Introduction
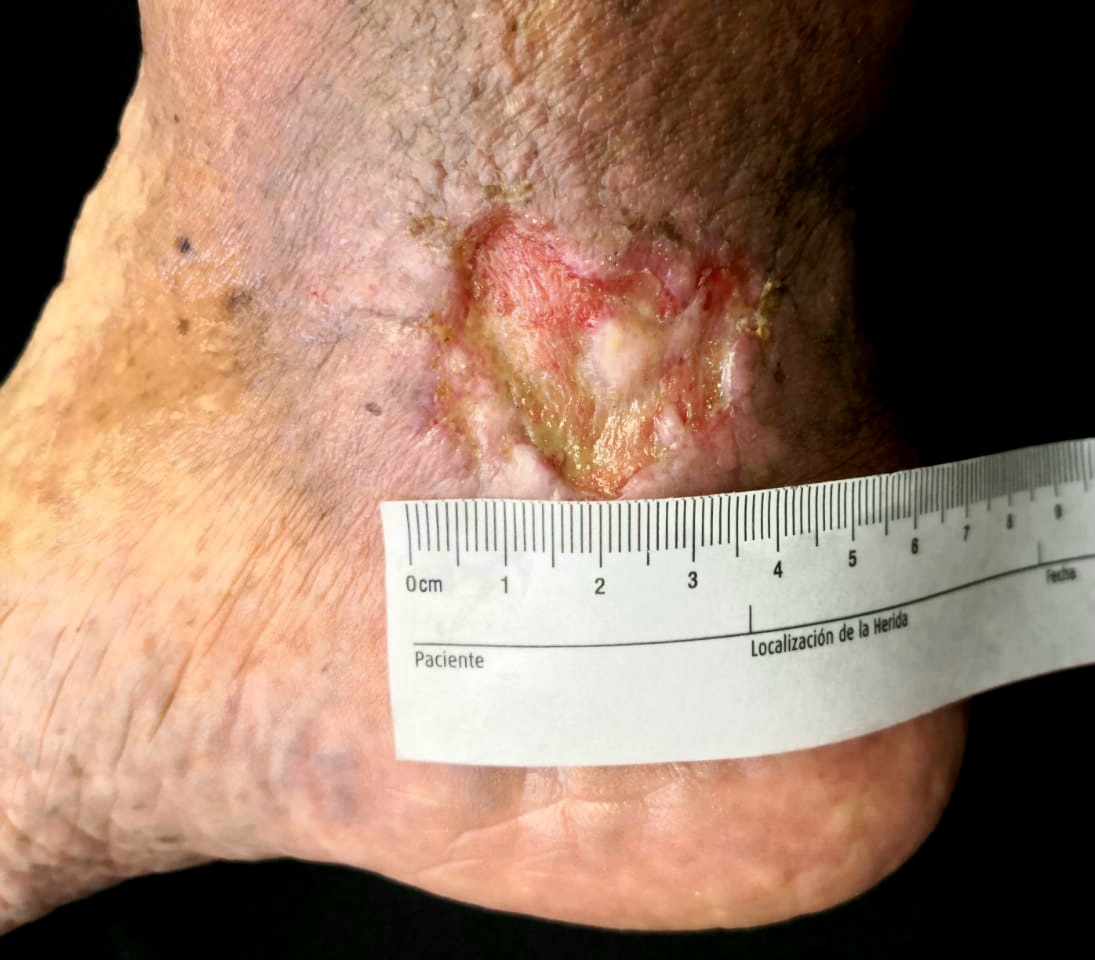
Venous leg ulcers (VLUs) are late indicators of chronic venous insufficiency (CVI) and venous hypertension.[1][2][3][4] Calf muscle contraction and intraluminal valves promote prograde flow while preventing blood reflux in normal conditions.[5] However, when retrograde flow, obstruction, or both exist, chronic venous hypertension is responsible for the dermatologic and vascular complications that form a VLU (See Image. Venous Leg Ulcer).[6]
VLUs are a costly medical condition that greatly affects worldwide healthcare systems.[7][8][9] In the United States (US), the annual cost of Medicare and commercial insurance for caring for patients with VLU is $18,986 and $13,653, respectively.[10] Together, these represent an annual burden of $14.9 billion for the US payers, a considerable increase from a previous $1 billion estimate.[11] The situation may worsen as the aging, obese, and sedentary population increases globally, with an expected rise in the incidence of CVI and VLUs.[12]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Although CVI is a well-known precipitant for VLU development, ulceration occurs rarely (5.1%) for unclear reasons.[13] CVI may develop due to blood reflux, obstruction, or both mechanisms, causing macro- and micro-circulatory dysfunction.[5] The increased intraluminal pressure causes protein extravasation and fibrin cuff formation, which impedes the diffusion of oxygen and growth factors and activates the inflammatory response.[14][15]
At a cellular level, mast cell degranulation, leukocyte recruitment, increased matrix metalloproteinase inhibitors, and prostacyclin, the acquisition of a non-contractile secretory phenotype by smooth muscle cells, and fibroblast differentiation into myofibroblasts participate in vein wall remodeling and varix formation. The proinflammatory microenvironment is maintained by M1 macrophages, mainly by releasing interleukin-1α, interferon-gamma, and transforming growth factor-beta 1. Chronic inflammation and incompetent blood flow favor thrombus formation, causing further fibrosis and valvular destruction.[16] Together, this inflammatory cascade of events impairs healing processes, which results in ulcer formation upon wounding.
Most risk factors for the development of VLUs are non-modifiable, and patients often present with more than 1. These involve a family history of CVI, advanced age, female sex, previous thrombosis or pulmonary embolism, multiparity, lipodermatosclerosis, and musculoskeletal and joint disease.[5][1] Modifiable risk factors such as obesity and sedentarism are also associated with venous disease.[17][18][19] Genetic traits may be an additional predisposing factor, presenting as an autosomal dominant trait with variable penetrance.[1][14] However, a specific gene or gene set has not been determined. Forkhead box C2, located on chromosome 16q24, is a candidate marker in subjects with varicose veins.[20]
Compared with healing VLUs, the gene expression profile of non-healing VLUs showed upregulation of secreted frizzled-related protein 4, branched-chain aminotransferase 1, dermatopontin, cytochrome P450, and 17 B hydroxysteroid dehydrogenase genes, which are involved in inflammation control, Wnt signaling, cell growth, extracellular matrix assembly, and steroidogenesis.[21][22][23][24] Conversely, collagen differentiation and epidermal repair genes such as collagen type 13α1, collagen 27α1, keratin 14, keratin 16, and heparin-binding epidermal growth factor were notoriously downregulated. Besides these, single nucleotide polymorphisms of high iron, ferroportin 1, and matrix metalloproteinase 12 indicate venous ulcer development susceptibility.[22][25][26] A gene expression study's results showed that VLUs treated with a bioengineered bilayered living cellular construct skin substitute displayed a shift from a chronic non-healing inflammatory profile to acute healing inflammation, highlighting the negative effects of chronic inflammation in wound healing.[27]
Epidemiology
VLUs are the most common type of chronic wound in the lower extremity.[28] There is an estimation that 1% to 3% of the older adult population is affected in the US and Europe.[29] An epidemiological survey from Asia, Eastern Europe, Latin America, and Western Europe showed that 2.21% of 99,359 patients with CVI had an active or healed VLU when visiting their primary care clinician for various reasons. Individual regional rates were reported at 1.27%, 2.87%, 3.97%, and 1.67%, respectively.[30] The overall incidence is higher for women than men, although the exact number is hard to establish as it depends on the cohort and place of study.[29][31] The average time from CVI diagnosis to ulceration is 5 years, according to the results from a 25-year population study.[32] Another study's results demonstrated that the 3-year risk for the first ulceration in people with CVI was 4.49%, ascending to 4.93% at the 5-year follow-up.[13]
Histopathology
Unless another diagnosis is suspected, a biopsy is not warranted.[33][34] If performed, specimens should be obtained from the wound edge and the base of the ulcer.
History and Physical
Identifying risk factors based on clinical history helps distinguish VLUs from other causes of non-healing wounds in the lower extremities. Patients with CVI and VLUs often refer to pruritus with or without a rash, aching pain in the gaiter area, evening pedal edema, and night cramps.[35][36] Early CVI physical findings must be identified on examination. The initial manifestations are telangiectases and reticular veins.[5] Varicose veins, brown-orange hyperpigmentation, chronic leg edema, stasis dermatitis, atrophie blanche, and lipodermatosclerosis are late indicators of venous insufficiency. VLUs typically affect the distal end of the legs over the medial aspect, appearing as shallow, irregular, well-defined ulcers with fibrinous material on the base.[37][38] The clinical, etiological, anatomical, and pathophysiological classification documents these findings for therapeutic and research purposes.[39] Other less-used instruments are the venous clinical severity score and the Widmer classification.[40][41]
Evaluation
Clinical assessment should describe the ulcer area, depth, edges, wound base, signs of infection, and peripheral skin changes.[33][42][34][42][34] Palpation of distal pulses and ankle-brachial pressure index (ABPI) are used to evaluate adequate arterial blood flow since approximately 20% of patients with VLU have coincident arterial disease. ABPI is obtained by dividing the systolic ankle pressure by the systolic arm pressure in the supine position. An ABPI greater than 1 to 1.3 is considered normal. Below this, cut-off values are used to classify disease severity and guide the appropriateness of compression therapy (see Table. Ankle-Brachial Pressure Index).[43] Paradoxically, an ABPI greater than 1.3 appears in patients with significant vascular calcification, indicating the need for urgent referral to a vascular specialist.[43]
Table. Ankle-Brachial Pressure Index
|
Ankle-brachial pressure index |
Arterial circulation |
Compression treatment |
|
Greater than 1-1.3 |
Normal |
Apply compression |
|
Equal to 0.8-1.0 |
Mild peripheral disease |
Apply compression with caution |
|
Less than or equal to 0.8-0.6 |
Significant arterial disease |
Use modified compression with caution and refer to a vascular specialist |
|
Less than 0.5 |
Critical ischemia |
Do not compress and urgently refer to a vascular specialist |
Color-flow duplex ultrasound is another inexpensive, non-invasive, and highly informative diagnostic test useful for superficial vein assessment.[34][44] The technique can identify thrombi presence and valve incompetence, declared when reflux time exceeds 0.5 seconds.[45] Computed tomography and magnetic resonance imaging are preferred for deeper vessels since they are often harder or impossible to evaluate with ultrasound. Phlebography, plethysmography, and phlebodynamometry are less used due to inferior accuracy and associated risks.[46][47]
Treatment / Management
The standard of care for VLUs relies on 2 strategies: compression therapy and direct wound management.[36] Reducing leg edema is crucial for the effectiveness of wound closure.[33] There are numerous conservative alternatives aimed at improving venous hypertension, such as medical compression, intermittent pneumatic compression (IPC), manual lymphatic drainage, and extracorporeal shockwave therapy. Compression therapy is the most practical, effective, and cost-conscious intervention for the treatment of VLUs.[6][34] This involves using various types of hosiery or bandages, which can be elastic, inelastic, single- or multi-layered.[48] Unlike inelastic wraps, elastic materials provide moldable compression during rest and physical activity.[49] According to a Cochrane review, multi-component, stretchy systems provide superior venous ulcer healing rates compared with single-layered systems.[50] Compression strength can be light (14 to 17 mm Hg), moderate (18 to 24 mm Hg), high (23 to 35 mm Hg), or extra-high (up to 60 mm Hg). High compression is the preferred strength and should reach the knee or higher.[34] Light, cautious compression can be used in mild or significant peripheral vascular disease cases, as the ABPI indicates.[43] Absolute contraindications for compression therapy include arterial occlusive disease, ABPI less than 0.5, serious uncontrolled high blood pressure, heart failure, suspected or documented thrombosis, extensive thrombophlebitis, and erysipelas.(A1)
IPC can be used in addition to compression therapy or in patients who are unable to tolerate the latter.[34] According to a systematic review, normal IPC and rapid IPC were superior to dressings and slow IPC delivery, respectively.[51] Lymphatic drainage and extracorporeal shockwave therapy have very low evidence in VLU treatment.[52][53] As an adjunct to compression therapy, treatment with pentoxifylline or micronized purified flavonoids is an effective ancillary measure.[54] Both drugs have shown improved healing in various systematic reviews and meta-analyses.[55][56][57][58] There is insufficient evidence to recommend other systemic treatments, namely aspirin, zinc, doxycycline, calcium dobesilate, stanozolol, and cilostazol.[54][59](A1)
Direct ulcer interventions involve cleansing, debridement, infection control, and applying dressings and topical substances.[38] Cleansing should be performed with a non-toxic substance to minimize damage to the viable tissue.[34] Debridement can be achieved by surgically removing non-viable tissue, which is strongly recommended.[49] Topical or injected anesthesia can be used to minimize discomfort; autolytic dressings, larval therapy, and enzymatic agents are painless debridement options, albeit results may take longer.[34][38] (A1)
Although VLUs are often colonized, frank infections are less common. Systemic antibiotic therapy should be considered if the infection is suspected to be a new-onset painful ulcer with erythema, tenderness, warmth, and systemic signs (eg, fever, chills). Tissue culture (as opposed to a swab) must be performed in fetid, purulent, or non-healing wounds to guide antimicrobial therapy. Dressings maintain adequate moisture, offer physical protection, and promote granulation and reepithelization. There are numerous absorptive (eg, alginates), moisture-retaining (eg, hydrocolloids), and antiseptic materials (eg, silver) that can be applied to dry, exudative, and infected wounds, respectively. Topical treatments are typically used as antiseptics (eg, cadexomer-iodine), antimicrobials (eg, silver sulfadiazine), and debriding agents such as collagenase or hydrogels).
In a Cochrane meta-analysis, sucralfate and silver dressings were the most highly ranked treatments. Interestingly, using silver dressings increased the probability of healing (risk ratio 2.43, 95% confidence interval 1.58 to 3.74) compared with nonadherent dressings.[60] However, this arose from low-certainty evidence, and the authors could not establish specific treatment recommendations. The benefit-cost balance must be considered since these are often more expensive materials. Adjunctive therapies are recommended for non-improving ulcers after 4 to 6 weeks of standard treatment.[34] Tissue-based products may be useful for these complicated scenarios. According to a systematic review, cultured bilayered skin grafting is more effective than conventional dressings when both were used with compression therapy; there is insufficient evidence to determine if other skin substitutes can effectively improve the healing of VLUs.[61](A1)
Invasive management intends to eliminate incompetent veins through various techniques, such as sclerotherapy, open vascular surgery, radiofrequency, and endovenous procedures. These are often reserved for advanced cases when standard therapies are insufficient.[5][62] In a multicenter study of 450 patients with venous leg ulcers, early endovenous ablation was associated with faster healing and a longer ulcer-free period compared to patients who had the procedure delayed until their ulcer healed or until 6 months if it remained unhealed.[63] (A1)
Differential Diagnosis
Other causes of lower extremity ulceration may be distinguished from VLUs based on the history and physical examination.[1] Arterial ulcers, caused by tissue ischemia, normally appear as deep, dry wounds affecting the toes, foot dorsum, and the anterior aspect of the leg, with abnormal distal pulses, ABPI, and cold extremities. Neuropathic or diabetic ulcers occur due to peripheral neuropathy and arterial disease, presenting as deep wounds surrounded by callous, mainly over the osseous prominences of the plantar surface.
Pressure ulcers arise in limited mobility, usually on the sacrum, coccyx, heels, hips, or anywhere else affected by prolonged pressure and high shear forces. Non-vascular diagnoses should be considered when ulcers show excessive granulation tissue, elevated borders, and failure to improve after standard treatment.[64] These include various types of skin cancers and pyoderma gangrenosum, for which a biopsy is essential.[65][66] Lower extremity ulceration may also occur in calciphylaxis, vasculitis, sickle-cell disease, and other infectious and connective-tissue diseases.
Staging
There is no specific staging instrument for VLUs, but the clinical, etiology, anatomy, pathophysiology (CEAP) classification is often used in patients with CVI patients. See Table. CEAP Classification.[39]
Table. CEAP Classification
|
Clinical |
|
|
C0 |
No visible or palpable signs of venous disease |
|
C1 |
Telangiectases or reticular veins |
|
C2 |
Varicose veins |
|
C3 |
Edema |
|
C4a |
Pigmentation or eczema |
|
C4b |
Lipodermatosclerosis or atrophie blanche |
|
C5 |
Healed venous ulcer |
|
C6 |
Active venous ulcer |
|
Etiological |
|
|
Ec |
Congenital |
|
Ep |
Primary |
|
Es |
Secondary (post-thrombotic) |
|
En |
No venous cause is identified. |
|
Anatomical |
|
|
As |
Superficial veins |
|
Ap |
Perforator veins |
|
Ad |
Deep veins |
|
An |
No venous location was identified. |
|
Pathophysiological |
|
|
Pr |
Reflux |
|
Po |
Obstruction |
|
Pr, o |
Reflux and obstruction |
|
Pn |
No venous pathophysiology identifiable |
Prognosis
Based on a prognostic model, wound area and chronicity are raw determinants for healing.[67] VLUs less than 1 year old and smaller than 10 cm2 at the first visit had a 29% chance of closure failure by the sixth month. The probability increased to 78% for wounds older than 1 year and larger than 10 cm2. Other reported risk factors associated with poor healing are advanced age, non-white race, high body mass index, calf muscle pump impairment, venous reflux, thrombosis, deep vein involvement, and lack of high compression.[68][69]
Results from a multicenter study found that VLUs with a greater than or equal to 3% increase within the first month of treatment had a 68% healing failure probability by the sixth month.[70] In another cohort, a reduction of less than 30% in size by the first month of treatment was associated with a low probability of healing by the third month.[71] Yet, healing predictions based on area or percentage reduction remain under scrutiny.[72][73] Healed ulcers have high recurrence rates, which considerably decrease with sustained compression therapy and vascular surgery.[6][74]
Complications
As with any chronic wound, the most common complications of VLUs include infection and pain, both of which should be controlled to improve healing outcomes and patient adherence.[36][75] Less frequently, skin cancer may develop in wounds that fail to improve over long periods of time.[65]
Deterrence and Patient Education
Individuals affected with VLUs can have an impaired quality of life, mainly related to disability.[76] Plus, heavy exudates can cause malodor, social isolation, and emotional disturbances that severely impact life quality.[76] Compliance is essential to achieve a successful outcome.[36] Patients should be educated because VLUs are chronic processes that require lifelong evaluation and care, even after wound closure. Additionally, conformity to diet and lifestyle modifications may reduce the risk of recurrence, but evidence is lacking.[77]
Enhancing Healthcare Team Outcomes
VLUs remain an unmet clinical need. Numerous guidelines showcase that a consensus between different specialties from various countries has not been reached. Despite this, there have been some attempts at analyzing the quality and consistency of the published literature.[78][79][80] These efforts also demonstrate that an interprofessional team approach is fundamental for a thorough evaluation and treatment strategy. Primary prevention is one crucial strategy in which clinicians should actively participate.[33][81][82]
Patients with limb-threatening diseases and severe infections who are unable to care for themselves should be immediately admitted to the hospital for advanced management and vascular surgery consultation. Mild to moderate cases can be treated in ambulatory wound care facilities.[38] Physiotherapists should also participate in the treatment plan, as a systematic review and meta-analysis demonstrated improved healing with both aerobic and anaerobic activity.[83] Finally, nutrition specialists can help malnourished and overweight patients with specific guidance on improving their overall health, although weight management outcomes remain unclear for VLU healing.[34][84]
Media
(Click Image to Enlarge)
References
Bonkemeyer Millan S, Gan R, Townsend PE. Venous Ulcers: Diagnosis and Treatment. American family physician. 2019 Sep 1:100(5):298-305 [PubMed PMID: 31478635]
Korstanje MJ. Venous stasis ulcers. Diagnostic and surgical considerations. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1995 Jul:21(7):635-40 [PubMed PMID: 7606378]
Molnar JA, Vlad LG, Gumus T. Nutrition and Chronic Wounds: Improving Clinical Outcomes. Plastic and reconstructive surgery. 2016 Sep:138(3 Suppl):71S-81S. doi: 10.1097/PRS.0000000000002676. Epub [PubMed PMID: 27556777]
Level 2 (mid-level) evidenceXie T, Ye J, Rerkasem K, Mani R. The venous ulcer continues to be a clinical challenge: an update. Burns & trauma. 2018:6():18. doi: 10.1186/s41038-018-0119-y. Epub 2018 Jun 15 [PubMed PMID: 29942813]
Santler B, Goerge T. Chronic venous insufficiency - a review of pathophysiology, diagnosis, and treatment. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2017 May:15(5):538-556. doi: 10.1111/ddg.13242. Epub [PubMed PMID: 28485865]
Nelson EA, Adderley U. Venous leg ulcers. BMJ clinical evidence. 2016 Jan 15:2016():. pii: 1902. Epub 2016 Jan 15 [PubMed PMID: 26771825]
Phillips CJ, Humphreys I, Thayer D, Elmessary M, Collins H, Roberts C, Naik G, Harding K. Cost of managing patients with venous leg ulcers. International wound journal. 2020 Aug:17(4):1074-1082. doi: 10.1111/iwj.13366. Epub 2020 May 7 [PubMed PMID: 32383324]
Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. International wound journal. 2018 Feb:15(1):29-37. doi: 10.1111/iwj.12814. Epub 2017 Dec 15 [PubMed PMID: 29243398]
Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. International wound journal. 2019 Feb:16(1):112-121. doi: 10.1111/iwj.13000. Epub 2018 Oct 5 [PubMed PMID: 30289621]
Level 2 (mid-level) evidenceRice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. Journal of medical economics. 2014 May:17(5):347-56. doi: 10.3111/13696998.2014.903258. Epub 2014 Mar 24 [PubMed PMID: 24625244]
Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vascular medicine (London, England). 1999:4(1):1-7 [PubMed PMID: 10355863]
Level 2 (mid-level) evidenceDavies AH. The Seriousness of Chronic Venous Disease: A Review of Real-World Evidence. Advances in therapy. 2019 Mar:36(Suppl 1):5-12. doi: 10.1007/s12325-019-0881-7. Epub 2019 Feb 13 [PubMed PMID: 30758738]
Level 3 (low-level) evidenceDarwin E, Liu G, Kirsner RS, Lev-Tov H. Examining risk factors and preventive treatments for first venous leg ulceration: A cohort study. Journal of the American Academy of Dermatology. 2021 Jan:84(1):76-85. doi: 10.1016/j.jaad.2019.12.046. Epub 2019 Dec 27 [PubMed PMID: 31884088]
Crawford JM, Lal BK, Durán WN, Pappas PJ. Pathophysiology of venous ulceration. Journal of vascular surgery. Venous and lymphatic disorders. 2017 Jul:5(4):596-605. doi: 10.1016/j.jvsv.2017.03.015. Epub [PubMed PMID: 28624002]
Brown J. The role of the fibrin cuff in the development of venous leg ulcers. Journal of wound care. 2005 Jul:14(7):324-7 [PubMed PMID: 16048219]
Mansilha A, Sousa J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. International journal of molecular sciences. 2018 Jun 5:19(6):. doi: 10.3390/ijms19061669. Epub 2018 Jun 5 [PubMed PMID: 29874834]
Meulendijks AM, Franssen WMA, Schoonhoven L, Neumann HAM. A scoping review on Chronic Venous Disease and the development of a Venous Leg Ulcer: The role of obesity and mobility. Journal of tissue viability. 2020 Aug:29(3):190-196. doi: 10.1016/j.jtv.2019.10.002. Epub 2019 Oct 9 [PubMed PMID: 31668667]
Level 2 (mid-level) evidenceRaffetto JD. Inflammation in chronic venous ulcers. Phlebology. 2013 Mar:28 Suppl 1():61-7. doi: 10.1177/0268355513476844. Epub [PubMed PMID: 23482537]
Rahmani J, Haghighian Roudsari A, Bawadi H, Thompson J, Khalooei Fard R, Clark C, Ryan PM, Ajami M, Rahimi Sakak F, Salehisahlabadi A, Abdulazeem HM, Jamali MR, Mirzay Razaz J. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: A systematic review and dose-response meta-analysis of cohort studies among four million participants. Thrombosis research. 2020 Aug:192():64-72. doi: 10.1016/j.thromres.2020.05.014. Epub 2020 May 15 [PubMed PMID: 32454303]
Level 1 (high-level) evidenceSerra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Annals of vascular surgery. 2012 Jul:26(5):636-42. doi: 10.1016/j.avsg.2011.11.036. Epub [PubMed PMID: 22664280]
Markovic JN, Shortell CK. Genomics of varicose veins and chronic venous insufficiency. Seminars in vascular surgery. 2013 Mar:26(1):2-13. doi: 10.1053/j.semvascsurg.2013.04.003. Epub [PubMed PMID: 23932556]
Level 2 (mid-level) evidenceCharles CA, Tomic-Canic M, Vincek V, Nassiri M, Stojadinovic O, Eaglstein WH, Kirsner RS. A gene signature of nonhealing venous ulcers: potential diagnostic markers. Journal of the American Academy of Dermatology. 2008 Nov:59(5):758-71. doi: 10.1016/j.jaad.2008.07.018. Epub 2008 Aug 20 [PubMed PMID: 18718692]
Ramelet AA, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti DP, Offord E, Mansourian R, Applegate LA. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Experimental gerontology. 2009 Mar:44(3):208-18. doi: 10.1016/j.exger.2008.11.004. Epub 2008 Nov 20 [PubMed PMID: 19049860]
Okamoto O, Fujiwara S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connective tissue research. 2006:47(4):177-89 [PubMed PMID: 16987749]
Level 3 (low-level) evidenceGemmati D, Federici F, Catozzi L, Gianesini S, Tacconi G, Scapoli GL, Zamboni P. DNA-array of gene variants in venous leg ulcers: detection of prognostic indicators. Journal of vascular surgery. 2009 Dec:50(6):1444-51. doi: 10.1016/j.jvs.2009.07.103. Epub [PubMed PMID: 19958990]
Level 3 (low-level) evidenceJacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2003 Jul-Aug:57(5-6):195-202 [PubMed PMID: 12888254]
Level 3 (low-level) evidenceStone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, Tomic-Canic M. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Science translational medicine. 2017 Jan 4:9(371):. doi: 10.1126/scitranslmed.aaf8611. Epub [PubMed PMID: 28053158]
Lal BK. Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens. Seminars in vascular surgery. 2015 Mar:28(1):3-5. doi: 10.1053/j.semvascsurg.2015.05.002. Epub 2015 May 8 [PubMed PMID: 26358303]
Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. Journal of the American Academy of Dermatology. 2002 Mar:46(3):381-6 [PubMed PMID: 11862173]
Level 2 (mid-level) evidenceVuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I. An Epidemiological Survey of Venous Disease Among General Practitioner Attendees in Different Geographical Regions on the Globe: The Final Results of the Vein Consult Program. Angiology. 2018 Oct:69(9):779-785. doi: 10.1177/0003319718759834. Epub 2018 Feb 26 [PubMed PMID: 29482348]
Level 2 (mid-level) evidenceBerenguer Pérez M, López-Casanova P, Sarabia Lavín R, González de la Torre H, Verdú-Soriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre-A time series study (2010-2014). International wound journal. 2019 Feb:16(1):256-265. doi: 10.1111/iwj.13026. Epub 2018 Nov 4 [PubMed PMID: 30393963]
Heit JA, Rooke TW, Silverstein MD, Mohr DN, Lohse CM, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. Journal of vascular surgery. 2001 May:33(5):1022-7 [PubMed PMID: 11331844]
Level 2 (mid-level) evidenceGillespie DL, Writing Group III of the Pacific Vascular Symposium 6, Kistner B, Glass C, Bailey B, Chopra A, Ennis B, Marston B, Masuda E, Moneta G, Nelzen O, Raffetto J, Raju S, Vedantham S, Wright D, Falanga V. Venous ulcer diagnosis, treatment, and prevention of recurrences. Journal of vascular surgery. 2010 Nov:52(5 Suppl):8S-14S. doi: 10.1016/j.jvs.2010.05.068. Epub 2010 Aug 3 [PubMed PMID: 20678885]
O'Donnell TF Jr, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P, Society for Vascular Surgery, American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. Journal of vascular surgery. 2014 Aug:60(2 Suppl):3S-59S. doi: 10.1016/j.jvs.2014.04.049. Epub 2014 Jun 25 [PubMed PMID: 24974070]
Level 1 (high-level) evidenceDi Bella S, Monticelli J, Luzzati R. Evaluation and Management of Lower-Extremity Ulcers. The New England journal of medicine. 2018 Jan 18:378(3):301. doi: 10.1056/NEJMc1715237. Epub [PubMed PMID: 29345447]
Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. The British journal of dermatology. 2015 Aug:173(2):379-90. doi: 10.1111/bjd.13953. Epub 2015 Aug 8 [PubMed PMID: 26257052]
Nicolaides AN. The Most Severe Stage of Chronic Venous Disease: An Update on the Management of Patients with Venous Leg Ulcers. Advances in therapy. 2020 Feb:37(Suppl 1):19-24. doi: 10.1007/s12325-020-01219-y. Epub 2020 Jan 22 [PubMed PMID: 31970660]
Level 3 (low-level) evidenceSinger AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. The New England journal of medicine. 2017 Oct 19:377(16):1559-1567. doi: 10.1056/NEJMra1615243. Epub [PubMed PMID: 29045216]
Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of vascular surgery. 2004 Dec:40(6):1248-52 [PubMed PMID: 15622385]
Level 3 (low-level) evidenceKolbach DN, Neumann HA, Prins MH. Definition of the post-thrombotic syndrome, differences between existing classifications. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005 Oct:30(4):404-14 [PubMed PMID: 16009579]
Vasquez MA, Munschauer CE. Revised venous clinical severity score: a facile measurement of outcomes in venous disease. Phlebology. 2012 Mar:27 Suppl 1():119-29 [PubMed PMID: 22312078]
Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, Woo K, Romanelli M, Kirsner RS. What's new: Management of venous leg ulcers: Approach to venous leg ulcers. Journal of the American Academy of Dermatology. 2016 Apr:74(4):627-40; quiz 641-2. doi: 10.1016/j.jaad.2014.10.048. Epub [PubMed PMID: 26979354]
Andriessen A, Apelqvist J, Mosti G, Partsch H, Gonska C, Abel M. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications - a review of present guidelines. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 Sep:31(9):1562-1568. doi: 10.1111/jdv.14390. Epub 2017 Jul 31 [PubMed PMID: 28602045]
Cavezzi A, Labropoulos N, Partsch H, Ricci S, Caggiati A, Myers K, Nicolaides A, Smith PC. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs--UIP consensus document. Part II. Anatomy. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2006 Mar:31(3):288-99 [PubMed PMID: 16230038]
Level 1 (high-level) evidenceLabropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M, Baker WH. Definition of venous reflux in lower-extremity veins. Journal of vascular surgery. 2003 Oct:38(4):793-8 [PubMed PMID: 14560232]
van Bemmelen PS, van Ramshorst B, Eikelboom BC. Photoplethysmography reexamined: lack of correlation with duplex scanning. Surgery. 1992 Sep:112(3):544-8 [PubMed PMID: 1519171]
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. Journal of vascular surgery. 2002 Apr:35(4):694-700 [PubMed PMID: 11932665]
Lurie F, Bittar S, Kasper G. Optimal Compression Therapy and Wound Care for Venous Ulcers. The Surgical clinics of North America. 2018 Apr:98(2):349-360. doi: 10.1016/j.suc.2017.11.006. Epub 2017 Dec 18 [PubMed PMID: 29502776]
Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, Woo K, Romanelli M, Kirsner RS. What's new: Management of venous leg ulcers: Treating venous leg ulcers. Journal of the American Academy of Dermatology. 2016 Apr:74(4):643-64; quiz 665-6. doi: 10.1016/j.jaad.2015.03.059. Epub [PubMed PMID: 26979355]
O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. The Cochrane database of systematic reviews. 2012 Nov 14:11(11):CD000265. doi: 10.1002/14651858.CD000265.pub3. Epub 2012 Nov 14 [PubMed PMID: 23152202]
Level 1 (high-level) evidenceNelson EA, Hillman A, Thomas K. Intermittent pneumatic compression for treating venous leg ulcers. The Cochrane database of systematic reviews. 2014 May 12:2014(5):CD001899. doi: 10.1002/14651858.CD001899.pub4. Epub 2014 May 12 [PubMed PMID: 24820100]
Level 1 (high-level) evidenceSzolnoky G, Tuczai M, Macdonald JM, Dosa-Racz E, Barsony K, Balogh M, Szabad G, Dobozy A, Kemeny L. Adjunctive role of manual lymph drainage in the healing of venous ulcers: A comparative pilot study. Lymphology. 2018:51(4):148-159 [PubMed PMID: 31119905]
Level 2 (mid-level) evidenceCooper B, Bachoo P. Extracorporeal shock wave therapy for the healing and management of venous leg ulcers. The Cochrane database of systematic reviews. 2018 Jun 11:6(6):CD011842. doi: 10.1002/14651858.CD011842.pub2. Epub 2018 Jun 11 [PubMed PMID: 29889978]
Level 1 (high-level) evidenceNair B. Venous leg ulcer: Systemic therapy. Indian dermatology online journal. 2014 Jul:5(3):374-7. doi: 10.4103/2229-5178.137821. Epub [PubMed PMID: 25165678]
Jull AB, Waters J, Arroll B. Oral pentoxifylline for treatment of venous leg ulcers. The Cochrane database of systematic reviews. 2000:(2):CD001733 [PubMed PMID: 10796661]
Level 1 (high-level) evidenceJull A, Waters J, Arroll B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet (London, England). 2002 May 4:359(9317):1550-4 [PubMed PMID: 12047963]
Level 1 (high-level) evidenceSmith PC. Daflon 500 mg and venous leg ulcer: new results from a meta-analysis. Angiology. 2005 Sep-Oct:56 Suppl 1():S33-9 [PubMed PMID: 16193225]
Level 1 (high-level) evidenceColeridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005 Aug:30(2):198-208 [PubMed PMID: 15936227]
Level 1 (high-level) evidencede Oliveira Carvalho PE, Magolbo NG, De Aquino RF, Weller CD. Oral aspirin for treating venous leg ulcers. The Cochrane database of systematic reviews. 2016 Feb 18:2(2):CD009432. doi: 10.1002/14651858.CD009432.pub2. Epub 2016 Feb 18 [PubMed PMID: 26889740]
Level 1 (high-level) evidenceNorman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. The Cochrane database of systematic reviews. 2018 Jun 15:6(6):CD012583. doi: 10.1002/14651858.CD012583.pub2. Epub 2018 Jun 15 [PubMed PMID: 29906322]
Level 1 (high-level) evidenceJones JE, Nelson EA, Al-Hity A. Skin grafting for venous leg ulcers. The Cochrane database of systematic reviews. 2013 Jan 31:2013(1):CD001737. doi: 10.1002/14651858.CD001737.pub4. Epub 2013 Jan 31 [PubMed PMID: 23440784]
Level 1 (high-level) evidenceMontminy ML, Jayaraj A, Raju S. A systematic review of the efficacy and limitations of venous intervention in stasis ulceration. Journal of vascular surgery. Venous and lymphatic disorders. 2018 May:6(3):376-398.e1. doi: 10.1016/j.jvsv.2017.11.007. Epub 2018 Feb 13 [PubMed PMID: 29452957]
Level 1 (high-level) evidenceGohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, Epstein DM, Nyamekye I, Poskitt KR, Renton S, Warwick J, Davies AH, EVRA Trial Investigators. A Randomized Trial of Early Endovenous Ablation in Venous Ulceration. The New England journal of medicine. 2018 May 31:378(22):2105-2114. doi: 10.1056/NEJMoa1801214. Epub 2018 Apr 24 [PubMed PMID: 29688123]
Level 1 (high-level) evidenceStansal A, Khayat K, Duchatelle V, Tella E, Gautier V, Sfeir D, Attal R, Lazareth I, Priollet P. [When to ask for a skin biopsy in a patient with leg ulcer? Retrospective study of 143 consecutive biopsies]. Journal de medecine vasculaire. 2018 Feb:43(1):4-9. doi: 10.1016/j.jdmv.2017.10.003. Epub 2017 Nov 21 [PubMed PMID: 29425540]
Level 2 (mid-level) evidenceTchanque-Fossuo CN, Millsop JW, Johnson MA, Dahle SE, Isseroff RR. Ulcerated Basal Cell Carcinomas Masquerading as Venous Leg Ulcers. Advances in skin & wound care. 2018 Mar:31(3):130-134. doi: 10.1097/01.ASW.0000530068.44631.dc. Epub [PubMed PMID: 29438147]
Level 3 (low-level) evidenceSenet P, Combemale P, Debure C, Baudot N, Machet L, Aout M, Vicaut E, Lok C, Angio-Dermatology Group Of The French Society Of Dermatology. Malignancy and chronic leg ulcers: the value of systematic wound biopsies: a prospective, multicenter, cross-sectional study. Archives of dermatology. 2012 Jun:148(6):704-8. doi: 10.1001/archdermatol.2011.3362. Epub [PubMed PMID: 22772403]
Level 2 (mid-level) evidenceMargolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2004 Mar-Apr:12(2):163-8 [PubMed PMID: 15086767]
Level 2 (mid-level) evidenceMelikian R, O'Donnell TF Jr, Suarez L, Iafrati MD. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Jan:7(1):98-105. doi: 10.1016/j.jvsv.2018.07.014. Epub 2018 Oct 24 [PubMed PMID: 30558732]
Parker CN, Finlayson KJ, Shuter P, Edwards HE. Risk factors for delayed healing in venous leg ulcers: a review of the literature. International journal of clinical practice. 2015 Sep:69(9):967-77. doi: 10.1111/ijcp.12635. Epub 2015 Apr 1 [PubMed PMID: 25831965]
Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. The British journal of dermatology. 2000 May:142(5):960-4 [PubMed PMID: 10809855]
Level 2 (mid-level) evidenceGelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. The Journal of investigative dermatology. 2002 Dec:119(6):1420-5 [PubMed PMID: 12485449]
Gorin DR, Cordts PR, LaMorte WW, Manzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. Journal of vascular surgery. 1996 Mar:23(3):524-8 [PubMed PMID: 8601898]
Level 2 (mid-level) evidenceGilman T. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. The British journal of dermatology. 2003 Oct:149(4):896-7; author reply 897-8 [PubMed PMID: 14616394]
Level 3 (low-level) evidenceMauck KF, Asi N, Elraiyah TA, Undavalli C, Nabhan M, Altayar O, Sonbol MB, Prokop LJ, Murad MH. Comparative systematic review and meta-analysis of compression modalities for the promotion of venous ulcer healing and reducing ulcer recurrence. Journal of vascular surgery. 2014 Aug:60(2 Suppl):71S-90S.e1-2. doi: 10.1016/j.jvs.2014.04.060. Epub 2014 May 28 [PubMed PMID: 24877851]
Level 1 (high-level) evidenceAtkin L, Martin R. An audience survey of practice relating to pain in the management of chronic venous leg ulcers. British journal of community nursing. 2020 Dec 1:25(Sup12):S20-S24. doi: 10.12968/bjcn.2020.25.Sup12.S20. Epub [PubMed PMID: 33300846]
Level 3 (low-level) evidenceAbbade LP, Lastória S, de Almeida Rollo H, Stolf HO. A sociodemographic, clinical study of patients with venous ulcer. International journal of dermatology. 2005 Dec:44(12):989-92 [PubMed PMID: 16409260]
Jindal R, Dekiwadia DB, Krishna PR, Khanna AK, Patel MD, Padaria S, Varghese R. Evidence-Based Clinical Practice Points for the Management of Venous Ulcers. The Indian journal of surgery. 2018 Apr:80(2):171-182. doi: 10.1007/s12262-018-1726-3. Epub 2018 Jan 27 [PubMed PMID: 29915484]
Couch KS, Corbett L, Gould L, Girolami S, Bolton L. The International Consolidated Venous Ulcer Guideline Update 2015: Process Improvement, Evidence Analysis, and Future Goals. Ostomy/wound management. 2017 May:63(5):42-46 [PubMed PMID: 28570248]
O'Donnell TF Jr, Balk EM. The need for an Intersociety Consensus Guideline for venous ulcer. Journal of vascular surgery. 2011 Dec:54(6 Suppl):83S-90S. doi: 10.1016/j.jvs.2011.06.001. Epub 2011 Sep 9 [PubMed PMID: 21908148]
Level 3 (low-level) evidenceTan MKH, Luo R, Onida S, Maccatrozzo S, Davies AH. Venous Leg Ulcer Clinical Practice Guidelines: What is AGREEd? European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2019 Jan:57(1):121-129. doi: 10.1016/j.ejvs.2018.08.043. Epub 2018 Oct 2 [PubMed PMID: 30287207]
Level 1 (high-level) evidenceMooij MC, Huisman LC. Chronic leg ulcer: does a patient always get a correct diagnosis and adequate treatment? Phlebology. 2016 Mar:31(1 Suppl):68-73. doi: 10.1177/0268355516632436. Epub [PubMed PMID: 26916772]
Flanagan M, Rotchell L, Fletcher J, Schofield J. Community nurses', home carers' and patients' perceptions of factors affecting venous leg ulcer recurrence and management of services. Journal of nursing management. 2001 May:9(3):153-9 [PubMed PMID: 11879462]
Jull A, Slark J, Parsons J. Prescribed Exercise With Compression vs Compression Alone in Treating Patients With Venous Leg Ulcers: A Systematic Review and Meta-analysis. JAMA dermatology. 2018 Nov 1:154(11):1304-1311. doi: 10.1001/jamadermatol.2018.3281. Epub [PubMed PMID: 30285080]
Level 1 (high-level) evidenceBarber GA, Weller CD, Gibson SJ. Effects and associations of nutrition in patients with venous leg ulcers: A systematic review. Journal of advanced nursing. 2018 Apr:74(4):774-787. doi: 10.1111/jan.13474. Epub 2017 Nov 9 [PubMed PMID: 28985441]
Level 1 (high-level) evidence