-right shunting, leading to pulmonary hypertension and Eisenmenger syndrome. In North America, elective VSD repair within the first 2 years of life is standard practice, with a mortality rate below 1% and most patients achieving a normal lifespan.[40]
Media
(Click Image to Enlarge)
References
Nguyen RT, Satish P, Atkins MD Jr, Goel SS. An Undiagnosed Ventricular Septal Rupture Presenting as New Onset Heart Failure: A Rare Complication of an Anterior Myocardial Infarction. Methodist DeBakey cardiovascular journal. 2022:18(1):113-116. doi: 10.14797/mdcvj.1157. Epub 2022 Dec 5 [PubMed PMID: 36561850]
Ghosh S, Sridhar A, Solomon N, Sivaprakasham M. Transcatheter closure of ventricular septal defect in aortic valve prolapse and aortic regurgitation. Indian heart journal. 2018 Jul-Aug:70(4):528-532. doi: 10.1016/j.ihj.2017.11.023. Epub 2017 Nov 27 [PubMed PMID: 30170648]
Hopkins MK, Goldstein SA, Ward CC, Kuller JA. Evaluation and Management of Maternal Congenital Heart Disease: A Review. Obstetrical & gynecological survey. 2018 Feb:73(2):116-124. doi: 10.1097/OGX.0000000000000536. Epub [PubMed PMID: 29480926]
Level 2 (mid-level) evidenceKenny D. Interventional Cardiology for Congenital Heart Disease. Korean circulation journal. 2018 May:48(5):350-364. doi: 10.4070/kcj.2018.0064. Epub 2018 Mar 29 [PubMed PMID: 29671282]
Ammash NM, Warnes CA. Ventricular septal defects in adults. Annals of internal medicine. 2001 Nov 6:135(9):812-24 [PubMed PMID: 11694106]
Vereczkey A, Gerencsér B, Czeizel AE, Szabó I. Association of certain chronic maternal diseases with the risk of specific congenital heart defects: a population-based study. European journal of obstetrics, gynecology, and reproductive biology. 2014 Nov:182():1-6. doi: 10.1016/j.ejogrb.2014.08.022. Epub 2014 Aug 27 [PubMed PMID: 25216447]
Gujral JS, Relan J, Naik N. An unusual cause of changing QRS morphology. Journal of electrocardiology. 2020 Sep-Oct:62():33-35. doi: 10.1016/j.jelectrocard.2020.08.009. Epub 2020 Aug 11 [PubMed PMID: 32799008]
Kohl A, Golan N, Cinnamon Y, Genin O, Chefetz B, Sela-Donenfeld D. A proof of concept study demonstrating that environmental levels of carbamazepine impair early stages of chick embryonic development. Environment international. 2019 Aug:129():583-594. doi: 10.1016/j.envint.2019.03.064. Epub 2019 Jun 4 [PubMed PMID: 31174146]
Level 2 (mid-level) evidenceWilliams LJ, Correa A, Rasmussen S. Maternal lifestyle factors and risk for ventricular septal defects. Birth defects research. Part A, Clinical and molecular teratology. 2004 Feb:70(2):59-64 [PubMed PMID: 14991912]
Level 2 (mid-level) evidenceLyu J, Zhao K, Xia Y, Zhao A, Yin Y, Hong H, Li S. Associations between maternal social support and stressful life event with ventricular septal defect in offspring: a case-control study. BMC pregnancy and childbirth. 2019 Nov 21:19(1):429. doi: 10.1186/s12884-019-2541-y. Epub 2019 Nov 21 [PubMed PMID: 31752736]
Level 2 (mid-level) evidenceKovalenko AA, Anda EE, Odland JØ, Nieboer E, Brenn T, Krettek A. Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study. International journal of environmental research and public health. 2018 Jun 24:15(7):. doi: 10.3390/ijerph15071320. Epub 2018 Jun 24 [PubMed PMID: 29937526]
Reece AS, Hulse GK. European epidemiological patterns of cannabis- and substance-related congenital cardiovascular anomalies: geospatiotemporal and causal inferential study. Environmental epigenetics. 2022:8(1):dvac015. doi: 10.1093/eep/dvac015. Epub 2022 Jul 5 [PubMed PMID: 35966825]
Level 2 (mid-level) evidenceFesslova V, Brankovic J, Lalatta F, Villa L, Meli V, Piazza L, Ricci C. Recurrence of congenital heart disease in cases with familial risk screened prenatally by echocardiography. Journal of pregnancy. 2011:2011():368067. doi: 10.1155/2011/368067. Epub 2011 Oct 1 [PubMed PMID: 21977323]
Level 3 (low-level) evidenceGelson E, Curry R, Gatzoulis MA, Swan L, Lupton M, Steer P, Johnson M. Effect of maternal heart disease on fetal growth. Obstetrics and gynecology. 2011 Apr:117(4):886-891. doi: 10.1097/AOG.0b013e31820cab69. Epub [PubMed PMID: 21422861]
Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJ, Vliegen HW, van Dijk AP, Voors AA, Yap SC, van Veldhuisen DJ, Pieper PG, ZAHARA Investigators. Predictors of pregnancy complications in women with congenital heart disease. European heart journal. 2010 Sep:31(17):2124-32. doi: 10.1093/eurheartj/ehq200. Epub 2010 Jun 28 [PubMed PMID: 20584777]
Muthialu N, Balakrishnan S, Sundar R. Single patch closure of multiple VSDs through right atrial approach. Indian heart journal. 2018 Jul-Aug:70(4):578-579. doi: 10.1016/j.ihj.2018.01.017. Epub 2018 Jan 11 [PubMed PMID: 30170657]
Durden RE, Turek JW, Reinking BE, Bansal M. Acquired ventricular septal defect due to infective endocarditis. Annals of pediatric cardiology. 2018 Jan-Apr:11(1):100-102. doi: 10.4103/apc.APC_130_17. Epub [PubMed PMID: 29440841]
Azab B, Aburizeg D, Ji W, Jeffries L, Isbeih NJ, Al-Akily AS, Mohammad H, Osba YA, Shahin MA, Dardas Z, Hatmal MM, Al-Ammouri I, Lakhani S. TBX5 variant with the novel phenotype of mixed‑type total anomalous pulmonary venous return in Holt‑Oram Syndrome and variable intrafamilial heart defects. Molecular medicine reports. 2022 Jun:25(6):. pii: 210. doi: 10.3892/mmr.2022.12726. Epub 2022 May 6 [PubMed PMID: 35514310]
Cresti A, Giordano R, Koestenberger M, Spadoni I, Scalese M, Limbruno U, Falorini S, Stefanelli S, Picchi A, De Sensi F, Malandrino A, Cantinotti M. Incidence and natural history of neonatal isolated ventricular septal defects: Do we know everything? A 6-year single-center Italian experience follow-up. Congenital heart disease. 2018 Jan:13(1):105-112. doi: 10.1111/chd.12528. Epub 2017 Aug 30 [PubMed PMID: 28857497]
Pugnaloni F, Felici A, Corno AF, Marino B, Versacci P, Putotto C. Gender differences in congenital heart defects: a narrative review. Translational pediatrics. 2023 Sep 18:12(9):1753-1764. doi: 10.21037/tp-23-260. Epub 2023 Sep 11 [PubMed PMID: 37814719]
Level 3 (low-level) evidencePatel ND, Kim RW, Pornrattanarungsi S, Wong PC. Morphology of intramural ventricular septal defects: Clinical imaging and autopsy correlation. Annals of pediatric cardiology. 2018 Sep-Dec:11(3):308-311. doi: 10.4103/apc.APC_139_17. Epub [PubMed PMID: 30271023]
Lopez L, Houyel L, Colan SD, Anderson RH, Béland MJ, Aiello VD, Bailliard F, Cohen MS, Jacobs JP, Kurosawa H, Sanders SP, Walters HL 3rd, Weinberg PM, Boris JR, Cook AC, Crucean A, Everett AD, Gaynor JW, Giroud J, Guleserian KJ, Hughes ML, Juraszek AL, Krogmann ON, Maruszewski BJ, St Louis JD, Seslar SP, Spicer DE, Srivastava S, Stellin G, Tchervenkov CI, Wang L, Franklin RCG. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases-Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. The Annals of thoracic surgery. 2018 Nov:106(5):1578-1589. doi: 10.1016/j.athoracsur.2018.06.020. Epub 2018 Jul 19 [PubMed PMID: 30031844]
Level 3 (low-level) evidencePinto NM, Waitzman N, Nelson R, Minich LL, Krikov S, Botto LD. Early Childhood Inpatient Costs of Critical Congenital Heart Disease. The Journal of pediatrics. 2018 Dec:203():371-379.e7. doi: 10.1016/j.jpeds.2018.07.060. Epub 2018 Sep 26 [PubMed PMID: 30268400]
Wasserman SM, Fann JI, Atwood JE, Burdon TA, Fadel BM. Acquired left ventricular-right atrial communication: Gerbode-type defect. Echocardiography (Mount Kisco, N.Y.). 2002 Jan:19(1):67-72 [PubMed PMID: 11884258]
GERBODE F, HULTGREN H, MELROSE D, OSBORN J. Syndrome of left ventricular-right atrial shunt; successful surgical repair of defect in five cases, with observation of bradycardia on closure. Annals of surgery. 1958 Sep:148(3):433-46 [PubMed PMID: 13571920]
Level 3 (low-level) evidenceSAKAKIBARA S, KONNO S. LEFT VENTRICULAR-RIGHT ATRIAL COMMUNICATION. Annals of surgery. 1963 Jul:158(1):93-9 [PubMed PMID: 14042644]
Manuel L, Freeman L, Nashef SA. Surgery for Eisenmenger syndrome: time for a rethink? Journal of the Royal Society of Medicine. 2019 Dec:112(12):512-513. doi: 10.1177/0141076819877551. Epub 2019 Sep 17 [PubMed PMID: 31526213]
Barradas-Pires A, Constantine A, Dimopoulos K. Preventing disease progression in Eisenmenger syndrome. Expert review of cardiovascular therapy. 2021 Jun:19(6):501-518. doi: 10.1080/14779072.2021.1917995. Epub 2021 May 6 [PubMed PMID: 33853494]
Maagaard M, Heiberg J, Eckerström F, Asschenfeldt B, Rex CE, Ringgaard S, Hjortdal VE. Biventricular morphology in adults born with a ventricular septal defect. Cardiology in the young. 2018 Dec:28(12):1379-1385. doi: 10.1017/S1047951118001361. Epub 2018 Aug 30 [PubMed PMID: 30160649]
Hadeed K, Hascoët S, Karsenty C, Ratsimandresy M, Dulac Y, Chausseray G, Alacoque X, Fraisse A, Acar P. Usefulness of echocardiographic-fluoroscopic fusion imaging in children with congenital heart disease. Archives of cardiovascular diseases. 2018 Jun-Jul:111(6-7):399-410. doi: 10.1016/j.acvd.2018.03.006. Epub 2018 May 28 [PubMed PMID: 29853351]
Zhao QM, Niu C, Liu F, Wu L, Ma XJ, Huang GY. Spontaneous Closure Rates of Ventricular Septal Defects (6,750 Consecutive Neonates). The American journal of cardiology. 2019 Aug 15:124(4):613-617. doi: 10.1016/j.amjcard.2019.05.022. Epub 2019 May 25 [PubMed PMID: 31208700]
Sendi P, Hasse B, Frank M, Flückiger U, Boggian K, Guery B, Jeger R, Zbinden S, Agyeman P, Knirsch W, Greutmann M. Infective endocarditis: prevention and antibiotic prophylaxis. Swiss medical weekly. 2021 Feb 15:151():w20473. doi: 10.4414/smw.2021.20473. Epub 2021 Feb 28 [PubMed PMID: 33705562]
Matteucci M, Ronco D, Corazzari C, Fina D, Jiritano F, Meani P, Kowalewski M, Beghi C, Lorusso R. Surgical Repair of Postinfarction Ventricular Septal Rupture: Systematic Review and Meta-Analysis. The Annals of thoracic surgery. 2021 Jul:112(1):326-337. doi: 10.1016/j.athoracsur.2020.08.050. Epub 2020 Nov 4 [PubMed PMID: 33157063]
Level 1 (high-level) evidenceGarg N, Nayyar M, Khouzam RN, Salem SA, Ardeshna D. Peri-procedural antibiotic prophylaxis in ventricular septal defect: a case study to re-visit guidelines. Annals of translational medicine. 2018 Jan:6(1):18. doi: 10.21037/atm.2017.11.11. Epub [PubMed PMID: 29404364]
Level 3 (low-level) evidenceCantinotti M, Assanta N, Murzi B, Lopez L. Controversies in the definition and management of insignificant left-to-right shunts. Heart (British Cardiac Society). 2014 Feb:100(3):200-5. doi: 10.1136/heartjnl-2013-304372. Epub 2013 Jul 25 [PubMed PMID: 23886608]
Goldberg JF. Long-term Follow-up of "Simple" Lesions--Atrial Septal Defect, Ventricular Septal Defect, and Coarctation of the Aorta. Congenital heart disease. 2015 Sep-Oct:10(5):466-74. doi: 10.1111/chd.12298. Epub 2015 Sep 14 [PubMed PMID: 26365715]
Elkattawy S, Alyacoub R, Noori MAM, Talpur A, Khimani K. A Rare Complication of Myocardial Infarction: Ventricular Septal Defect. Cureus. 2020 Aug 13:12(8):e9725. doi: 10.7759/cureus.9725. Epub 2020 Aug 13 [PubMed PMID: 32944444]
Hoashi T, Yazaki S, Kagisaki K, Kitano M, Shimada M, Shiraishi I, Ichikawa H. Importance of multidisciplinary management for pulmonary atresia, ventricular septal defect, major aorto-pulmonary collateral arteries and completely absent central pulmonary arteries. General thoracic and cardiovascular surgery. 2017 Jun:65(6):337-342. doi: 10.1007/s11748-017-0765-1. Epub 2017 Mar 4 [PubMed PMID: 28260150]
Schuh M, Schendel S, Islam S, Klassen K, Morrison L, Rankin KN, Robert C, Mackie AS. Parent readiness for discharge from a tertiary care pediatric cardiology unit. Journal for specialists in pediatric nursing : JSPN. 2016 Jul:21(3):139-46. doi: 10.1111/jspn.12148. Epub 2016 Jul 4 [PubMed PMID: 27373700]
Rao PS, Harris AD. Recent advances in managing septal defects: ventricular septal defects and atrioventricular septal defects. F1000Research. 2018:7():. pii: F1000 Faculty Rev-498. doi: 10.12688/f1000research.14102.1. Epub 2018 Apr 26 [PubMed PMID: 29770201]
Level 3 (low-level) evidence