Introduction
Sleep-disordered breathing disorders (SDB) encompass a spectrum of chronic conditions ranging from snoring, upper airway resistance syndrome (UARS), obstructive sleep apnea (OSA), and central sleep apnea (CSA) and its sub-types.[1] These terminologies have been collectively described as abnormal breathing during sleep, historically based on the recording technologies and knowledge of the time.[2] UARS has been discussed and researched for many years, yet, there is still no clear consensus on what diagnostic criteria should be used or whether UARS represents a distinct syndrome from OSA.[3]
While OSA and CSA are defined by the number of apnea and hypopnea episodes per hour of sleep (apnea-hypopnea index, AHI), UARS is defined in general as airflow limitation due to increased respiratory effort leading to arousals from sleep without significant desaturation (i.e., RERAs) associated with daytime symptoms.[4] UARS has also been more specifically defined as apnea-hypopnea index < 5 events/h, oxygen saturation ≥ 92%, and respiratory effort–related arousal index ≥ 5/hour.[5] Another study used a slightly different definition when AHI <5/hour, minimum SpO ≥ 92%, the presence of airflow limitation during sleep for ≥5% of total sleep time, and daytime sleepiness and/or fatigue.[6]
The etiology, epidemiology, history and physical presentation, evaluation, management, differential diagnosis, and complications of UARS are reviewed here. The topics of OSA and CSA are described separately.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
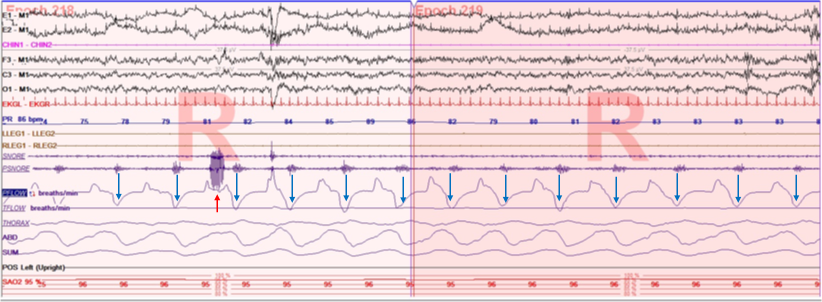
Factors contributing to upper airway narrowing during sleep are disadvantageous anatomic factors and/or reduced neuromuscular compensatory mechanisms to maintain airway patency. Upper airway resistance syndrome occurs in the upper airway most commonly due to partial narrowing and increased resistance in the retropalatal (between the hard palate and the uvula) and retroglossal (between the uvula and the epiglottis) locations.[7] The increased efforts associated with increased resistance and inspiratory flow limitation can lead to multiple arousals from sleep (both cortical or autonomic) and disrupt natural sleep.[8] These frequent respiratory-related arousal (i.e., RERAs) cause non-refreshing sleep, excessive daytime sleepiness, or unexplained daytime tiredness.[4] One of the main parameters of UARS includes flow limitation during sleep without significant desaturation and not meeting the definition of hypopnea (Figure 1).[9]
UARS may represent an early stage of the OSA condition and could help elucidate the natural progression of sleep-disordered breathing. Still, there are limited epidemiological data to assess the role of non-apneic respiratory events; these are mainly associated with prolonged flow limitation and effort-related arousals.[8] Worsening airflow during sleep can lead to partial upper airway narrowing in susceptible individuals with unfavorable upper airway anatomy. In susceptible patients, the low arousal threshold during sleep could contribute to the mechanism of sleep disturbances and fragmented sleep dispersed by periods of flow limitation and SDB.[10]
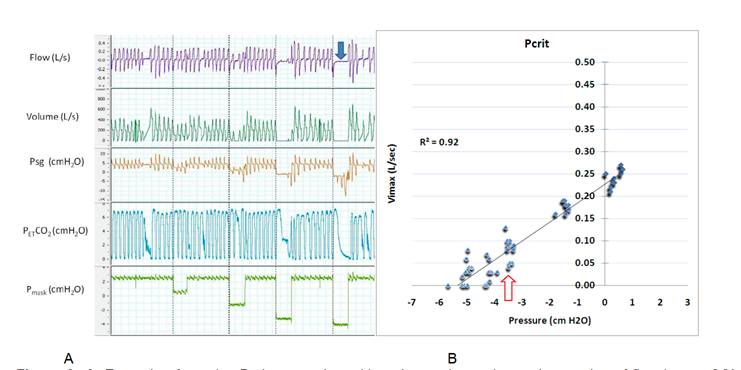
The flow limitation can be inspiratory, expiratory, or in both respiratory cycle phases.[11] The collapsibility of the airway also influences upper airway patency. Upper airway collapsibility can be determined by applying progressively negative pressure to the upper airway until the intraluminal pressure reaches a critical closing pressure (Pcrit). The Pcrit is usually negative pressure in normal healthy individuals and progressively increases toward positive ranges in patients with UARS, obstructive hypopnea, and apnea (Pcrit>0 cmH2O).[12]
The airflow and upper airway resistance (Rua) is governed by Poiseuille's law or what is known as the "Starling resistor" model of the upper airway.[13] Airway resistance is inversely related to the diameter (radius) of the tube and proportionally related to the difference between upstream pressure (Pus) and Pcrit (Rua=Pus-Pcrit/Vmax).[1] When Pus (i.e., Nasal pressure) approaches the Pcrit, the collapsible segment of the upper airway narrows down, and airflow diminishes until no flow occurs (during apnea). During sleep in UARS, inspiratory and expiratory flows could become limited due to the gradient between the upstream pressure and Pcrit. Expiratory snoring and flow limitation can occur in chronic obstructive lung diseases like asthma and COPD.[11] One study reported that expiratory snoring alone predicted lower airflow obstruction (defined as FEV/FVC < 70).[11][14]
Epidemiology
Using a specific definition of UARS (AHI <5/h, minimum SpO ≥ 92%, the presence of airflow limitation during sleep for ≥5% of total sleep time, and daytime sleepiness and/or fatigue), the prevalence of the UARS in a recent study was reported being of 3.1% (4.4% in women and 1.5% in men).[6] However, the prevalence of at least mild OSA (defined by AHI>5 events/hour) is 24% of men and 9% of women, with approximately one billion individuals affected worldwide, as found in recent international studies.[15][16]
The prevalence of UARS is not known; however, it is reported that about 5.3% of all obstructive non-apneic events in patients with OSA are due to RERAs.[17] UARS is more common in pre and perimenopausal women than in men or postmenopausal women.[18] In addition, women with UARS were found to have a higher reported need for sleep (approximately 30 minutes more) than men with UARS.
History and Physical
Patients with upper airway resistance syndrome present with complaints of snoring, fatigue, daytime tiredness, morning headache, depressive symptoms, and excessive daytime sleepiness, without definitive witnessed apnea or gasping episodes.[19] In addition, the patient may complain of sleep disruptions and unexplained awakening from sleep, mainly after 2 to 3 hours of sleep. These frequent unexplained arousals are associated with increased respiratory effort and lead to sleep fragmentation, resulting in fatigue and excessive daytime sleepiness.[3] In addition, individuals with URAS and OSA have exhibited a low quality of life compared to the general population (5 to 6 times worse).[5]
Evaluation
To evaluate adequately individuals suspected to have upper airway resistance syndrome, a full PSG is recommended. Home sleep apnea testing may underestimate the severity of SDB [14], while PSG allows for detecting flow limitation and associated arousal, which are required for confirming this diagnosis. In contrast to OSA, patients with UARS have more slow-wave sleep activity and less awakening from sleep on their PSG studies.[20]
Additionally, patients with UARS present with apneas or hypopneas and have fewer episodes of desaturation, but they have episodes of inspiratory flow limitation associated with arousals on PSG. UARS has often been overlooked because RERAs are underestimated and scored during PSG studies. Therefore, the PSG study for these patients may be interpreted as a normal or mild OSA. UARS can also be easily identified by measuring esophageal pressure (Pes) or supraglottic catheter, which is not commonly used in clinical laboratory settings.[21]
Untreated UARS individuals can present a low quality of life and, over time, can develop cardiovascular consequences. Over time, these symptoms usually increase in untreated individuals with UARS. UARS could cause hypertension, cardiovascular, and metabolic consequences if left untreated. Prompt diagnosis and proper treatment should be offered to UARS patients as early as possible to avoid the consequences previously mentioned.
Treatment / Management
The treatment options for upper airway resistance syndrome include addressing the underlying causes of the upper airway anatomical problems such as nasal allergies, dental malocclusion, and abnormal lifestyles. The mainstay therapy is continuous positive airway pressure (CPAP), with many studies reporting subjective, clinical, and physiological improvements.[19] In recent studies, CPAP application improved upper airway resistance, reduced arousal frequency, and respiratory-related heart rate changes.[8][10] It is important to note that in-laboratory CPAP titration is preferred over auto-CPAP to eliminate all periods of flow limitation and restore normal sleep.
Oral appliances are also an excellent treatment option for those patients with UARS who are intolerant of CPAP or those who refuse surgery.[22] The device moves the mandibular bone and soft tissue forward, allowing more space behind the tongue. Long-term therapy for 1.5 years decreases stress symptoms of UARS, including measured by the inventory of Stress Symptom.[23] Other oral appliances used to treat patients who suffer from UARS include tongue retaining devices, soft palate lifters, and tongue retainers.
Surgical treatment for upper airway obstruction is considered when a patient is not able to tolerate other therapies (i.e., CPAP and oral appliances). Many surgical procedures are used in UARS, including palatal surgeries.[24]
Pharmacological treatments have been considered investigational therapies, but no drug has FDA approval for this indication to date. Hypnotics are attractive options as they may reduce respiratory-related arousal, improving sleep fragmentation. Studies using nonbenzodiazepines (e.g., zolpidem) have shown mixed results. While some studies showed that zolpidem decreases respiratory arousal threshold and SDB severity, others reported no effect on arousal threshold.[25][26][27] Therefore, due to the lack of large definitive studies, this pharmacological treatment remains at best investigational with the potential for a synergistic effect with other therapies.(B3)
Differential Diagnosis
Several pathologies can present themselves as upper airway resistance syndrome, such as obstructive sleep apnea syndrome, sleep-related breathing disorder, respiratory sleep disorder, and sleep-disordered breathing. Even though all these terminologies have different titles, they all can be summed up by describing a patient who experiences abnormal breathing during sleep.
Prognosis
UARS is the intermediate between that of normal subjects and that of patients with mild-to-moderate sleep apnea syndrome. The best outcome for the patient is strongly dependent on the early discovery and help of the patient's physician. However, some studies suggest that untreated UARS has an increased risk of arterial hypertension. It can also evolve into obstructive sleep apnea, particularly with increased weight or additional comorbidities and age.[28]
Complications
The repetitive episodes of flow limitation usually result in arousals due to increased respiratory effort leading to sleep fragmentation. Sleep fragmentation then results in excessive daytime sleepiness for patients suffering from UARS.[3] On the other hand, prolonged periods of an inspiratory flow limitation during sleep are associated with increased carbon dioxide levels (highest levels compared to eupneic breathing or hypopnea and apnea), indicating the possibility of UARS playing in subsequent daytime symptoms and neurocognitive impairment. [29] If left undiagnosed and untreated, UARS patients usually present with a low quality of life and daytime symptoms ranging from fatigue, insomnia, and depressive mood.[30]
A retrospective study from Peru comparing patients (n=93) with UARS (defined as apnea-hypopnea index < 5 events/hour, oxygen saturation ≥ 92%, respiratory effort–related arousal index ≥ 5/h) to individuals with OSA (AHI >5/h) (n= 795) and the general public (n=641) found that those with UARS have a poor quality of life (low total HRQoL score) similar to those with OSA but much lower than the general public.[5] Moreover, individuals with UARS and one or more of the following symptoms: muscle pain, psychotropic medication, obesity, and depression, were negatively associated with quality of life scores. In addition, individuals with untreated UARS have been reported to suffer from hypertension and complicated cardiovascular consequences even without significant hypoxemia.[31]
UARS patient's main characteristic of esophagic pressure (Pes) negativity can cause a diastolic leftward shift of the interventricular heart septum with pulsus paradoxus simultaneously occurring when peak end-inspiratory esophageal pressure reported exceeding more negative than -35 cm H2O.[31] These cardiovascular consequences directly result from the long-lasting flow limitation episodes a patient experiences during sleep. These flow limitation episodes can slightly increase end-tidal carbon dioxide (PetCO2). This slight increase in end-tidal carbon dioxide can stimulate sympathetic nervous system activity and increase arterial hypertension risk.[32]
Deterrence and Patient Education
Patient counseling for upper airway resistance syndrome includes explaining the pathophysiology and possible sequelae in laypeople's terms so the patient has a reasonable understanding of their condition, explaining the various treatment options, and demonstrating how to use any mechanical devices (e.g., CPAP< oral appliances), and answering patient questions.
Pearls and Other Issues
Historically, sleep apnea, or upper airway resistance syndrome, has been diagnosed by a sleep medicine physician. However, as discussed in his article, its management is multidisciplinary. The primary care providers, otolaryngologists, and dentists have the vital role of first screening patients for OSA or URAS risk factors. Risk factors for OSA or UARS include retrognathia, a palate with an extremely high arch, consistently enlarged tonsils, or a tongue larger than normal for the patient's oral anatomy. Probing questions asked by the patient-physician regarding poor sleep, sleep position, obesity, hypertension, morning headaches, or orofacial pain are also necessary identifiers. Most importantly, as discussed at length in this article, the more critical identification of upper airway examination. Next, the clinician is responsible for referring to an appropriate health professional, as the case indicates.
The monitoring is one of the most critical steps because if a patient isn't comfortable with the appropriately chosen treatment regimen, the patient isn't going to use it, resulting in the patient not benefiting from treatment, potentially leading to cardiovascular and metabolic consequences. In addition to an adequately chosen oral appliance based on the patients' needs and goals, it requires provider expertise in all fields of medicine to appropriately and successfully treat our patients suffering from UARS. Oral appliance effectiveness is vital in managing OSA caused by UARS.
Enhancing Healthcare Team Outcomes
Effectively identifying and ultimately treating the risk factors of SDB and UARS in patients suffering from diurnal or nocturnal symptoms require individualized therapy and effective interprofessional communication throughout the entire diagnosing process.
UARS requires an interprofessional team approach to diagnosis and management. When discussing UARS, the interprofessional communication could begin with the dental professional identifying the patient's risk factors during the dental exam, a routine visit with a primary care clinician (MD, DO, NP, or PA), or a specialist. Further, follow-up questioning is performed by the patient's healthcare provider, then a visit to the sleep medicine doctor, where if no changes in the patient's breathing and oxygen levels are detected during periods of sleep, that patient's symptoms can be managed and treated by an appropriate device such as positive airway pressure (PAP) or simple oral device, created by their dentist. Nursing staff will also be involved in patient education, demonstrating how to use any prescribed devices and serving as a contact point between various clinicians involved in the patient's care. Any team member who notes an issue or signs of therapeutic failure should document their findings in the patient's record and report it to the other team members so appropriate action can be taken. Better patient outcomes are achievable with interprofessional team-based care coordination and open communication. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
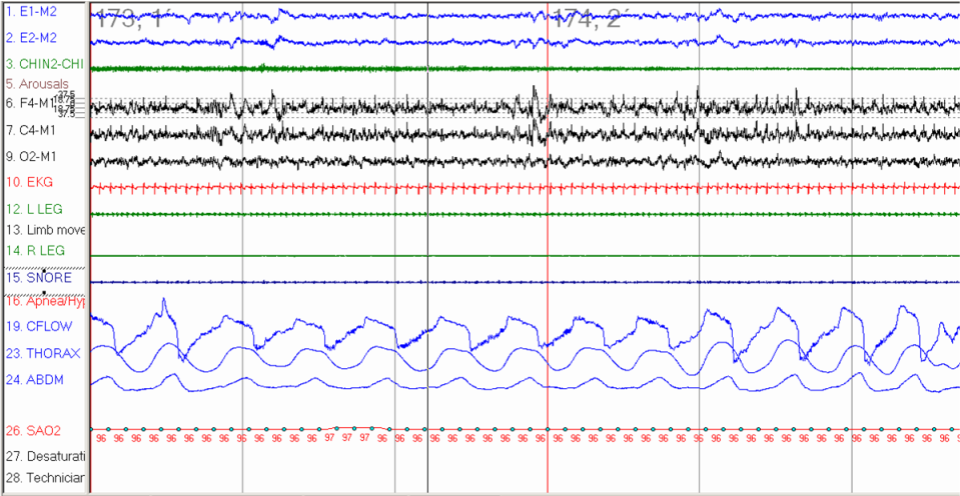
A polygraph illustrating the protocol for determining the critical closing pressure of upper airway (Pcrit) during sleep. The mask pressure is progressively reduced until the air flow is reduced and stopped (apnea as indicated in blue arrow). Note the effort measured by supraglottic pressure catheter (Psg) during the closure of the airway. Pcrit can be determined fro the relationship between flow and mask pressure when flow is < 0.05 L/min as indicated by red arrow.
Contributed by Abdulghani Sankari, MD, PhD
(Click Image to Enlarge)
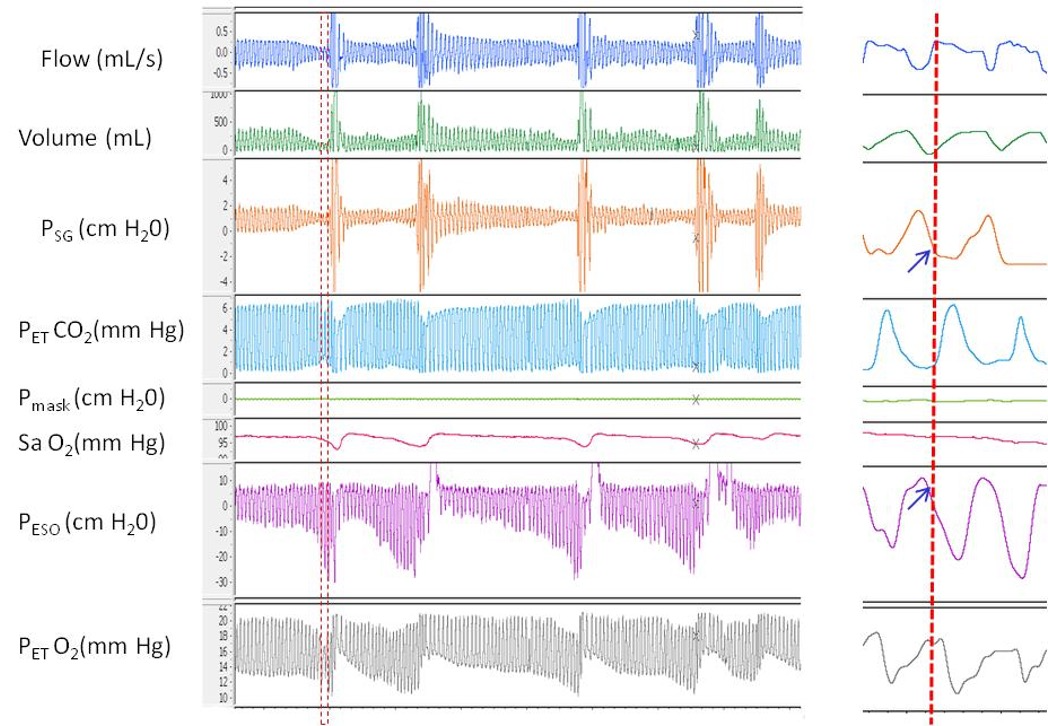
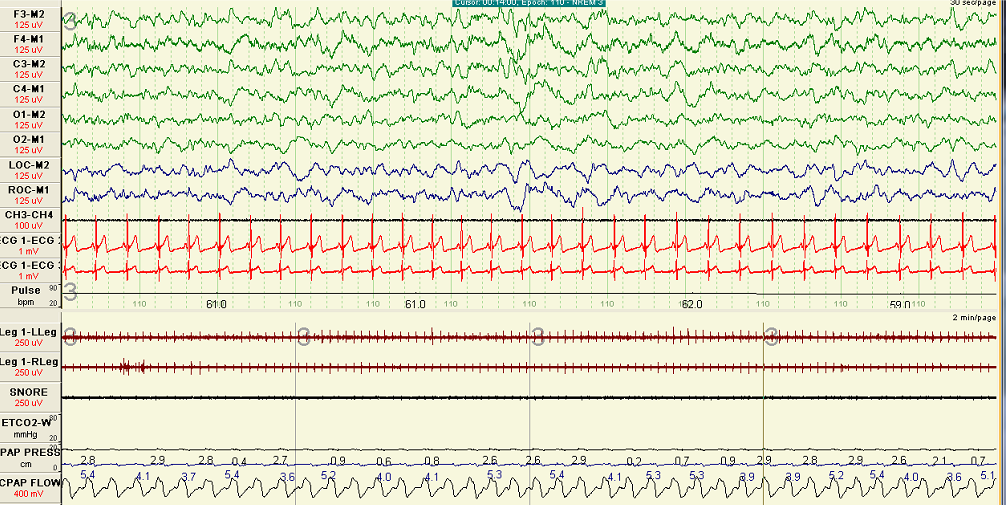
A polygraph illustrating the pressure flow relationship during repetitive episodes of inspiratory flow limitation. In this example two pressure catheters were used on to measure upper airway pressure at supraglottic area (Psg) and another catheter in the esophagus (Pes). Notice that in the magnified partition, at the onset of peak inspiratory flow there is deceleration and flattening in the inspiratory limb coinciding with flattening of Psg but significant decrease in Pes. These changes collectively indicate significant increase in effort and upper airway resistance below the supraglottic area. Contributed by Abdulghani Sankari, MD, PhD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sankri-Tarbichi AG. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna journal of medicine. 2012 Jan:2(1):3-8. doi: 10.4103/2231-0770.94803. Epub [PubMed PMID: 23210013]
Arnold WC, Guilleminault C. Upper airway resistance syndrome 2018: non-hypoxic sleep-disordered breathing. Expert review of respiratory medicine. 2019 Apr:13(4):317-326. doi: 10.1080/17476348.2019.1575731. Epub 2019 Feb 6 [PubMed PMID: 30689957]
Chervin RD, Guilleminault C. Obstructive sleep apnea and related disorders. Neurologic clinics. 1996 Aug:14(3):583-609 [PubMed PMID: 8871978]
Ogna A, Tobback N, Andries D, Preisig M, Vollenweider P, Waeber G, Marques-Vidal P, Haba-Rubio J, Heinzer R. Prevalence and Clinical Significance of Respiratory Effort-Related Arousals in the General Population. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018 Aug 15:14(8):1339-1345. doi: 10.5664/jcsm.7268. Epub 2018 Aug 15 [PubMed PMID: 30092888]
Vizcarra-Escobar D, Duque KR, Barbagelata-Agüero F, Vizcarra JA. Quality of life in upper airway resistance syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 May 1:18(5):1263-1270. doi: 10.5664/jcsm.9838. Epub [PubMed PMID: 34931609]
Level 2 (mid-level) evidenceTufik SB, Pires GN, Palombini L, Andersen ML, Tufik S. Prevalence of upper airway resistance syndrome in the São Paulo Epidemiologic Sleep Study. Sleep medicine. 2022 Mar:91():43-50. doi: 10.1016/j.sleep.2022.02.004. Epub 2022 Feb 14 [PubMed PMID: 35255282]
Trudo FJ, Gefter WB, Welch KC, Gupta KB, Maislin G, Schwab RJ. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. American journal of respiratory and critical care medicine. 1998 Oct:158(4):1259-70 [PubMed PMID: 9769290]
Level 2 (mid-level) evidenceSankari A, Pranathiageswaran S, Maresh S, Hosni AM, Badr MS. Characteristics and Consequences of Non-apneic Respiratory Events During Sleep. Sleep. 2017 Jan 1:40(1):. doi: 10.1093/sleep/zsw024. Epub [PubMed PMID: 28364453]
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. AASM Scoring Manual Updates for 2017 (Version 2.4). Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017 May 15:13(5):665-666. doi: 10.5664/jcsm.6576. Epub 2017 May 15 [PubMed PMID: 28416048]
Rizwan A, Sankari A, Bascom AT, Vaughan S, Badr MS. Nocturnal swallowing and arousal threshold in individuals with chronic spinal cord injury. Journal of applied physiology (Bethesda, Md. : 1985). 2018 Aug 1:125(2):445-452. doi: 10.1152/japplphysiol.00641.2017. Epub 2018 Apr 19 [PubMed PMID: 29672224]
Alchakaki A, Riehani A, Shikh-Hamdon M, Mina N, Badr MS, Sankari A. Expiratory Snoring Predicts Obstructive Pulmonary Disease in Patients with Sleep-disordered Breathing. Annals of the American Thoracic Society. 2016 Jan:13(1):86-92. doi: 10.1513/AnnalsATS.201507-413OC. Epub [PubMed PMID: 26630563]
Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. Journal of applied physiology (Bethesda, Md. : 1985). 2007 Feb:102(2):547-56 [PubMed PMID: 17008440]
Level 2 (mid-level) evidenceSankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. American journal of respiratory and critical care medicine. 2009 Feb 15:179(4):313-9. doi: 10.1164/rccm.200805-741OC. Epub 2008 Nov 21 [PubMed PMID: 19201929]
Sankari A, Badr MS. Sleep-Disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2015 Sep:12(9):1419-20. doi: 10.1513/AnnalsATS.201506-313LE. Epub [PubMed PMID: 26372809]
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29:328(17):1230-5 [PubMed PMID: 8464434]
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet. Respiratory medicine. 2019 Aug:7(8):687-698. doi: 10.1016/S2213-2600(19)30198-5. Epub 2019 Jul 9 [PubMed PMID: 31300334]
Cracowski C, Pépin JL, Wuyam B, Lévy P. Characterization of obstructive nonapneic respiratory events in moderate sleep apnea syndrome. American journal of respiratory and critical care medicine. 2001 Sep 15:164(6):944-8 [PubMed PMID: 11587975]
Stoohs R, Janicki J, Hohenhorst W. [Obstructive sleep apnea syndrome and upper airway resistance syndrome. Gender-related differences]. HNO. 2007 Oct:55(10):792-7 [PubMed PMID: 17287938]
Anttalainen U, Tenhunen M, Rimpilä V, Polo O, Rauhala E, Himanen SL, Saaresranta T. Prolonged partial upper airway obstruction during sleep - an underdiagnosed phenotype of sleep-disordered breathing. European clinical respiratory journal. 2016:3():31806. doi: 10.3402/ecrj.v3.31806. Epub 2016 Sep 6 [PubMed PMID: 27608271]
Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, Kushida C. Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome. The European respiratory journal. 2001 May:17(5):838-47 [PubMed PMID: 11488314]
Badr MS, Zahn BR. Images in clinical medicine. Upper-airway resistance syndrome. The New England journal of medicine. 2000 May 11:342(19):1408 [PubMed PMID: 10805826]
Level 3 (low-level) evidenceNerfeldt P, Friberg D. Effectiveness of Oral Appliances in Obstructive Sleep Apnea with Respiratory Arousals. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2016 Aug 15:12(8):1159-65. doi: 10.5664/jcsm.6058. Epub 2016 Aug 15 [PubMed PMID: 27397661]
de Godoy LBM, Sousa KMM, Palombini LO, Poyares D, Dal-Fabbro C, Guimarães TM, Tufik S, Togeiro SM. Long term oral appliance therapy decreases stress symptoms in patients with upper airway resistance syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2020 Nov 15:16(11):1857-1862. doi: 10.5664/jcsm.8698. Epub [PubMed PMID: 32686643]
Newman JP, Clerk AA, Moore M, Utley DS, Terris DJ. Recognition and surgical management of the upper airway resistance syndrome. The Laryngoscope. 1996 Sep:106(9 Pt 1):1089-93 [PubMed PMID: 8822711]
Messineo L, Eckert DJ, Lim R, Chiang A, Azarbarzin A, Carter SG, Carberry JC. Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. The Journal of physiology. 2020 Oct:598(20):4681-4692. doi: 10.1113/JP280173. Epub 2020 Aug 30 [PubMed PMID: 32864734]
Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - An open-label pilot study. Journal of sleep research. 2019 Dec:28(6):e12853. doi: 10.1111/jsr.12853. Epub 2019 Apr 10 [PubMed PMID: 30968498]
Level 3 (low-level) evidenceSmith PR, Sheikh KL, Costan-Toth C, Forsthoefel D, Bridges E, Andrada TF, Holley AB. Eszopiclone and Zolpidem Do Not Affect the Prevalence of the Low Arousal Threshold Phenotype. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017 Jan 15:13(1):115-119. doi: 10.5664/jcsm.6402. Epub 2017 Jan 15 [PubMed PMID: 27784413]
M'saad S, Yangui I, Feki W, Abid N, Bahloul N, Marouen F, Chakroun A, Kammoun S. [The syndrome of increased upper airways resistance: What are the clinical features and diagnostic procedures?]. Revue des maladies respiratoires. 2015 Dec:32(10):1002-15. doi: 10.1016/j.rmr.2015.08.001. Epub 2015 Oct 30 [PubMed PMID: 26525135]
Rimpilä V, Hosokawa K, Huhtala H, Saaresranta T, Salminen AV, Polo O. Transcutaneous carbon dioxide during sleep-disordered breathing. Respiratory physiology & neurobiology. 2015 Dec:219():95-102. doi: 10.1016/j.resp.2015.10.002. Epub 2015 Oct 22 [PubMed PMID: 26474829]
de Godoy LB, Palombini LO, Guilleminault C, Poyares D, Tufik S, Togeiro SM. Treatment of upper airway resistance syndrome in adults: Where do we stand? Sleep science (Sao Paulo, Brazil). 2015 Jan-Mar:8(1):42-8. doi: 10.1016/j.slsci.2015.03.001. Epub 2015 Mar 20 [PubMed PMID: 26483942]
Guilleminault C, Stoohs R, Shiomi T, Kushida C, Schnittger I. Upper airway resistance syndrome, nocturnal blood pressure monitoring, and borderline hypertension. Chest. 1996 Apr:109(4):901-8 [PubMed PMID: 8635368]
Guilleminault C, Los Reyes VD. Upper-airway resistance syndrome. Handbook of clinical neurology. 2011:98():401-9. doi: 10.1016/B978-0-444-52006-7.00026-5. Epub [PubMed PMID: 21056201]