Introduction
Oysters, clams, mussels, scallops, and, rarely, crustaceans, gastropods, and some fish may be contaminated by dinoflagellate or cyanobacteria toxins. Consumption of these marine food sources can cause poisoning, producing neurologic and gastrointestinal symptoms.[1][2] The action sites of marine toxins include ion channels, kainate receptors, and protein phosphatases, which are crucial to nerve and muscle function. Disruption of these molecules' functions causes the symptoms observed in patients with shellfish toxicity.
Shellfish poisoning may be classified into 4 clinical syndromes, depending on the specific etiology and clinical presentation: paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), amnesic shellfish poisoning (ASP), and diarrheic shellfish poisoning (DSP). PSP is associated with saxitoxins.[3] NSP is produced by brevetoxin.[4] ASP is due to domoic acid ingestion.[5] DSP is caused by okadaic acid.[6] These toxins and their associated illnesses are related to harmful algal blooms.[7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Shellfish toxicity occurs mostly during “red tide” algal blooms and is primarily a foodborne illness. Brevetoxin poisoning is an exception, as this toxicant may be ingested or inhaled in aerosol form. Algal blooms are more common during the summer, hence the increased risk of exposure to marine poisons during this season.
The marine toxins causing human illnesses are synthesized by dinoflagellates and certain cyanobacteria, accumulating in shellfish that serve as marine food sources. Saxitoxins and the related compounds neosaxitoxin and ganyautoxin are collectively called "paralytic shellfish toxins" (PSTs). PSTs are heat-stable, produced by the dinoflagellate genera Alexandrium, Gymnodinium, and Pyrodinium, and certain cyanobacteria. Brevetoxin is synthesized by Karenia brevis. Diatoms of the genus Pseudonitzschia make domoic acid. Okadaic acid and its analogs, the dinophysistoxins (DTXs), are produced by Prorocentrum lima and Dinophysis.[8]
Epidemiology
Individuals at risk for shellfish toxicity are those who consume shellfish harvested from temperate and tropical marine coasts during summer.[9] Human cases of PSP in Canada,[10][11] Chile,[12][13] France,[14] Guatemala,[15] Malaysia,[16] Nicaragua,[17] the Philippines,[18][19] Portugal,[20] Spain,[21] Tasmania,[22], the United Kingdom,[23] and the United States [24][25][26] have been published. Beyond these occurrences, PSTs have been identified in shellfish and other organisms globally without published cases of human exposure. Of the shellfish toxicity syndromes, PSP has produced the most fatalities.
Blooms of K. brevis are endemic to the Gulf of Mexico. Some cases have been documented in other parts of the world, but most NSP occurrences have been published in the southwest United States.[27][28][29][30] Besides gastrointestinal involvement, cases of pulmonary irritation from aerosolized brevetoxin have been reported from the same geographic region.[31][32][33]
The largest collection of human ASP reports came from Prince Edward Island, Canada in 1987.[34] Chronic domoic acid exposure has been implicated in memory changes observed in patients.[35] Following the 1987 outbreak, several public health programs have been instituted to mitigate further human ASP outbreaks.
Human cases of DSP have been reported in Argentina, Canada, Chile, Denmark, France, Ireland, Japan, New Zealand, Norway, Portugal, the United Kingdom,[36] and the United States.[37]
Pathophysiology
Saxitoxins and PSTs inhibit ion conductance by binding to the outer mouth of voltage-gated sodium channels at the site referred to as "site 1." Tetrodotoxin binds in a similar fashion on the same location (see Image. A-Subunit and Neurotoxin Binding). Blocking these ion channels reduces sodium flow into the cell.[38] Saxitoxins and PSTs also target potassium and calcium channels to a lesser extent. The muscle weakness characteristic of PSP results from sodium conduction inhibition.[39]
Brevetoxins bind with high affinity to the same voltage-gated sodium channels but at receptor site 5, located in the intramembranous portion of the ion channel. In contrast to saxitoxin, brevetoxins induce sodium ion influx. The increased sodium conductance results in membrane depolarization, spontaneous firing, and neuroexcitation.[4] Brevetoxin's chemical structure is similar to ciguatoxin, and both molecules have similar receptor activity.[40]
Domoic acid is a kainoid analog with an affinity for some kainate receptor subclasses. Kainate receptors are widely distributed in the mammalian brain but are particularly abundant in the human hippocampal CA3 region—an area highly involved in memory. In animal studies, domoic acid exposure has been shown to overstimulate hippocampal neuronal firing, resulting in neuronal necrosis. Hippocampal degradation in animals paralleled the autopsy findings on individuals affected by the 1987 outbreak. Domoic acid-induced neuroexcitotoxicity in the hippocampus is presumed to underlie the ASP symptoms.
Okadaic acid and DTXs are inhibitors of protein phosphatases 2A, 1B, and 2B. The mechanism of okadaic acid-induced diarrhea is incompletely described. Inhibition of protein phosphatases 2A and 1B increases the phosphorylation of myosin and related proteins, resulting in smooth muscle contraction and increased intestinal sodium secretion. Increased intraluminal sodium drives intraluminal fluid accumulation and abdominal cramping.
Other sources report that okadaic acid's direct phosphorylation of the proteins controlling sodium secretion is causing the intraluminal electrolyte and fluid shift. The toxin also alters cell morphology, destroys the cellular cytoskeleton, disrupts the cell cycle, and induces apoptosis. Okadaic acid has been reported to be carcinogenic in animal studies, though correlations to specific human malignancies have not been described.[41]
Toxicokinetics
Most toxicokinetic data for the dinoflagellate toxins found in shellfish are from animal studies. Saxitoxin is well-absorbed orally with rapid distribution throughout the body, including the brain. Inhalational absorption in mice has also been reported. Saxitoxin clearance is primarily urinary with no biliary elimination.[42][43] Rodent studies revealed that saxitoxin LD50 was 3-9 µg/kg intravenously and 263 µg/kg orally.
Brevetoxins are also rapidly absorbed and distributed throughout the body. The highest concentrations are found in the liver following absorption. The toxin is eliminated through both biliary and renal pathways, with the biliary route constituting most of the early stages of clearance.[44] Brevotoxin LD50 values in mice are 94 µg/kg intravenously and 520 µg/kg orally.
Domoic acid toxicokinetic studies are somewhat limited by the small experimental group sizes. The toxin is poorly absorbed from the gut and widely distributed in the body. The primary route of elimination is the feces. An intraperitoneal LD50 of 2.4 mg/kg was reported in mice.
Okadaic acid is well-absorbed from the gastrointestinal tract. Though this toxin is widely distributed, it is usually highly concentrated in intestinal tissue. Clearance is by both urinary and fecal routes, though okadaic acid can also undergo enterohepatic recirculation.[45] This toxin's intraperitoneal LD50 in mice was reported to be 192 µg/kg.
History and Physical
Some shellfish poisoning cases can be fatal. Patients may present apneic, unresponsive, and pulseless, needing immediate resuscitation. The airway, breathing, and circulation (ABCs) must be stabilized after finishing the primary survey. The secondary survey may be completed once emergencies have been ruled out.
On history, the common feature of shellfish poisoning syndromes is recent shellfish or seafood ingestion. The condition can occur in endemic regions throughout the year but is most frequent in the summer when algal overgrowth is most common. Shellfish toxicity can cause an outbreak if many people have been exposed to the same contaminated marine source.
For PSP, the initial symptoms may include facial or fingertip paresthesias, dizziness, diplopia, circumoral numbness, ataxia, and weakness. A “floating sensation” may also be reported. Early gastrointestinal symptoms such as nausea, vomiting, diarrhea, hypersalivation, and abdominal pain may be less prominent in PSP than the other shellfish toxicity syndromes. Symptom onset ranges from 20 minutes to 5 hours of ingestion. Severity correlates with the amount of shellfish consumed. Neurologic symptoms may progress to generalized weakness with possible ventilatory compromise.
The median time to symptom resolution is 24 hours, ranging from 30 minutes to 10 days. Death can occur between 3.5 and 8 hours after onset. Fatalities from PSP have been reported secondary to respiratory failure.
Neurotoxic shellfish poisoning presents with both gastrointestinal and neurologic symptoms. Patients may report mild-to-moderate nausea, vomiting, diarrhea, and facial and extremity numbness and paresthesia. Hot-and-cold sensation reversal, similar to exposure to ciguatoxin, has also been reported.[46] Symptoms usually occur 1 to 3 hours after ingestion of contaminated shellfish.[47] However, NSP manifestations may be delayed for up to 18 hours following ingestion.
The risk of generalized muscle weakness, respiratory compromise, and death is less with NSP than with PSP. Airborne brevetoxin in aerosolized seawater can cause pulmonary irritation, mucosal irritation, nonproductive cough, bronchoconstriction, wheezing, and asthma exacerbations.
Characterization of ASP's clinical presentation is largely based on the 1987 Canadian outbreak. Early gastrointestinal symptoms were seen in most individuals, with symptoms ranging from mild abdominal discomfort to severe nausea and vomiting. About 43% of the patients reported a headache, while 25% had short-term memory loss. Myoclonus, generalized seizures, psychomotor seizures, and focal motor seizures were also documented. Three deaths were observed in this cohort. Outside of this outbreak, verbal memory decline has been reported in people who consume domoic acid-containing razor clams.
DSP symptoms include nausea, abdominal pain, vomiting, headache, chills, and fever. These manifestations appear between 0.5 and 4 hours after consuming contaminated shellfish and may last up to 72 hours. DSP's acute symptoms are not always severe. Okadaic acid has been observed to be carcinogenic in animals.
On physical examination, tachypnea, tachycardia, and low-to-borderline blood pressure may be early signs of dehydration. Patients with severe vomiting or diarrhea may have sunken eyeballs, dry oral mucosa, poor capillary refill, and hyperactive bowel sounds. Hypoxemia and adventitious breath sounds are more consistent with brevetoxin-related illness.
Patients suspected to have PSP, NSP, or ASP must be given a full neurologic evaluation. These individuals must be closely monitored for progression, as the manifestations of shellfish poisoning are sometimes delayed. Clinicians must have a low threshold for intubation in patients who may have PSP. If respiratory muscle weakness is suspected, performing a test for negative inspiratory force is reasonable.
Evaluation
Patients with suspected shellfish poisoning are evaluated primarily based on history and clinical assessment. A detailed history should be obtained, with special attention to local public health alerts about algal blooms or known shellfish contamination. Patients who harvest and consume shellfish while these alerts are in effect may have a greater risk of developing the condition.[42]
High-pressure liquid chromatography (HPLC) and enzyme-linked immunosorbent assays (ELISA) can be used to detect saxitoxin,[3] brevetoxin,[4] domoic acid,[5] and okadaic acid.[37] However, these tests may not be readily available in small healthcare centers. Clinicians may have to rely on history and clinical assessment if testing results cannot be obtained within a reasonable time frame.
Initial blood tests that may be obtained for patients with shellfish toxicity include a complete blood count (CBC), coagulation studies, and a complete metabolic panel (CMP). The CBC may show neutrophilia with or without total leukocyte elevation, possibly related to vomiting or acute systemic inflammation. Increased hemoglobin, hematocrit, and sodium levels may be signs of dehydration.
A high blood urea nitrogen level with a normal creatinine level is more consistent with acute dehydration than kidney disease. However, the elevation of both parameters could mean dehydration has started affecting kidney function. The CMP can provide information about baseline liver function, which may be used for assessing the effects of toxins eliminated by the biliary route.
Chest x-rays may be obtained for patients made ill by brevetoxin inhalation. The findings may be nonspecific but can help rule out conditions like pneumonia, which warrant a different treatment approach.
Treatment / Management
The treatment for all shellfish toxicity syndromes is supportive. Activated charcoal may be considered if the patient has not vomited most of the ingested shellfish.[48] However, activated charcoal's shellfish toxin-adsorptive ability is unknown. Cathartics and gastric lavage are not universally recommended because evidence for their benefits for patients with this condition is lacking.[49][50](B3)
The respiratory status of individuals with PSP must be monitored closely. Endotracheal intubation and ventilatory support are warranted if respiratory compromise develops. Ventilatory management may continue for several days until motor weakness resolves. Saxitoxin antibodies have been tested in animals to reverse respiratory failure but not humans.[51](B3)
For NSP, managing gastrointestinal symptoms and sensory changes is an important component of supportive treatment.
Mannitol may be considered in brevetoxin poisoning treatment given its use in ciguatera fish poisoning and the similarities between brevetoxin and ciguatoxin. However, data for mannitol use in ciguatera is limited, and no information exists for its use in NSP.[52] Brevenal is a natural brevetoxin inhibitor found in K. brevis that may be a potential treatment. However, this compound has not been tested, and its pharmaceutical role is only theoretical.[53](B3)
For exposure to aerosolized brevetoxin, β-adrenergic agonists like albuterol may be used for treating bronchoconstriction or asthma exacerbation. Animal studies show that histamine antagonists and atropine also effectively block brevetoxin-induced bronchospasm. Patients recover quickly after vacating the location where the aerosolized toxin has spread. Advising patients to avoid such places may aid in preventing the condition.[32](B3)
ASP and DSP treatment is supportive. No specific antidote for either condition has been identified.
Differential Diagnosis
Shellfish toxicity has the following differential diagnosis:
- Botulism
- Tetrodotoxin toxicity
- Conotoxin toxicity
- Crown-of-thorns starfish sting
- Seabather’s eruption
- Ciguatera toxicity
- Scombroid
- Infectious enterocolitis
- Food poisoning
- Guillain-Barre syndrome
- Seizure disorder
A complete medical evaluation will help distinguish between these conditions.
Deterrence and Patient Education
The following measures may prevent shellfish poisoning:
- Purchasing from reputable sources
- Checking local advisories
- Avoiding harvesting during red-tide seasons
- Proper food storage and refrigeration
- Cooking shellfish thoroughly
- Discarding damaged or dead shellfish
Patients must be counseled to seek medical attention promptly if symptoms occur after consuming seafood.
Pearls and Other Issues
The most important points to remember in shellfish poisoning management are the following:
- Shellfish toxicity has 4 syndromes: PSP, NSP, ASP, and DSP. While each has a unique constellation of symptoms, these syndromes' common clinical features include a history of recent shellfish or seafood consumption and gastrointestinal symptoms. The exception is brevetoxin poisoning.
- Brevetoxin exposure may take the gastrointestinal or pulmonary route. Other shellfish toxins enter the body primarily through the digestive system.
- The manifestations of shellfish poisoning depend on the toxin causing the pathology.
- Shellfish toxicity can be seasonal and regional, often related to algal blooms. Harvesting in areas with high toxin levels, especially in the summer, must be avoided.
- Health departments and regulatory bodies monitor shellfish harvesting areas for increasing toxin levels. Authorities issue warnings and close affected locations to protect public health.
- Shellfish species like mussels, clams, oysters, and scallops are more prone to accumulating toxins than others.
- Cooking shellfish can destroy bacteria but does not always neutralize toxins.
- Patients must be counseled to seek immediate medical attention if they develop poisoning symptoms after consuming shellfish.
- Suspected cases must be reported to local health authorities to protect public health.
The treatment for this condition is mainly supportive. Prevention is the best way to protect individuals and communities.
Enhancing Healthcare Team Outcomes
Interprofessional communication is important in the management of shellfish toxicity. After examining the patient, healthcare providers should inform local public health officials about the diagnosis and patient history. The location and time of poisoning and the shellfish source must be reported to start mitigation measures in the affected areas.
Care coordination among multiple professionals and facilities may be necessary for large outbreaks. In the field, a multidisciplinary team of epidemiologists, marine biologists, and public health officials can help contain outbreaks. At the hospital, the interprofessional team rendering care to patients includes emergency medicine physicians, nurses, toxicologists, pharmacists, intensivists, gastroenterologists, respiratory therapists, pulmonologists, neurologists, nutritionists, and rehabilitation specialists. Outbreak information can be disseminated to local providers by public health officials to increase awareness of signs and symptoms and prompt inquiries for potential exposures.[54]
Media
(Click Image to Enlarge)
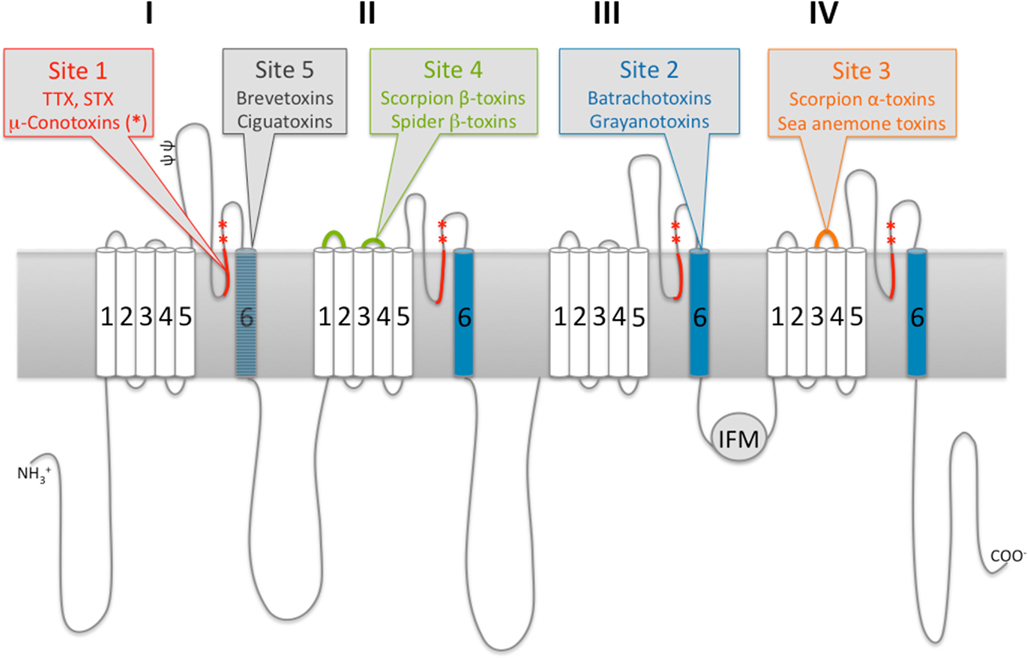
A-Subunit and Neurotoxin Binding. A schematic 2-dimensional representation of the functional A-subunit of voltage-gated ion channels and identification of known neurotoxin binding areas.
Stevens M, Peigneur S, Tytgat J. Front Pharmacol. Neurotoxins and their binding areas on voltage-gated sodium channels. 2011;2:71.doi: 10.3389/fphar.2011.00071.
References
Deeds JR, Landsberg JH, Etheridge SM, Pitcher GC, Longan SW. Non-traditional vectors for paralytic shellfish poisoning. Marine drugs. 2008 Jun 10:6(2):308-48. doi: 10.3390/md20080015. Epub 2008 Jun 10 [PubMed PMID: 18728730]
Level 3 (low-level) evidenceRasmussen SA, Andersen AJ, Andersen NG, Nielsen KF, Hansen PJ, Larsen TO. Chemical Diversity, Origin, and Analysis of Phycotoxins. Journal of natural products. 2016 Mar 25:79(3):662-73. doi: 10.1021/acs.jnatprod.5b01066. Epub 2016 Feb 22 [PubMed PMID: 26901085]
Cusick KD, Sayler GS. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Marine drugs. 2013 Mar 27:11(4):991-1018. doi: 10.3390/md11040991. Epub 2013 Mar 27 [PubMed PMID: 23535394]
Level 3 (low-level) evidenceWatkins SM, Reich A, Fleming LE, Hammond R. Neurotoxic shellfish poisoning. Marine drugs. 2008:6(3):431-55. doi: 10.3390/md20080021. Epub 2008 Jul 12 [PubMed PMID: 19005578]
Level 3 (low-level) evidenceJeffery B, Barlow T, Moizer K, Paul S, Boyle C. Amnesic shellfish poison. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2004 Apr:42(4):545-57 [PubMed PMID: 15019178]
Level 3 (low-level) evidenceValdiglesias V, Prego-Faraldo MV, Pásaro E, Méndez J, Laffon B. Okadaic acid: more than a diarrheic toxin. Marine drugs. 2013 Oct 31:11(11):4328-49. doi: 10.3390/md11114328. Epub 2013 Oct 31 [PubMed PMID: 24184795]
Level 3 (low-level) evidenceGrattan LM, Holobaugh S, Morris JG Jr. Harmful Algal Blooms and Public Health. Harmful algae. 2016 Jul:57(B):2-8 [PubMed PMID: 27616971]
Potera C. Red tide chokehold. Environmental health perspectives. 2007 Apr:115(4):A188 [PubMed PMID: 17450197]
Level 3 (low-level) evidenceGrattan LM, Holobaugh S, Morris JG Jr. Harmful algal blooms and public health. Harmful algae. 2016 Jul:57(Pt B):2-8. doi: 10.1016/j.hal.2016.05.003. Epub 2016 Aug 30 [PubMed PMID: 28918886]
Acres J, Gray J. Paralytic shellfish poisoning. Canadian Medical Association journal. 1978 Nov 18:119(10):1195-7 [PubMed PMID: 570450]
Level 3 (low-level) evidenceMcIntyre L, Miller A, Kosatsky T. Changing Trends in Paralytic Shellfish Poisonings Reflect Increasing Sea Surface Temperatures and Practices of Indigenous and Recreational Harvesters in British Columbia, Canada. Marine drugs. 2021 Oct 14:19(10):. doi: 10.3390/md19100568. Epub 2021 Oct 14 [PubMed PMID: 34677468]
García C, del Carmen Bravo M, Lagos M, Lagos N. Paralytic shellfish poisoning: post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon : official journal of the International Society on Toxinology. 2004 Feb:43(2):149-58 [PubMed PMID: 15019474]
Level 3 (low-level) evidenceLagos N. Microalgal blooms: a global issue with negative impact in Chile. Biological research. 1998:31(4):375-86 [PubMed PMID: 10029901]
Level 3 (low-level) evidenceSinno-Tellier S, Abadie E, de Haro L, Paret N, Langrand J, Le Roux G, Labadie M, Boels D, French PCC Research Group, Bloch J, Delcourt N. Human poisonings by neurotoxic phycotoxins related to the consumption of shellfish: study of cases registered by the French Poison Control Centres from 2012 to 2019. Clinical toxicology (Philadelphia, Pa.). 2022 Jun:60(6):759-767. doi: 10.1080/15563650.2022.2034840. Epub 2022 Feb 8 [PubMed PMID: 35130811]
Level 3 (low-level) evidenceRodrigue DC, Etzel RA, Hall S, de Porras E, Velasquez OH, Tauxe RV, Kilbourne EM, Blake PA. Lethal paralytic shellfish poisoning in Guatemala. The American journal of tropical medicine and hygiene. 1990 Mar:42(3):267-71 [PubMed PMID: 2316796]
Level 3 (low-level) evidenceSuleiman M, Jelip J, Rundi C, Chua TH. Case Report: Paralytic Shellfish Poisoning in Sabah, Malaysia. The American journal of tropical medicine and hygiene. 2017 Dec:97(6):1731-1736. doi: 10.4269/ajtmh.17-0589. Epub 2017 Oct 5 [PubMed PMID: 29016314]
Level 3 (low-level) evidenceCallejas L, Darce AC, Amador JJ, Conklin L, Gaffga N, Schurz Rogers H, DeGrasse S, Hall S, Earley M, Mei J, Rubin C, Aldighieri S, Backer LC, Azziz-Baumgartner E. Paralytic shellfish poisonings resulting from an algal bloom in Nicaragua. BMC research notes. 2015 Mar 10:8():74. doi: 10.1186/s13104-015-1012-4. Epub 2015 Mar 10 [PubMed PMID: 25890043]
Hartigan-Go K, Bateman DN. Redtide in the Philippines. Human & experimental toxicology. 1994 Dec:13(12):824-30 [PubMed PMID: 7718301]
Level 3 (low-level) evidenceChing PK, Ramos RA, de los Reyes VC, Sucaldito MN, Tayag E. Lethal paralytic shellfish poisoning from consumption of green mussel broth, Western Samar, Philippines, August 2013. Western Pacific surveillance and response journal : WPSAR. 2015 Apr-Jun:6(2):22-6. doi: 10.5365/WPSAR.2015.6.1.004. Epub 2015 May 8 [PubMed PMID: 26306212]
de Carvalho M, Jacinto J, Ramos N, de Oliveira V, Pinho e Melo T, de Sá J. Paralytic shellfish poisoning: clinical and electrophysiological observations. Journal of neurology. 1998 Aug:245(8):551-4 [PubMed PMID: 9747920]
Level 3 (low-level) evidenceAnderson DM, Sullivan JJ, Reguera B. Paralytic shellfish poisoning in northwest Spain: the toxicity of the dinoflagellate Gymnodinium catenatum. Toxicon : official journal of the International Society on Toxinology. 1989:27(6):665-74 [PubMed PMID: 2749763]
Level 3 (low-level) evidenceTurnbull A, Harrison R, McKeown S. Paralytic shellfish poisoning in south eastern Tasmania. Communicable diseases intelligence quarterly report. 2013 Mar 31:37(1):E52-4 [PubMed PMID: 23692159]
Level 3 (low-level) evidenceIngham HR, Mason J, Wood PC. Distribution of toxin in molluscan shellfish following the occurrence of mussel toxicity in North-East England. Nature. 1968 Oct 5:220(5162):25-7 [PubMed PMID: 5677440]
Level 3 (low-level) evidence. The red tide--a public-health emergency. The New England journal of medicine. 1973 May 24:288(21):1126-7 [PubMed PMID: 4697944]
Level 3 (low-level) evidenceGessner BD, Middaugh JP. Paralytic shellfish poisoning in Alaska: a 20-year retrospective analysis. American journal of epidemiology. 1995 Apr 15:141(8):766-70 [PubMed PMID: 7709919]
Level 3 (low-level) evidenceHurley W, Wolterstorff C, MacDonald R, Schultz D. Paralytic shellfish poisoning: a case series. The western journal of emergency medicine. 2014 Jul:15(4):378-81. doi: 10.5811/westjem.2014.4.16279. Epub [PubMed PMID: 25035737]
Level 3 (low-level) evidenceJames KJ, Carey B, O'Halloran J, van Pelt FN, Skrabáková Z. Shellfish toxicity: human health implications of marine algal toxins. Epidemiology and infection. 2010 Jul:138(7):927-40. doi: 10.1017/S0950268810000853. Epub 2010 Apr 23 [PubMed PMID: 20412612]
Level 3 (low-level) evidenceAbraham A, Flewelling LJ, El Said KR, Odom W, Geiger SP, Granholm AA, Jackson JT, Bodager D. An occurrence of neurotoxic shellfish poisoning by consumption of gastropods contaminated with brevetoxins. Toxicon : official journal of the International Society on Toxinology. 2021 Feb:191():9-17. doi: 10.1016/j.toxicon.2020.12.010. Epub 2020 Dec 16 [PubMed PMID: 33338449]
Morris PD, Campbell DS, Taylor TJ, Freeman JI. Clinical and epidemiological features of neurotoxic shellfish poisoning in North Carolina. American journal of public health. 1991 Apr:81(4):471-4 [PubMed PMID: 2003627]
Level 3 (low-level) evidencePoli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Neurotoxic shellfish poisoning and brevetoxin metabolites: a case study from Florida. Toxicon : official journal of the International Society on Toxinology. 2000 Jul:38(7):981-93 [PubMed PMID: 10728835]
Level 3 (low-level) evidenceLee RU, Woessner KM, Mathison DA. Surfer's asthma. Allergy and asthma proceedings. 2009 Mar-Apr:30(2):202-5. doi: 10.2500/aap.2009.30.3211. Epub [PubMed PMID: 19463209]
Level 3 (low-level) evidenceFleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Reich A, Zaias J, Cheng YS, Pierce R, Naar J, Abraham WM, Baden DG. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest. 2007 Jan:131(1):187-94 [PubMed PMID: 17218574]
Level 3 (low-level) evidenceMilian A, Nierenberg K, Fleming LE, Bean JA, Wanner A, Reich A, Backer LC, Jayroe D, Kirkpatrick B. Reported respiratory symptom intensity in asthmatics during exposure to aerosolized Florida red tide toxins. The Journal of asthma : official journal of the Association for the Care of Asthma. 2007 Sep:44(7):583-7 [PubMed PMID: 17885863]
Level 3 (low-level) evidencePerl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. The New England journal of medicine. 1990 Jun 21:322(25):1775-80 [PubMed PMID: 1971709]
Level 3 (low-level) evidenceStuchal LD, Grattan LM, Portier KM, Kilmon KA, Manahan LM, Roberts SM, Morris JG Jr. Dose-response assessment for impaired memory from chronic exposure to domoic acid among native American consumers of razor clams. Regulatory toxicology and pharmacology : RTP. 2020 Nov:117():104759. doi: 10.1016/j.yrtph.2020.104759. Epub 2020 Aug 6 [PubMed PMID: 32768666]
Young N, Robin C, Kwiatkowska R, Beck C, Mellon D, Edwards P, Turner J, Nicholls P, Fearby G, Lewis D, Hallett D, Bishop T, Smith T, Hyndford R, Coates L, Turner A. Outbreak of diarrhetic shellfish poisoning associated with consumption of mussels, United Kingdom, May to June 2019. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2019 Aug:24(35):. doi: 10.2807/1560-7917.ES.2019.24.35.1900513. Epub [PubMed PMID: 31481146]
Lloyd JK, Duchin JS, Borchert J, Quintana HF, Robertson A. Diarrhetic shellfish poisoning, Washington, USA, 2011. Emerging infectious diseases. 2013 Aug:19(8):1314-6. doi: 10.3201/eid1908.121824. Epub [PubMed PMID: 23876232]
Level 3 (low-level) evidenceLlewellyn LE. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Natural product reports. 2006 Apr:23(2):200-22 [PubMed PMID: 16572228]
Level 3 (low-level) evidenceEtheridge SM. Paralytic shellfish poisoning: seafood safety and human health perspectives. Toxicon : official journal of the International Society on Toxinology. 2010 Aug 15:56(2):108-22. doi: 10.1016/j.toxicon.2009.12.013. Epub 2009 Dec 24 [PubMed PMID: 20035780]
Level 3 (low-level) evidenceDechraoui MY, Tiedeken JA, Persad R, Wang Z, Granade HR, Dickey RW, Ramsdell JS. Use of two detection methods to discriminate ciguatoxins from brevetoxins: application to great barracuda from Florida Keys. Toxicon : official journal of the International Society on Toxinology. 2005 Sep 1:46(3):261-70 [PubMed PMID: 15982699]
Level 3 (low-level) evidenceFu LL, Zhao XY, Ji LD, Xu J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon : official journal of the International Society on Toxinology. 2019 Mar 15:160():1-7. doi: 10.1016/j.toxicon.2018.12.007. Epub 2019 Jan 11 [PubMed PMID: 30639658]
Andrinolo D, Michea LF, Lagos N. Toxic effects, pharmacokinetics and clearance of saxitoxin, a component of paralytic shellfish poison (PSP), in cats. Toxicon : official journal of the International Society on Toxinology. 1999 Mar:37(3):447-64 [PubMed PMID: 10080350]
Level 3 (low-level) evidenceStafford RG, Hines HB. Urinary elimination of saxitoxin after intravenous injection. Toxicon : official journal of the International Society on Toxinology. 1995 Nov:33(11):1501-10 [PubMed PMID: 8744989]
Level 3 (low-level) evidenceCattet M, Geraci JR. Distribution and elimination of ingested brevetoxin (PbTx-3) in rats. Toxicon : official journal of the International Society on Toxinology. 1993 Nov:31(11):1483-6 [PubMed PMID: 8310449]
Level 3 (low-level) evidenceMatias WG, Traore A, Creppy EE. Variations in the distribution of okadaic acid in organs and biological fluids of mice related to diarrhoeic syndrome. Human & experimental toxicology. 1999 May:18(5):345-50 [PubMed PMID: 10372758]
Level 3 (low-level) evidenceMcFarren EF, Silva FJ, Tanabe H, Wilson WB, Campbell JE, Lewis KH. The occurrence of a ciguatera-like poison in oysters, clams, and Gymnodinium breve cultures. Toxicon : official journal of the International Society on Toxinology. 1965 Nov:3(2):111-23 [PubMed PMID: 5867066]
Level 3 (low-level) evidenceHughes JM, Merson MH. Current concepts fish and shellfish poisoning. The New England journal of medicine. 1976 Nov 11:295(20):1117-20 [PubMed PMID: 988478]
Level 3 (low-level) evidenceChyka PA, Seger D, Krenzelok EP, Vale JA, American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: Single-dose activated charcoal. Clinical toxicology (Philadelphia, Pa.). 2005:43(2):61-87 [PubMed PMID: 15822758]
Level 3 (low-level) evidence. Position paper: cathartics. Journal of toxicology. Clinical toxicology. 2004:42(3):243-53 [PubMed PMID: 15362590]
Level 3 (low-level) evidenceVale JA. Position statement: gastric lavage. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. Journal of toxicology. Clinical toxicology. 1997:35(7):711-9 [PubMed PMID: 9482426]
Level 3 (low-level) evidenceBenton BJ, Rivera VR, Hewetson JF, Chang FC. Reversal of saxitoxin-induced cardiorespiratory failure by a burro-raised alpha-STX antibody and oxygen therapy. Toxicology and applied pharmacology. 1994 Jan:124(1):39-51 [PubMed PMID: 8291060]
Level 3 (low-level) evidenceMullins ME, Hoffman RS. Is mannitol the treatment of choice for patients with ciguatera fish poisoning? Clinical toxicology (Philadelphia, Pa.). 2017 Nov:55(9):947-955. doi: 10.1080/15563650.2017.1327664. Epub 2017 May 23 [PubMed PMID: 28535116]
Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JL, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cellular and molecular neurobiology. 2004 Aug:24(4):553-63 [PubMed PMID: 15233378]
Level 3 (low-level) evidenceVelayudhan A, Nayak J, Murhekar MV, Dikid T, Sodha SV, Working Group*. Shellfish poisoning outbreaks in Cuddalore District, Tamil Nadu, India. Indian journal of public health. 2021 Jan:65(Supplement):S29-S33. doi: 10.4103/ijph.IJPH_1070_20. Epub [PubMed PMID: 33753589]