Introduction
Transposition of the great arteries (TGA) is a congenital cardiac defect caused by an embryological discordance between the aorta and pulmonary trunk or by both atrioventricular and ventriculoarterial discordance—a condition often termed "double discordance."
Dextro-TGA (d-TGA) occurs during cardiac development when the conotruncal septum, which normally spirals toward the aortic sac, forms linearly. This anomaly interferes with the normal division of the truncus arteriosus into the pulmonary and aortic channels, which form the pulmonary arteries and aorta. As a result, the aorta arises from the right ventricle, and the pulmonary trunk emerges from the left ventricle. As the most common form of TGA, d-TGA is characterized by the right ventricle being positioned to the right of the left ventricle, with the aorta arising anterior and rightward to the pulmonary artery, creating 2 parallel circulatory circuits (see Image. Dextro-Transposition of the Great Arteries).
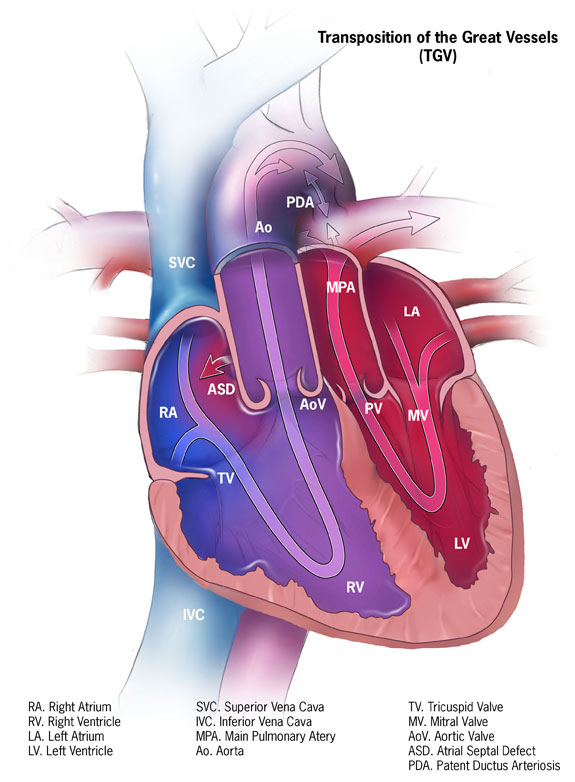
In the systemic circuit of d-TGA, deoxygenated blood returns to the right atrium, flows through the tricuspid valve and is then pumped back into systemic circulation by the contraction of the right ventricle as it enters the abnormally formed aorta. The pulmonary circuit, on the other hand, involves oxygenated blood from the pulmonary veins draining into the left atrium, passing through the mitral valve, and then being pushed back into the lungs via the contraction of the left ventricle and the pulmonary arteries. Patients with d-TGA typically present with cyanosis within the first 30 days of life. Completely parallel circulatory circuits are incompatible with life and require a patent ductus arteriosus (PDA) or an atrial (ASD) or ventricular septal defect (VSD) to allow the mixing of oxygenated and deoxygenated blood (see Image. Dextro-Transposition of the Great Arteries and Parallel Circulation).[1]
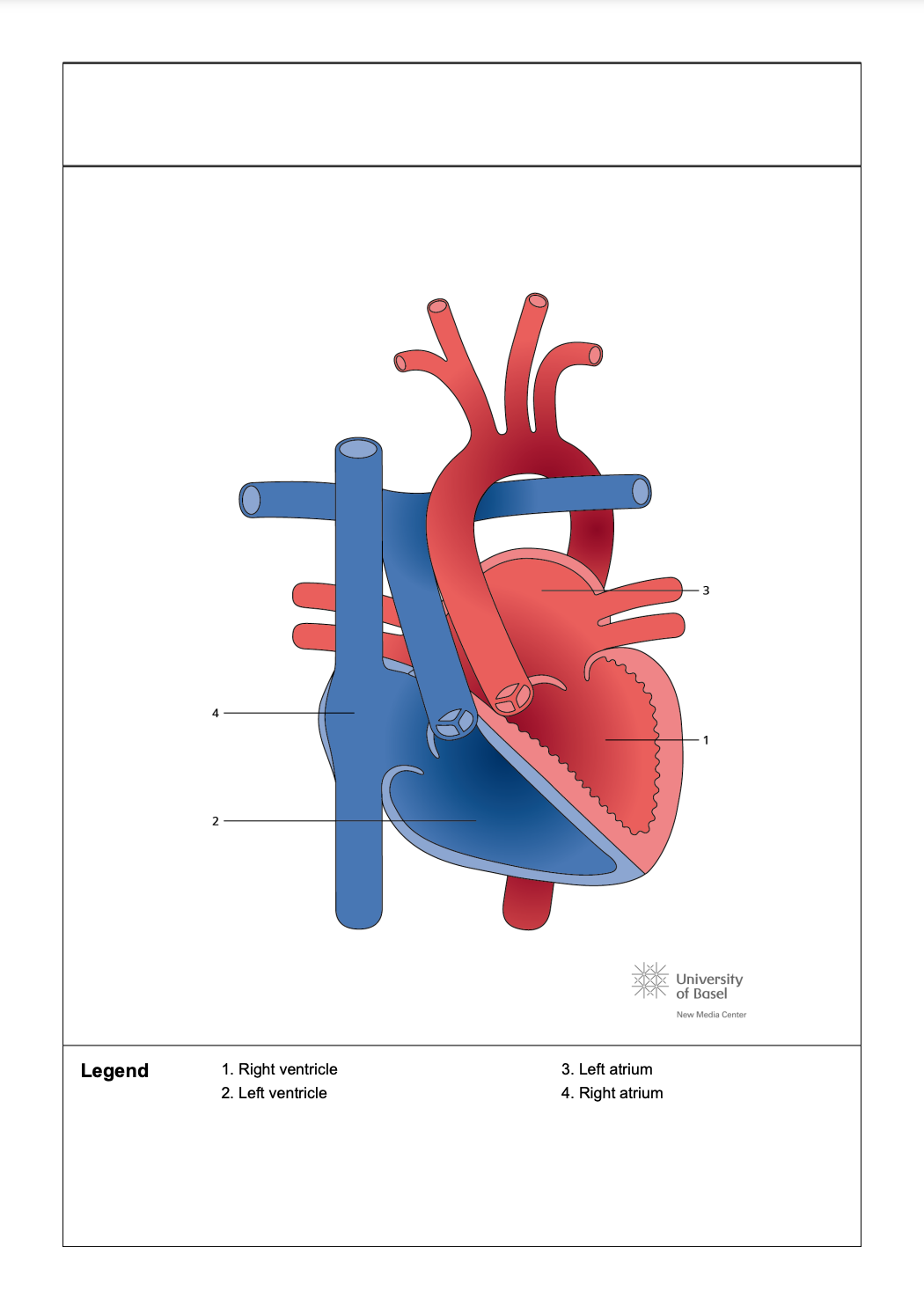
Congenitally corrected TGA (ccTGA) is a complex congenital heart defect (CHD) characterized by misalignment of the ventricles and transposition of the great arteries, resulting in ventricular discordance where the ventricles are not properly aligned with their corresponding atria. This condition is highly variable and can present with a range of anatomical features and clinical manifestations. CcTGA may be associated with other cardiac anomalies, including mirror-image atrial arrangement, atrial isomerism, dextrocardia, VSDs, an Ebstein-like tricuspid valve, pulmonary stenosis, and atresia (see Image. Congenitally Corrected Transposition of the Great Arteries).
Symptomatic infants often have additional cardiac defects that require early intervention. In contrast, patients without significant intracardiac anomalies may remain asymptomatic during childhood and maintain good health well into adulthood. However, over time, the right ventricle and tricuspid valve, which support systemic circulation, can gradually deteriorate, leading to dysfunction and eventual failure.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The exact cause of d-TGA remains unclear, but it is believed to result from multiple complex factors. Understanding the pathogenesis is challenging, particularly due to difficulties replicating the condition in animal models. Traditionally, 2 main theories have been proposed to explain the embryological development of d-TGA. The "extracardiac theory" suggests that d-TGA arises from abnormal spiralization of the aortopulmonary septum during heart development, where the conotruncal septum follows a straight path instead of its normal spiral trajectory.[3][4]
In contrast, the "infundibular theory" proposes that d-TGA results from improper resorption or underdevelopment of the subpulmonary conus, along with the persistence of the subaortic conus. This anomaly leads to the aorta being positioned over the anterior right ventricle.[5] Several potential risk factors have been identified, including gestational diabetes, maternal exposure to rodenticides and herbicides, and the use of antiepileptic drugs during pregnancy.[6]
The uncommon occurrence of ccTGA has made research on this condition equally challenging, with the underlying cause remaining unidentified. The variability in age at diagnosis, along with a wide range of associated cardiac defects and comorbidities, further complicates studies.[7] Patients exhibit significant diversity; individuals with major accompanying heart defects, such as pulmonary stenosis or VSD, are often diagnosed early in life, while those without such complications may not be identified until adulthood.[8]
Epidemiology
D-TGA is a CHD occurring in about 1 in 4000 live births. Although some cases of d-TGA have been linked to rare variants in specific genes, the genetic basis for most cases remains unclear. The pattern of familial recurrence, along with the sporadic nature of the majority of d-TGA cases, suggests polygenic inheritance.[9] TGA accounts for 3% of all CHDs and 20% of cyanotic heart diseases.[10]
CcTGA is an uncommon and complex CHD, occurring in about 0.05% of all CHD cases, with an estimated incidence of approximately 1 in every 33,000 live births.[11] An international study examining the largest cohort of ccTGA cases identified nonsporadic occurrences, including familial clusters. The study also found associations between ccTGA, d-TGA, laterality defects, and, in some instances, primary ciliary dyskinesia. These findings suggest a potential common pathogenetic pathway involving laterality genes in the development of ccTGA.[12]
CcTGA is more prevalent in male infants. Due to the physiologically corrected circulation, isolated ccTGA typically remains asymptomatic during childhood and may go unnoticed until the sixth decade of life. At this stage, ccTGA can present with right ventricular dysfunction and heart block.[13] Approximately 90% of individuals with ccTGA have additional cardiac abnormalities that affect clinical symptoms and prognosis. Commonly associated conditions include VSD in 80% of cases, pulmonary stenosis in 40% to 50%, left ventricular outflow tract obstruction (LVOTO) in 30%, and Ebstein anomaly in 30%. Other potential anomalies include situs inversus, dextrocardia, complete heart block, and reentrant tachycardias. Symptoms may manifest as bradycardia, fatigue, and reduced exercise tolerance.
Pathophysiology
Understanding the normal formation of the conotruncal septum is essential for grasping the development of d-TGA. In the fifth week of gestation, pairs of opposing ridges emerge in the truncus arteriosus, specifically the right superior and left inferior truncus swellings. The right superior truncus swelling grows distally to the left, while the left inferior truncus swelling grows distally to the right. This interaction results in the twisting of the swellings around one another, setting the stage for the formation of an anatomically normal spiral septum.
Simultaneously, swellings form in the dorsal and ventral walls of the conus cordis, growing toward each other and distally. These swellings eventually fuse with the truncus septum, dividing the conus cordis into the anterolateral (right ventricular outflow tract) and posteromedial (left ventricular outflow tract) portions. An equally crucial process for septal formation is the migration of neural crest cells through pharyngeal arches 3, 4, and 6 to the heart. These cells are critical in forming the endocardial cushions in the truncus arteriosus and conus cordis and extending the outflow tracts. Disruption of normal neural crest cell migration can lead to congenital anomalies such as tetralogy of Fallot, truncus arteriosus, and TGA. Because neural crest cells also contribute to craniofacial development, it is not uncommon to see cardiac and craniofacial defects occur together in the same individual.
TGA results from the failure of the aorticopulmonary septum to spiral, which can arise from defects in the maturation of the right superior and left inferior truncus swellings, improper fusion of the conus swellings with the aorticopulmonary septum, or abnormalities in neural crest development or migration. These pathological changes in d-TGA disrupt normal cardiac physiology. Because the 2 circuits run parallel, deoxygenated blood continuously recirculates through the systemic circulation, while oxygenated blood is confined to the pulmonary circuit.
Parallel channels are incompatible with life unless mixing between deoxygenated and oxygenated blood occurs. Mixing can occur via an ASD, VSD, PDA, or collateral bronchopulmonary circulation. Interventional cardiologists may also perform a balloon atrial septostomy to facilitate mixing between the atria. Cardiac anomalies that can occur in patients with d-TGA include VSD, LVOTO, mitral and tricuspid valve abnormalities, and coronary artery variations.
A less common form of TGA is ccTGA, also known as levo-TGA. This condition is characterized by the left ventricle being positioned to the right of the right ventricle, resulting in the ventricles being on opposite sides of the heart. Although the pulmonary trunk and aorta originate from their anatomically correct positions, the reversal of the ventricles causes the aorta to fuse with the right ventricle and the pulmonary trunk to connect with the left ventricle.
In ccTGA, deoxygenated blood enters the anatomically correct right atrium, passes through the mitral valve into the left ventricle, and is then pumped into the pulmonary trunk toward the lungs. Oxygenated blood from the lungs returns to the left atrium, flows through the tricuspid valve into the right ventricle, and is subsequently pumped into the aorta. Because blood flow in ccTGA follows the normal systemic and pulmonary pathways, the condition is sometimes referred to as "anatomically corrected TGA."[14]
History and Physical
Antenatal Course
Diagnosing TGA antenatally is challenging, as routine screening ultrasounds do not typically detect this condition in utero.
Postnatal Course
The clinical features of d-TGA depend solely on the degree of mixing between the parallel circuits. Most patients present with signs and symptoms during the neonatal period or within the first 30 days of life. Typical clinical manifestations include the below conditions.
- Cyanosis: The severity of cyanosis depends on the degree of intracardiac mixing between the two parallel circuits, which is influenced by the size and presence of an ASD or VSD. Cyanosis typically does not change with exertion or supplemental oxygen.[15]
- Tachypnea: Patients often exhibit a respiratory rate exceeding 60 breaths per minute, but they do not show signs of retractions, grunting, or nasal flaring. Despite the elevated rate, they typically appear comfortable.
- Heart sounds: The first heart sound (S1) generally has normal intensity, as ventricular contraction remains unaffected. The second heart sound (S2) is often more pronounced and may be single, attributed to the anterior positioning of the aorta.
- Murmurs: Murmurs are usually absent unless a small ventricular septal defect (VSD) or pulmonary stenosis is present. When a VSD is involved, it produces a pansystolic murmur that is prominent at the lower left sternal border. In the case of pulmonary stenosis, a systolic ejection murmur is heard at the upper left sternal border.[16]
In ccTGA, associated anatomical cardiac abnormalities may include VSD, pulmonary and subpulmonary obstructions, coarctation of the aorta, and Ebstein's anomaly of the tricuspid valve.[17] Surgical correction, tailored to address the specific underlying cardiac anomalies, can involve procedures such as a double switch operation that combines an atrial switch (Senning or Mustard) with an arterial switch. Alternatively, an atrial switch can be paired with a Rastelli operation, which includes VSD closure, a baffle to the aorta, and a conduit connecting the right ventricle to the pulmonary artery.
Another approach involves the hemi-Mustard procedure combined with a bidirectional Glenn shunt. These anatomical repairs aim to position the left ventricle as the systemic ventricle, thereby reducing the long-term risk of systemic right ventricular failure and the likelihood of progressive systemic tricuspid valve insufficiency.[18][19] Individuals with ccTGA often remain asymptomatic until later in life when the right ventricle can no longer compensate for the increased afterload of systemic circulation. At this point, these patients may present with signs and symptoms of heart failure.
Right ventricular hypertrophy (RVH) shifts the QRS vector toward the right and anteriorly, often resulting in a delayed R-wave peak in the right precordial leads. Several criteria, primarily based on the amplitudes of the R and S waves in leads I, V1, and V6, as well as the R-wave peak time in V1, have been proposed for diagnosing RVH. Electrocardiographic (ECG) RVH has been categorized based on various patterns observed in CHD. One such pattern resembles an incomplete right bundle branch block, indicating volume overload. In contrast, another pattern is characterized by predominantly tall R waves (seen in Rs, R, or QR-type complexes) in the right precordial leads, suggesting pressure overload. Both patterns are often associated with right-axis deviation.[20]
Evaluation
As previously stated, TGA is challenging to detect on fetal ultrasound due to the lack of size differences between the ventricles. D-TGA can be diagnosed in utero using specialized views of the outflow tracts during fetal ultrasound.[21] Imaging is crucial for assessing d-TGA before and after surgical or nonsurgical interventions. The primary objectives of initial imaging are to confirm the diagnosis, detail the anatomy, evaluate hemodynamic abnormalities, assess the degree of mixing between pulmonary and systemic circulations, and identify any associated anomalies. Postprocedural or postsurgical imaging evaluates cardiac function and detects potential complications. The choice of imaging modalities depends on the patient's condition and the available technology and expertise at the medical facility.[22]
Commonly used imaging techniques are listed below.
- Electrocardiography: Usually appears normal but may demonstrate right-axis deviation and right ventricular hypertrophy (RVH). Several criteria, primarily based on the R and S wave amplitudes in leads I, V1, and V6, along with the R-wave peak time in V1, have been established for diagnosing RVH.
- Chest radiography: The classic radiographic feature of TGA is the "egg on a string" appearance.
- Echocardiography: Transthoracic echocardiography is the primary imaging modality for TGA, providing essential information on the morphology, function, and hemodynamics of the ventricles and valves.[23]
- Cardiac catheterization: Angiography is rarely used to diagnose TGA but remains the gold standard for determining the origins of the coronary arteries. Cardiac catheterization is routinely utilized in d-TGA to perform balloon atrial septostomy in patients with severe cyanosis and may also assist in identifying coronary arteries.[24]
- Chest computed tomography imaging: Computed tomography (CT) is often used in postsurgical patients who cannot undergo magnetic resonance imaging (MRI) due to contraindications or the presence of devices that may cause artifacts, such as pacemakers in individuals who have had an atrial switch operation (ASO).[25] CT imaging provides detailed information on the morphology of cardiovascular structures, including baffles, conduits, and coronary arteries.[26] Although CT can provide quantification of ventricular function, it does not assess hemodynamics or blood flow.[27]
- Cardiac magnetic resonance imaging: MRI offers detailed information on the anatomy and hemodynamics of cardiovascular structures, including baffles and conduits, and allows for the quantification of ventricular size, function, and valvular performance.[28] Although invasive angiography was historically the primary method for assessing coronary arterial anatomy, CT and MRI can now effectively evaluate the coronary arteries. The MRI protocol is typically tailored to the patient's specific clinical needs. Furthermore, with appropriate safety measures and image optimization, MRI can be safely performed in patients with various cardiac implantable devices.[29]
Following an ASO, the outflow tracts must be meticulously identified during echocardiographic evaluation to assess for potential supravalvar narrowing and neovalvar regurgitation. This evaluation should utilize subxiphoid, apical, and parasternal views, with the apical and parasternal perspectives being particularly effective for evaluating regional wall motion abnormalities. When evaluating the translocated coronary arteries, the parasternal short-axis view should be utilized with a very low color scale to enhance color flow detection. Doppler interrogation of the branch pulmonary arteries should be performed, particularly from a high parasternal vantage point.
For long-term follow-up, the neoaortic root should be measured using the parasternal long-axis view, while neoaortic and neopulmonary valve regurgitation should be assessed from multiple angles. Echocardiographic imaging can be challenging in adult patients post-ASO, making additional cross-sectional imaging modalities such as CT scans or MRI necessary for accurate visualization of the pulmonary artery branches and coronary arteries.[30]
Echocardiograms reveal the atypical connections associated with ccTGA. Although chest radiography is generally nonspecific, it may display an abnormal bulge along the left heart border due to the leftward positioning of the aorta, right ventricular outflow tract, and juxtaposed atrial appendage. MRI and CT scans often demonstrate the most common anatomical configuration of ccTGA, described using the Van Praagh notation system as {S, L, L}. The notation indicates situs solitus (atria in their normal positions), l-looped ventricles (right ventricle on the left and left ventricle on the right), and l-transposed great arteries (aorta positioned anteriorly and to the left of the pulmonary artery) (see Image. Congenitally Corrected Transposition of the Great Arteries With Double Discordance).
The aorta and pulmonary artery typically run parallel in the sagittal or coronal plane. The aorta connects to the thick-walled morphologic right ventricle on the left, which may initially suggest a normal connection. Therefore, if an abnormal relationship between these vessels is observed, images must be carefully assessed to determine whether the left-sided ventricle is morphologically left or right.
Treatment / Management
Initial management of patients with d-TGA focuses on ensuring adequate oxygenation. Administration of prostaglandin E1 and balloon atrial septostomy stabilizes patients by maintaining patency of the ductus arteriosus. Corrective surgery d-TGA is typically performed once the patient is hemodynamically stable, usually within the first week of life.[31][32](B3)
The 2 commonly used surgical procedures for d-TGA are listed below.
- Arterial switch operation: The ASO is the standard procedure for patients with d-TGA who do not have major pulmonic stenosis. During the ASO, the surgeon transects the pulmonary trunk and aorta, repositioning these vessels to their correct anatomical locations. The coronary arteries are mobilized and reimplanted into the aortic trunk. Following this, a Lecompte maneuver is performed after implanting the coronary vessels into the proximal portion of the pulmonary trunk, which is now referred to as the neoaorta. This maneuver repositions the pulmonary trunk, originally located behind the aorta, to a new position in front of the ascending aorta. Additionally, any existing VSD is repaired during this procedure.[33] (B3)
- Rastelli procedure: The Rastelli procedure is indicated for patients with d-TGA, a large VSD, and pulmonary stenosis. In this operation, the VSD is closed using a baffle, directing oxygenated blood from the left ventricle into the aorta. A conduit is then placed from the right ventricle to the pulmonary artery to shunt deoxygenated blood into the pulmonary artery.[34] (B3)
Other corrective procedures include the Mustard and Senning, Nikaidoh, Réparation à l'Etage Ventriculaire (REV), and Yasui procedures, although these are less commonly performed.[35] In patients with simple TGA, pulmonary blood flow gradually increases after birth, reaching nearly twice the normal level by 2 months of age. During the first few weeks of life, the left ventricular wall thickness decreases rapidly—a process known as left ventricular involution. Consequently, performing an ASO on patients older than 2 weeks with TGA and an intact ventricular septum is considered risky, as numerous studies have shown.[36](B2)
For ccTGA, a physiological repair strategy that preserves the right ventricle as the systemic ventricle is generally considered safe, but it often results in progressive congestive heart failure (CHF) over time. According to Graham et al, 67% of patients with ccTGA and associated abnormalities develop CHF by age 45.[37] Hraska et al report that the 10-year survival rate for individuals following physiological repair is only 68%.[38] Anatomic repair is often considered unsuitable for older patients, especially those whose morphological left ventricles may not sustain systemic pressures or adequately respond to pulmonary artery band training.[39] Additional factors predicting poor outcomes after anatomic repair include significant preexisting tricuspid regurgitation, right ventricular dysfunction, and the need for pacing.[40]
In this condition, the heart maintains a normal atrial position but has abnormal atrioventricular connections, resulting in reversed positions of the left and right ventricles. The anatomical left ventricle is located on the right side and connected to the pulmonary artery, while the anatomical right ventricle is on the left side and connected to the aorta. These structural abnormalities can disrupt the heart's electrical system. Research indicates that approximately 30% of patients with ccTGA develop a complete atrioventricular block, potentially due to the anterior and superior displacement of the atrioventricular node or a conduction defect below the node.[41]
Differential Diagnosis
The differential diagnoses for TGA include the following conditions:
- Double-outlet right ventricle
- Tricuspid atresia
- Pulmonary atresia
- Tetralogy of Fallot
- Total anomalous pulmonary venous return
- Truncus arteriosus
A thorough clinical investigation and appropriate imaging studies are essential to differentiate TGA from these conditions and guide management.
Prognosis
Surgical repair for d-TGA should be performed within the first week of life. The two most commonly used procedures are the standard ASO and the Rastelli procedure, which is recommended for patients with d-TGA, a large VSD, and pulmonary stenosis. Studies report a survival rate exceeding 95% at 15 to 25 years post-discharge.[42][43] Other corrective procedures, such as the Mustard, Senning, Nakaidoh, and REV, are available but less commonly performed.
Postoperative complications can result from the underlying pathophysiology, surgical intervention, or residual defects, leading to adverse effects such as right ventricular dysfunction, tricuspid valve regurgitation, supraventricular arrhythmias, interatrial and interventricular septal dysfunction, and, less commonly, pulmonary hypertension. Despite these potential complications, most treated patients reach adulthood, with a 20-year survival rate nearing 90%. The primary cause of death is sudden cardiac death, followed by anatomical right ventricular dysfunction.
Significant concerns exist regarding the ability of the anatomical right ventricle to sustain systemic circulation in patients with TGA who have undergone the Mustard procedure. In this intraatrial procedure, the anatomical right ventricle becomes the systemic ventricle, responsible for pumping blood into the main artery and managing pressures 3 to 4 times higher than those in the pulmonary circuit, leading to a considerable pressure load. Over time, the anatomical right ventricle often struggles to maintain systemic circulation, leading to a gradual decline in the clinical condition of patients following Mustard repair.[44] These late complications are associated with poor outcomes, emphasizing the importance of timely intervention.
Additionally, conduction and electrical stimulation disorders are common complications in adults who have undergone intraatrial surgery for d-TGA. These issues may arise from congenital anomalies of the sinus node and conductive fibers, damage to these structures during surgery, or injury to the coronary arteries supplying these areas. Over time, the inevitable degeneration of tissues also contributes to these complications. Notably, only 40% to 50% of patients who underwent the Senning procedure maintain sinus rhythm after 20 years of follow-up.[45]
In ccTGA, the improved long-term survival associated with anatomic repair must be carefully weighed against the short-term safety of a physiological repair strategy, which focuses solely on correcting associated abnormalities.[46] However, surgical mortality in the physiological repair population is not insignificant. For instance, a series from the Mayo Clinic reported a 3% surgical mortality rate for operations performed after 1986, with an overall mortality rate of 16%. Similarly, a Dutch series by Bogers et al documented a 6.7% mortality rate.[47][48] An alternative approach for managing cc-TGA is staged single-ventricle palliation with Fontan completion. While short-term outcomes regarding survival and symptoms are comparable, the long-term complications are significant, with many patients experiencing serious morbidities.[49]
Reinterventions after anatomic correction for cc-TGA are common.[50] In particular, conduit replacements are especially frequent in the atrial switch-Rastelli cohort, with most patients likely requiring at least one exchange to a larger conduit suitable for transcatheter replacement. After the initial reoperation, procedures such as multiple transcatheter replacements are often performed, reducing the need for further surgeries. Complications related to baffles, such as residual leaks or stenosis, are well-documented after Mustard or Senning operations and are a common reason for reoperations following anatomic repair for ccTGA. However, in the current era, most baffle-related issues can be effectively managed with transcatheter techniques, minimizing the need for additional surgeries. Residual LVOTO is also a known complication following both types of anatomic repair.[51]
Complications
Untreated TGA can result in CHF, life-threatening arrhythmias, and death. Complications that may arise from corrective procedures for TGA include:
- Arrhythmias [52]
- Obstruction or leakage of the baffle (following a Rastelli procedure)
- Pulmonary artery stenosis
- Coronary artery stenosis
- Aortic root dilation
- Aortic regurgitation [53]
Timely intervention and regular follow-up care can help minimize complications and improve outcomes for patients with TGA.
Deterrence and Patient Education
Patients with corrected d-TGA often experience slightly reduced exercise capacity. Predictors of poor exercise performance include VSD repair, diminished left ventricular function, and repair performed before the development of the ASO. However, reduced exercise capacity has not been shown to impact daily living activities.[54] Additionally, studies indicate that patients who have undergone ASO are at a higher risk of neurodevelopmental impairments.
A center reports that 65% of adolescents who underwent ASO as infants required special education services. Additionally, the same center found that patients with surgically corrected ASO were more likely to be diagnosed with attention-deficit/hyperactivity disorder (ADHD). The American Heart Association recommends continued screening and referral for neurodevelopmental impairments in children who have undergone corrective surgery for d-TGA.[55]
Pearls and Other Issues
Understanding the timing and nature of potential complications is crucial for implementing treatments that can extend the lifespan of patients with TGA into adulthood. Therefore, an interprofessional approach is essential for managing this condition, ensuring that appropriate treatment options are selected and advanced care is provided when necessary.
Enhancing Healthcare Team Outcomes
Managing TGA can be challenging. While the primary responsibility for correcting this condition lies with a pediatric cardiothoracic surgeon, involvement from other specialists is crucial for comprehensive patient care. Interventional pediatric cardiology should be consulted promptly to assess cyanosis in a newborn, enabling the timely administration of prostaglandin E1 and balloon atrial septostomy. Additionally, interventional cardiology can perform angiography concurrently to identify any anomalies in the coronary arteries.
General pediatrics should be involved in the infant's care by providing routine preventive measures and neurodevelopmental screening throughout treatment and after discharge. If neurodevelopmental deficiencies are identified during annual assessments, consultations with pediatric neurology and psychiatry should follow. Additionally, pharmacists should assist with medication reconciliation and pain management in the preoperative phase.
To achieve optimal outcomes, the nurse caring for a patient with corrected TGA should monitor for the following:
- Cardiac dysrhythmias or heart palpitations
- New heart murmurs, bruits, or extra heart sounds
- Fatigue, shortness of breath, or chest pain
- Changes in blood pressure (increasing or decreasing)
- Decrease in oxygen saturation or development of cyanosis
- Swelling in the lower extremities
These symptoms may indicate a severe complication and should be promptly addressed to the clinical team. Additionally, the nurse should conduct frequent assessments of physical, cardiac, respiratory, and neurological status, monitor vital signs and hemodynamics, and evaluate fluid and electrolyte balance. Patient and family education is crucial during the preoperative, postoperative, and discharge periods. Optimal outcomes are achieved when nurses, pharmacists, and surgeons collaborate to evaluate and monitor the patient while providing coordinated education to both the patient and their family.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
Dextro-Transposition of the Great Arteries and Parallel Circulation. This diagram illustrates the anatomical structures involved in the parallel circulation resulting from dextro-transposition of the great arteries.
Abbreviations: PA = Pulmonary artery; LV = Left ventricle; LA = Left atrium; RA = Right atrium; RV = Right ventricle.
Contributed by P Rajiah, MD
(Click Image to Enlarge)
Congenitally Corrected Transposition of the Great Arteries With Double Discordance. This illustration depicts the anatomical structures involved in congenitally corrected transposition of the great arteries with double discordance.
Abbreviations: RA = Right atrium; LV = Left ventricle; PA = Pulmonary artery; LA = Left atrium; RV = Right ventricle.
Contributed by P Rajiah, MD
(Click Video to Play)
D-Transposition of the Great Arteries. Normal location of the ventricles and abnormal origin of the aorta from the right ventricle and main pulmonary artery from the left ventricle (ventriculoarterial discordance). Additionally, there is a right-sided aortic arch with two-vessel mirror-image branching. Common trunk trifurcates to form bilateral common carotid arteries and left subclavian artery.
Contributed by Aby Thomas, MD
(Click Video to Play)
D-Transposition of the Great Arteries. Normal location of the ventricles and abnormal origin of the aorta from the right ventricle and main pulmonary artery from the left ventricle (ventriculoarterial discordance). Additionally, there is a right-sided aortic arch with two-vessel mirror-image branching. Common trunk trifurcates to form bilateral common carotid arteries and left subclavian artery.
Contributed by Aby Thomas, MD
(Click Video to Play)
D-Transposition of the Great Arteries. Status post arterial switch procedure with LeCompte maneuver. Post surgical changes from arterial switch with aorta now arising from the left ventricle and main pulmonary artery arising from the right ventricle. Branch pulmonary arteries are seen draping across the aorta.
Contributed by Aby Thomas, MD
References
Warnes CA. Transposition of the great arteries. Circulation. 2006 Dec 12:114(24):2699-709 [PubMed PMID: 17159076]
Ladouceur M. Congenitally corrected transposition of the great arteries: have we shifted the disease 'trajectory? European heart journal. 2023 Sep 7:44(34):3292-3294. doi: 10.1093/eurheartj/ehad482. Epub [PubMed PMID: 37592741]
de la Cruz MV, Arteaga M, Espino-Vela J, Quero-Jiménez M, Anderson RH, Díaz GF. Complete transposition of the great arteries: types and morphogenesis of ventriculoarterial discordance. American heart journal. 1981 Aug:102(2):271-81 [PubMed PMID: 7258100]
Level 3 (low-level) evidenceZubrzycki M, Schramm R, Costard-Jäckle A, Morshuis M, Gummert JF, Zubrzycka M. Pathogenesis and Surgical Treatment of Dextro-Transposition of the Great Arteries (D-TGA): Part II. Journal of clinical medicine. 2024 Aug 15:13(16):. doi: 10.3390/jcm13164823. Epub 2024 Aug 15 [PubMed PMID: 39200964]
Goor DA, Edwards JE. The spectrum of transposition of the great arteries: with specific reference to developmental anatomy of the conus. Circulation. 1973 Aug:48(2):406-15 [PubMed PMID: 4726219]
Martins P, Castela E. Transposition of the great arteries. Orphanet journal of rare diseases. 2008 Oct 13:3():27. doi: 10.1186/1750-1172-3-27. Epub 2008 Oct 13 [PubMed PMID: 18851735]
Kowalik E. Management of congenitally corrected transposition from fetal diagnosis to adulthood. Expert review of cardiovascular therapy. 2023 Jun:21(6):389-396. doi: 10.1080/14779072.2023.2211264. Epub 2023 May 9 [PubMed PMID: 37143366]
van Dissel AC, Opotowsky AR, Burchill LJ, Aboulhosn J, Grewal J, Lubert AM, Antonova P, Shah S, Cotts T, John AS, Kay WA, DeZorzi C, Magalski A, Han F, Baker D, Kay J, Yeung E, Vonder Muhll I, Pylypchuk S, Kuo MC, Nicolarsen J, Sarubbi B, Fusco F, Jameson SM, Cramer J, Gupta T, Gallego P, O'Donnell C, Hannah J, Dellborg M, Kauling RM, Ginde S, Krieger EV, Rodriguez F, Dehghani P, Kutty S, Wong J, Wilson WM, Rodriguez-Monserrate CP, Roos-Hesselink J, Celermajer DS, Khairy P, Broberg CS. End-stage heart failure in congenitally corrected transposition of the great arteries: a multicentre study. European heart journal. 2023 Sep 7:44(34):3278-3291. doi: 10.1093/eurheartj/ehad511. Epub [PubMed PMID: 37592821]
Škorić-Milosavljević D, Tadros R, Bosada FM, Tessadori F, van Weerd JH, Woudstra OI, Tjong FVY, Lahrouchi N, Bajolle F, Cordell HJ, Agopian AJ, Blue GM, Barge-Schaapveld DQCM, Gewillig M, Preuss C, Lodder EM, Barnett P, Ilgun A, Beekman L, van Duijvenboden K, Bokenkamp R, Müller-Nurasyid M, KORA-Study Group, Vliegen HW, Konings TC, van Melle JP, van Dijk APJ, van Kimmenade RRJ, Roos-Hesselink JW, Sieswerda GT, Meijboom F, Abdul-Khaliq H, Berger F, Dittrich S, Hitz MP, Moosmann J, Riede FT, Schubert S, Galan P, Lathrop M, Munter HM, Al-Chalabi A, Shaw CE, Shaw PJ, Morrison KE, Veldink JH, van den Berg LH, Evans S, Nobrega MA, Aneas I, Radivojkov-Blagojević M, Meitinger T, Oechslin E, Mondal T, Bergin L, Smythe JF, Altamirano-Diaz L, Lougheed J, Bouma BJ, Chaix MA, Kline J, Bassett AS, Andelfinger G, van der Palen RLF, Bouvagnet P, Clur SB, Breckpot J, Kerstjens-Frederikse WS, Winlaw DS, Bauer UMM, Mital S, Goldmuntz E, Keavney B, Bonnet D, Mulder BJ, Tanck MWT, Bakkers J, Christoffels VM, Boogerd CJ, Postma AV, Bezzina CR. Common Genetic Variants Contribute to Risk of Transposition of the Great Arteries. Circulation research. 2022 Jan 21:130(2):166-180. doi: 10.1161/CIRCRESAHA.120.317107. Epub 2021 Dec 10 [PubMed PMID: 34886679]
Centers for Disease Control and Prevention (CDC). Improved national prevalence estimates for 18 selected major birth defects--United States, 1999-2001. MMWR. Morbidity and mortality weekly report. 2006 Jan 6:54(51):1301-5 [PubMed PMID: 16397457]
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2011 Nov 15:58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. Epub [PubMed PMID: 22078432]
Level 1 (high-level) evidenceTortigue M, Nield LE, Karakachoff M, McLeod CJ, Belli E, Babu-Narayan SV, Prigent S, Boet A, Conway M, Elder RW, Ladouceur M, Khairy P, Kowalik E, Kalfa DM, Barron DJ, Mussa S, Hiippala A, Temple J, Abadir S, Le Gloan L, Lachaud M, Sanatani S, Thambo JB, Gronier CG, Amedro P, Vaksmann G, Charbonneau A, Koutbi L, Ovaert C, Houeijeh A, Combes N, Maury P, Duthoit G, Hiel B, Erickson CC, Bonnet C, Van Hare GF, Dina C, Karsenty C, Fournier E, Le Bloa M, Pass RH, Liberman L, Happonen JM, Perry JC, Romefort B, Benbrik N, Hauet Q, Fraisse A, Gatzoulis MA, Abrams DJ, Dubin AM, Ho SY, Redon R, Bacha EA, Schott JJ, Baruteau AE. Familial Recurrence Patterns in Congenitally Corrected Transposition of the Great Arteries: An International Study. Circulation. Genomic and precision medicine. 2022 Jun:15(3):e003464. doi: 10.1161/CIRCGEN.121.003464. Epub 2022 May 12 [PubMed PMID: 35549293]
Hornung TS, Calder L. Congenitally corrected transposition of the great arteries. Heart (British Cardiac Society). 2010 Jul:96(14):1154-61. doi: 10.1136/hrt.2008.150532. Epub [PubMed PMID: 20610462]
Hornung TS, Bernard EJ, Celermajer DS, Jaeggi E, Howman-Giles RB, Chard RB, Hawker RE. Right ventricular dysfunction in congenitally corrected transposition of the great arteries. The American journal of cardiology. 1999 Nov 1:84(9):1116-9, A10 [PubMed PMID: 10569681]
Oster ME, Aucott SW, Glidewell J, Hackell J, Kochilas L, Martin GR, Phillippi J, Pinto NM, Saarinen A, Sontag M, Kemper AR. Lessons Learned From Newborn Screening for Critical Congenital Heart Defects. Pediatrics. 2016 May:137(5):. doi: 10.1542/peds.2015-4573. Epub 2016 Apr 15 [PubMed PMID: 27244826]
Van Praagh R, Geva T, Kreutzer J. Ventricular septal defects: how shall we describe, name and classify them? Journal of the American College of Cardiology. 1989 Nov 1:14(5):1298-9 [PubMed PMID: 2808986]
Wallis GA, Debich-Spicer D, Anderson RH. Congenitally corrected transposition. Orphanet journal of rare diseases. 2011 May 14:6():22. doi: 10.1186/1750-1172-6-22. Epub 2011 May 14 [PubMed PMID: 21569592]
Kutty S, Danford DA, Diller GP, Tutarel O. Contemporary management and outcomes in congenitally corrected transposition of the great arteries. Heart (British Cardiac Society). 2018 Jul:104(14):1148-1155. doi: 10.1136/heartjnl-2016-311032. Epub 2018 Jan 11 [PubMed PMID: 29326110]
Chatterjee A, Miller NJ, Cribbs MG, Mukherjee A, Law MA. Systematic review and meta-analysis of outcomes of anatomic repair in congenitally corrected transposition of great arteries. World journal of cardiology. 2020 Aug 26:12(8):427-436. doi: 10.4330/wjc.v12.i8.427. Epub [PubMed PMID: 32879705]
Level 1 (high-level) evidenceLehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest. 1988 Apr:93(4):839-42 [PubMed PMID: 2964996]
Ravi P, Mills L, Fruitman D, Savard W, Colen T, Khoo N, Serrano-Lomelin J, Hornberger LK. Population trends in prenatal detection of transposition of great arteries: impact of obstetric screening ultrasound guidelines. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2018 May:51(5):659-664. doi: 10.1002/uog.17496. Epub [PubMed PMID: 28436133]
Cohen MS, Eidem BW, Cetta F, Fogel MA, Frommelt PC, Ganame J, Han BK, Kimball TR, Johnson RK, Mertens L, Paridon SM, Powell AJ, Lopez L. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016 Jul:29(7):571-621. doi: 10.1016/j.echo.2016.04.002. Epub [PubMed PMID: 27372954]
Canan A, Ashwath R, Agarwal PP, François C, Rajiah P. Multimodality Imaging of Transposition of the Great Arteries. Radiographics : a review publication of the Radiological Society of North America, Inc. 2021 Mar-Apr:41(2):338-360. doi: 10.1148/rg.2021200069. Epub 2021 Jan 22 [PubMed PMID: 33481689]
Gopalakrishnan A, Krishnamoorthy KM, Sivasubramonian S. Balloon atrial septostomy at the bedside versus the catheterisation laboratory. Cardiology in the young. 2019 Mar:29(3):454. doi: 10.1017/S1047951118002214. Epub 2019 Jan 28 [PubMed PMID: 30688192]
Ranganath P, Singh S, Abbara S, Agarwal PP, Rajiah P. Computed Tomography in Adult Congenital Heart Disease. Radiologic clinics of North America. 2019 Jan:57(1):85-111. doi: 10.1016/j.rcl.2018.08.013. Epub [PubMed PMID: 30454820]
Tomasian A, Malik S, Shamsa K, Krishnam MS. Congenital heart diseases: post-operative appearance on multi-detector CT-a pictorial essay. European radiology. 2009 Dec:19(12):2941-9. doi: 10.1007/s00330-009-1474-7. Epub [PubMed PMID: 19513718]
Yamasaki Y, Nagao M, Yamamura K, Yonezawa M, Matsuo Y, Kawanami S, Kamitani T, Higuchi K, Sakamoto I, Shiokawa Y, Yabuuchi H, Honda H. Quantitative assessment of right ventricular function and pulmonary regurgitation in surgically repaired tetralogy of Fallot using 256-slice CT: comparison with 3-Tesla MRI. European radiology. 2014 Dec:24(12):3289-99. doi: 10.1007/s00330-014-3344-1. Epub 2014 Aug 12 [PubMed PMID: 25113649]
Tsai-Goodman B, Geva T, Odegard KC, Sena LM, Powell AJ. Clinical role, accuracy, and technical aspects of cardiovascular magnetic resonance imaging in infants. The American journal of cardiology. 2004 Jul 1:94(1):69-74 [PubMed PMID: 15219512]
Rajiah P, Kay F, Bolen M, Patel AR, Landeras L. Cardiac Magnetic Resonance in Patients With Cardiac Implantable Electronic Devices: Challenges and Solutions. Journal of thoracic imaging. 2020 Jan:35(1):W1-W17. doi: 10.1097/RTI.0000000000000462. Epub [PubMed PMID: 31855948]
Cohen MS, Mertens LL. EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Echocardiographic assessment of transposition of the great arteries and congenitally corrected transposition of the great arteries. Echo research and practice. 2019 Dec 1:6(4):R107-R119. doi: 10.1530/ERP-19-0047. Epub 2019 Dec 1 [PubMed PMID: 31729212]
Freed MD, Heymann MA, Lewis AB, Roehl SL, Kensey RC. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation. 1981 Nov:64(5):899-905 [PubMed PMID: 7285305]
Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA. 1966 Jun 13:196(11):991-2 [PubMed PMID: 4160716]
Level 3 (low-level) evidenceJatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. The Journal of thoracic and cardiovascular surgery. 1976 Sep:72(3):364-70 [PubMed PMID: 957754]
Level 3 (low-level) evidenceRastelli GC, Wallace RB, Ongley PA. Complete repair of transposition of the great arteries with pulmonary stenosis. A review and report of a case corrected by using a new surgical technique. Circulation. 1969 Jan:39(1):83-95 [PubMed PMID: 5782810]
Level 3 (low-level) evidenceHazekamp MG, Gomez AA, Koolbergen DR, Hraska V, Metras DR, Mattila IP, Daenen W, Berggren HE, Rubay JE, Stellin G, European Congenital Heart Surgeons Association. Surgery for transposition of the great arteries, ventricular septal defect and left ventricular outflow tract obstruction: European Congenital Heart Surgeons Association multicentre study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010 Dec:38(6):699-706. doi: 10.1016/j.ejcts.2010.03.030. Epub 2010 May 13 [PubMed PMID: 20466558]
Level 2 (mid-level) evidenceDuncan BW, Poirier NC, Mee RB, Drummond-Webb JJ, Qureshi A, Mesia CI, Graney JA, Malek CL, Latson LA. Selective timing for the arterial switch operation. The Annals of thoracic surgery. 2004 May:77(5):1691-6; discussion 1697 [PubMed PMID: 15111168]
Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. Journal of the American College of Cardiology. 2000 Jul:36(1):255-61 [PubMed PMID: 10898443]
Hraska V, Duncan BW, Mayer JE Jr, Freed M, del Nido PJ, Jonas RA. Long-term outcome of surgically treated patients with corrected transposition of the great arteries. The Journal of thoracic and cardiovascular surgery. 2005 Jan:129(1):182-91 [PubMed PMID: 15632841]
Myers PO, del Nido PJ, Geva T, Bautista-Hernandez V, Chen P, Mayer JE Jr, Emani SM. Impact of age and duration of banding on left ventricular preparation before anatomic repair for congenitally corrected transposition of the great arteries. The Annals of thoracic surgery. 2013 Aug:96(2):603-10. doi: 10.1016/j.athoracsur.2013.03.096. Epub 2013 Jun 29 [PubMed PMID: 23820627]
Bautista-Hernandez V, Myers PO, Cecchin F, Marx GR, Del Nido PJ. Late left ventricular dysfunction after anatomic repair of congenitally corrected transposition of the great arteries. The Journal of thoracic and cardiovascular surgery. 2014 Jul:148(1):254-8. doi: 10.1016/j.jtcvs.2013.08.047. Epub 2013 Oct 5 [PubMed PMID: 24100093]
Baruteau AE, Abrams DJ, Ho SY, Thambo JB, McLeod CJ, Shah MJ. Cardiac Conduction System in Congenitally Corrected Transposition of the Great Arteries and Its Clinical Relevance. Journal of the American Heart Association. 2017 Dec 21:6(12):. doi: 10.1161/JAHA.117.007759. Epub 2017 Dec 21 [PubMed PMID: 29269355]
Hutter PA, Kreb DL, Mantel SF, Hitchcock JF, Meijboom EJ, Bennink GB. Twenty-five years' experience with the arterial switch operation. The Journal of thoracic and cardiovascular surgery. 2002 Oct:124(4):790-7 [PubMed PMID: 12324738]
Tobler D, Williams WG, Jegatheeswaran A, Van Arsdell GS, McCrindle BW, Greutmann M, Oechslin EN, Silversides CK. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. Journal of the American College of Cardiology. 2010 Jun 29:56(1):58-64. doi: 10.1016/j.jacc.2010.03.031. Epub [PubMed PMID: 20620718]
Andrade L, Carazo M, Wu F, Kim Y, Wilson W. Mechanisms for heart failure in systemic right ventricle. Heart failure reviews. 2020 Jul:25(4):599-607. doi: 10.1007/s10741-019-09902-1. Epub [PubMed PMID: 31853794]
Love BA, Mehta D, Fuster VF. Evaluation and management of the adult patient with transposition of the great arteries following atrial-level (Senning or Mustard) repair. Nature clinical practice. Cardiovascular medicine. 2008 Aug:5(8):454-67. doi: 10.1038/ncpcardio1252. Epub 2008 Jul 1 [PubMed PMID: 18594551]
Hirose K, Nishina T, Kanemitsu N, Mizuno A, Yasumizu D, Yada M, Onga Y, Yamanaka K. The long-term outcomes of physiologic repair for ccTGA (congenitally corrected transposition of the great arteries). General thoracic and cardiovascular surgery. 2015 Sep:63(9):496-501. doi: 10.1007/s11748-015-0550-y. Epub 2015 May 12 [PubMed PMID: 25964161]
Biliciler-Denktas G, Feldt RH, Connolly HM, Weaver AL, Puga FJ, Danielson GK. Early and late results of operations for defects associated with corrected transposition and other anomalies with atrioventricular discordance in a pediatric population. The Journal of thoracic and cardiovascular surgery. 2001 Aug:122(2):234-41 [PubMed PMID: 11479495]
Bogers AJ, Head SJ, de Jong PL, Witsenburg M, Kappetein AP. Long term follow up after surgery in congenitally corrected transposition of the great arteries with a right ventricle in the systemic circulation. Journal of cardiothoracic surgery. 2010 Sep 28:5():74. doi: 10.1186/1749-8090-5-74. Epub 2010 Sep 28 [PubMed PMID: 20920167]
Sun J, Brizard C, Winlaw D, Alphonso N, d'Udekem Y, Eastaugh L, Marathe S, Bell D, Ayer J. Biventricular repair versus Fontan completion for patients with d- or l-transposition of the great arteries with ventricular septal defect and left ventricular outflow tract obstruction. The Journal of thoracic and cardiovascular surgery. 2019 Oct:158(4):1158-1167.e1. doi: 10.1016/j.jtcvs.2019.05.061. Epub 2019 Jun 10 [PubMed PMID: 31301903]
Caughron H, Kim D, Kamioka N, Lerakis S, Yousef A, Maini A, Reginauld S, Sahu A, Shashidharan S, Jokhadar M, Rodriguez FH 3rd, Book WM, McConnell M, Block PC, Babaliaros V. Repeat Pulmonary Valve Replacement: Similar Intermediate-Term Outcomes With Surgical and Transcatheter Procedures. JACC. Cardiovascular interventions. 2018 Dec 24:11(24):2495-2503. doi: 10.1016/j.jcin.2018.07.042. Epub 2018 Nov 28 [PubMed PMID: 30503596]
Chatterjee A, Miller NJ, Cribbs MG, Law MA. Transcatheter Repair of Pulmonary Venous Baffle Stenosis. JACC. Cardiovascular interventions. 2018 Aug 27:11(16):e129-e130. doi: 10.1016/j.jcin.2018.06.031. Epub 2018 Aug 1 [PubMed PMID: 30077679]
Gatzoulis MA, Walters J, McLaughlin PR, Merchant N, Webb GD, Liu P. Late arrhythmia in adults with the mustard procedure for transposition of great arteries: a surrogate marker for right ventricular dysfunction? Heart (British Cardiac Society). 2000 Oct:84(4):409-15 [PubMed PMID: 10995411]
Schwartz ML, Gauvreau K, del Nido P, Mayer JE, Colan SD. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004 Sep 14:110(11 Suppl 1):II128-32 [PubMed PMID: 15364851]
Level 2 (mid-level) evidenceSamos F, Fuenmayor G, Hossri C, Elias P, Ponce L, Souza R, Jatene I. Exercise Capacity Long-Term after Arterial Switch Operation for Transposition of the Great Arteries. Congenital heart disease. 2016 Mar-Apr:11(2):155-9. doi: 10.1111/chd.12303. Epub 2015 Nov 11 [PubMed PMID: 26556777]
Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr, Li J, Smith SE, Bellinger DC, Mahle WT, American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012 Aug 28:126(9):1143-72 [PubMed PMID: 22851541]