Introduction
Brain herniation is defined as the movement of brain tissue from one intracranial compartment to another. There are three key intracranial compartments created by two rigid in-folds of dura known as the falx cerebri and the tentorium. The falx cerebri separates each brain hemisphere. The tentorium separates the cranial vault into supratentorial and infratentorial compartments. The infratentorial compartment is also known as the posterior fossa. Within the posterior fossa are the cerebellum and brainstem. There are two openings in the infratentorial compartment: the foramen magnum and the tentorial notch. The tentorial notch allows communication between the infratentorial and supratentorial compartments. The foramen magnum allows passage out of the posterior fossa and into the spinal canal.[1]
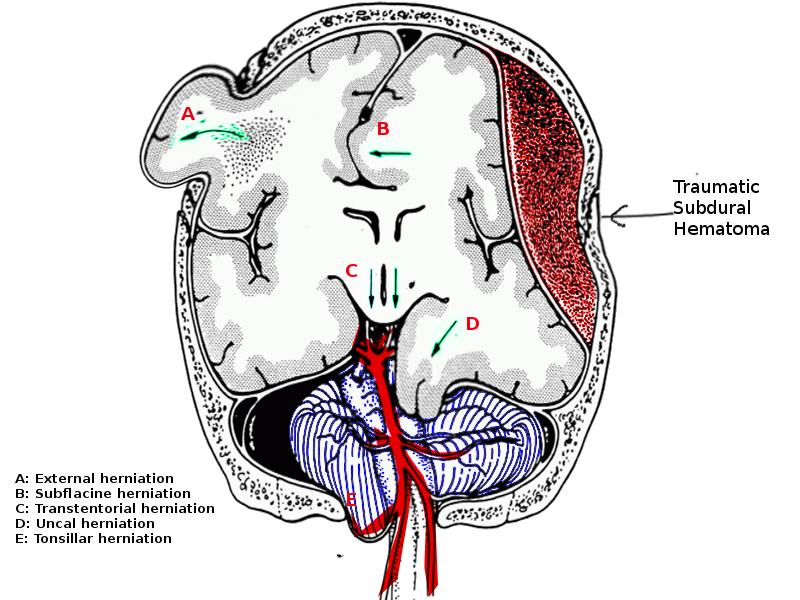
A tonsillar herniation is characterized by the descent of the cerebellar tonsils through the foramen magnum, which compresses the medulla against the clivus/odontoid process. It is described as "coning" as the brain tissue is squeezed down through the foramen like being squeezed into a cone. (See media image 1) Clinically, progressive compression of the medulla causes Cushing's reflex, eventually resulting in death.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Any intracranial pathology which causes an increase in intracranial pressure (ICP) can cause tonsillar herniation, particularly posterior fossa pathologies. These include:[3][4]
- Hematoma:
- Intracerebral (particularly cerebellar)
- Subdural
- Extradural
- Intraventricular
- Subarachnoid hemorrhage
- Posterior fossa space-occupying lesions:
- Tumor
- Abscess
- Hydrocephalus
- Diffuse brain swelling:
- Diffuse axonal injury
- Malignant middle cerebral artery stroke syndrome
- Cerebrospinal fluid (CSF) over drainage by lumbar puncture (LP)
Historically, LP was a more common cause of tonsillar herniation. If a patient has raised ICP, then lowering the pressure within the spinal canal can increase the pressure gradient between the posterior fossa and the spinal canal, resulting in tonsillar herniation through the foramen magnum. Before the advent of computed tomographic (CT) scan in the 1970s, patients with signs of raised intracranial pressure had "therapeutic lumbar taps," where a large volume of CSF was removed. In modern practice, if a patient is symptomatic with signs of raised ICP (headache, nausea, reduced consciousness, or focal neurological deficit), then intracranial imaging with CT scan or magnetic resonance imaging (MRI) is indicated before LP. This allows an assessment as to whether it is safe or not to proceed with an LP.[5][6]
Epidemiology
The incidence of tonsillar herniation is not documented, as it is a physiological response to a variety of underlying pathological processes. The most common cause is traumatic brain injury (TBI), but other causes include intracerebral hemorrhage and subarachnoid hemorrhage. The incidence of TBI worldwide is estimated to be 69 million individuals each year.[7]
Pathophysiology
The Monroe-Kellie doctrine states that the contents of the cranial cavity have fixed volumes. There is approximately 1400 mL of brain tissue, 150 mL of CSF, and 150 mL of blood within the cerebral vasculature.[8] The skull is a fixed structure once childhood sutures have fused at around 18 months of age. The only remaining outlet from the cranial vault is through the foramen magnum at the base of the posterior fossa.[3] As per the Monroe-Kellie doctrine, if there is an increase in one of the components, the others must compensate. For example, if the brain parenchyma swells following an ischemic stroke, the total brain tissue volume increases. To compensate, the CSF and blood volumes decrease. Typically the volume of CSF is reduced first by being pushed out through the foramen magnum into the spinal canal. This is followed by venous and capillary blood, which is emptied predominantly via the jugular veins. This ability for the cranial cavity to compensate for increased volumes is known as intracranial compliance. Once this mechanism has been exhausted, brain parenchyma will herniate from one compartment to another, or posterior fossa herniation occurs through the foramen magnum. When this occurs, there is a loss of intracranial compliance.[3]
A particularly important example for tonsillar herniation is a cerebellar hemorrhage, which can cause rapid herniation of the cerebellar tonsils down through the foramen magnum where it compresses the medulla. In 1901, Cushing published his paper "Concerning a definite regulatory mechanism of the vasomotor center, which controls blood pressure during cerebral compression", describing the physiological changes that occur during compression of the medulla. This later became known as Cushing's reflex, which gives rise to Cushing's triad of elevated blood pressure, bradycardia, and irregular breathing.[2] Cushing demonstrated that increased intracranial pressure increases blood pressure to a level slightly above the pressure that is being exerted upon the medulla, and postulated that the increase in blood pressure was a reflex response to brainstem ischemia.
Cerebral perfusion pressure (CPP) is equal to the mean arterial pressure (MAP) of the carotid arteries minus the ICP [CPP = MAP - ICP].[9] As the ICP increases, the CPP drops. CPP is the force that pushes blood through the cerebral vasculature. Intracranial pathology that increases the ICP reduces blood flow to the brain tissue, and thus, a sympathetically driven compensatory rise in MAP is initiated to increase the CPP.[10][11] There are two proposed theories to explain the subsequent bradycardia. First, the increased blood pressure activates baroreceptors in the aortic arch, which triggers parasympathetic activation. Second, intracranial compression of the vagus nerve causes bradycardia.[12][13][14]
Regardless of which mechanism explains the bradycardia, this is considered a terminal stage as blood pressure continues to rise to perfuse the brainstem as it is compressed against the clivus/odontoid process by the cerebellar tonsils. As further damage occurs to the brainstem, the heart rate may become tachycardic, and breathing slows with apneic episodes.[12] Eventually, the breathing may become agonal before the patient enters respiratory and cardiac arrest.[15]
History and Physical
Generally, with raised intracranial pressure and brainstem compression, the patient is in a coma. On examination, the patient may have abnormal posturing, such as decortication or decerebration. However, with late tonsillar herniation, the patient will have flaccid paralysis. Pupils are likely to be abnormal with varying patterns depending on the underlying pathology. Midbrain compression tends to give midsized and unreactive pupils, while a pontine hemorrhage gives pinpoint and unreactive pupils. If a supratentorial lesion with tentorial herniation occurred first, the patient might have unequal pupils due to oculomotor compression.[3]
Cushing described four stages of intracranial hypertension:[2]
- Compensation - intracranial pressure rises without physiological changes
- Early manifestations - headache and irritability develop
- Maximum manifestation - severe symptoms with raised blood pressure, bradycardia and slowed breathing
- Paralysis - deep coma with low blood pressure and tachycardia before apnea and death
Evaluation
Neurological assessment in patients with TBI is based on the Glasgow coma scale, which is accepted worldwide. However, it does have some criticisms; in particular, the scale is not ordinal or linear. For instance, losing two points in speech may represent a focal neurological deficit with injury to a brain region responsible for speech as opposed to representing a disorder of consciousness.[16] A head computed tomography (CT) scan is mandatory in patients presenting with coma unless a known and reversible medical or metabolic cause is found. Patients with reduced consciousness and unequal pupils are very likely to have a so-called "surgical lesion." Therefore, urgent imaging is required to diagnose intracranial pathology that may be amenable to surgical intervention.[17]
Signs of mass effect on the head CT scan include midline shift, obliteration of the basal cisterns, effacement of the ventricles, obstructive hydrocephalus, and sulcal effacement. The mass effect should prompt the clinician to look for possible herniation. Tonsillar herniation is visualized on coronal imaging by crowding of the foramen magnum, effacement of the CSF cisterns surrounding the brainstem and, on sagittal views, the descent of the cerebellar tonsils below the level of the foramen magnum.[3][17] There may also be signs of obstructive hydrocephalus due to compression of the fourth ventricle.
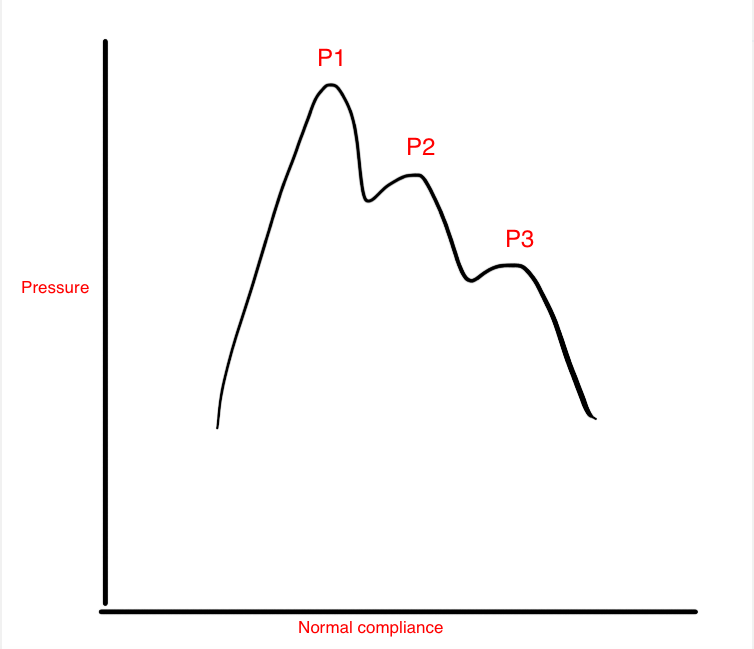
If the patient needs acute management, the placement of an ICP monitoring system may be indicated. This is usually in the form of an ICP bolt or intraventricular catheter. With herniation syndromes, ICP is typically high, at least over 20 mm Hg. The waveform will likely indicate a loss in intracranial compliance. On the ICP waveform, there are three waves; the first (P1) represents systole and is known as the "percussion wave" or "arterial kick." The second (P2) represents the "tidal wave" of brain compliance, and the third (P3) is the venous wave or "dicrotic notch." In normal physiology, P1 should be higher than P2, which is higher than the P3. (see media image 2) When P2 is the highest wave, compliance is lost. This indicates an intracranial compartment that is no longer able to compensate for increased volumes, and so, the pressure rises.[3] An additional benefit to placing an intraventricular catheter to monitor the ICP is that CSF can be therapeutically drained, as required, to lower ICP.
Treatment / Management
A tonsillar herniation is indicative of an underlying pathology that may include trauma, hemorrhage, tumor, or hydrocephalus. Therefore, treatment is directed at the underlying pathology. Firstly, as with all comatose patients, supportive measures are initiated. These include:
- Airway protection if Glasgow coma scale (GCS) of 8 or less.[18]
- Oxygenation and avoidance of hypoxia (O2 saturations target >90%).[19]
- Adequate ventilation for a target paCO2 of 35-40 mmHg. A short period of hyperventilation with a paCO2 of 30-35 mmHg is only used as a temporizing measure to lower paCO2, causing arterial vasoconstriction and reducing cerebral blood flow. This reduces ICP acutely as a life-saving method and may be especially useful for a patient who is acutely deteriorating with signs of herniation while arranging a CT scan or transfer to the operating room.[18][20][21][22][23] A paCO2 below 25 mmHg will cause cerebral ischemia.[23][24]
- Fluid balance with MAP targets of 60 to 70 mm Hg. Avoidance of hypotension (systolic blood pressure <90mm Hg) as it doubles mortality in TBI.[25]
- Osmotic therapy is another temporizing measure that can be initiated when a patient has acute signs of herniation. Again, this works by lowering the ICP and thus improving brain physiology and increasing the CPP. Typical agents used are mannitol (0.25 - 1 gm/ kg body weight) or hypertonic 3% saline (2-5 mL/kg over 10-20 minutes).[18][26]
- Normothermia, treatment of sepsis, and adequate nutrition are initiated.[27][28] (A1)
Surgical interventions are based on three broad principles:
- Removal of the lesion causing mass effect such as hematoma or tumor.[29]
- CSF diversion to reduce ICP, such as insertion of an external ventricular drain.
- Decompressive craniectomy to combat the Monroe-Kellie doctrine of a fixed space allows intracranial contents to swell out of the craniectomy site. There is level III evidence for the use of decompressive craniectomy for refractory raised ICP with evidence of herniation.[30][31][32][33][34] (A1)
Differential Diagnosis
Congenital abnormalities like Chiari malformation show tonsillar descent below the foramen magnum and may be found incidentally on imaging. Though Chiari can be symptomatic, it is not an acute phenomenon and does not cause acute brainstem compression.[35]
Autonomic dysreflexia can also cause high blood pressure with reflexive bradycardia; however, these patients are not comatose, and their breathing is unaffected.[36]
Acute spinal injury with neurogenic shock, if cervical, can cause bradycardia, but with associated hypotension.[37]
Prognosis
Tonsillar herniation is the final stage of an aggressive intracranial process. However, Cushing described four stages of tonsillar herniation, and some studies have suggested surgical intervention early in the process could prevent mortality. One case series looked at early recognition of tonsillar herniation in posterior fossa tumor cases and showed death could be avoided with early surgical decompression.[29]
Tonsillar herniation is typically considered a terminal event if not treated promptly. When it occurs, the chance of recovery is significantly reduced. When a herniation syndrome produces respiratory compromise, as found in Cushing's triad, there is no chance of a meaningful recovery.[3] Brain herniation following TBI has a poor prognosis, and herniation with signs of brainstem compression is "uncommonly reversible".[38] Patients in a coma after gunshot wounds with clinical evidence of brainstem impairment had a 100% mortality.[39]
Complications
Other than the underlying neurological deficit that may follow tonsillar herniation (permanent brain damage, brain death, respiratory arrest, coma, and death), other complications include the sequelae of critical illness. These include ventilator-associated pneumonia, deep vein thrombosis, pulmonary embolus, and critical illness myopathy. Approximately 5% of patients with TBI have an associated visual system injury.[40] TBI with skull base fractures can also present later with hypopituitarism.[41]
Consultations
- Neurosurgeon
- Neurologist
- Intensivist
- Respiratory therapy
- Physical medicine and rehabilitation
Deterrence and Patient Education
Measures to reduce road traffic collisions, workplace accidents, and traumatic brain injury are vital at a national public health level. Such measures include mandatory wearing of seatbelts and helmets on motorcycles and bicycles.[7][42]
Tonsillar herniation and all other cerebral herniation syndromes cause a significant burden to the patient and the relatives and produce a substantial economic cost to the health care system. A prolonged hospitalization and extensive rehabilitation to the patient are the principal factors involved.
Pearls and Other Issues
Tonsillar herniation is usually considered a terminal event if not treated promptly.
Prevention and adequate management of increased ICP is necessary to reduce the risk of tonsillar herniation.
Cerebral perfusion pressure is equal to mean arterial pressure minus the intracranial pressure. [CPP = MAP - ICP]
Cushing’s triad includes:
- Increased blood pressure with a widened pulse pressure
- Bradycardia
- Irregular breathing pattern
Enhancing Healthcare Team Outcomes
Tonsillar herniation is a medical emergency and requires urgent assessment. Intervention may require a wide variety of interprofessional team members, including emergency medicine clinicians, anesthesiologists, intensivists, traumatologists, and neurosurgeons. Patients with intracranial pathologies require close nursing observations and monitoring. Nurses will identify early signs of herniation and will consult with the intensivist and neurosurgery for adequate management. If the patient survives the acute episode, rehabilitation will likely be required. This includes a variety of healthcare professionals, including physiotherapists, occupational therapists, speech therapists, dieticians, and psychologists. If timely treated, a functional recovery is indicated by a return to activities of daily living.
Collaboration between the healthcare team members and communication are crucial elements for good results. The interprofessional care provided to the patient must follow an evidence-based approach to improve outcomes.
Media
(Click Image to Enlarge)
References
Adler DE, Milhorat TH. The tentorial notch: anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. Journal of neurosurgery. 2002 Jun:96(6):1103-12 [PubMed PMID: 12066913]
Level 3 (low-level) evidenceMeyer GA, Winter DL. Spinal cord participation in the Cushing reflex in the dog. Journal of neurosurgery. 1970 Dec:33(6):662-75 [PubMed PMID: 5482797]
Level 3 (low-level) evidencePlum F, Posner JB. The diagnosis of stupor and coma. Contemporary neurology series. 1972:10():1-286 [PubMed PMID: 4664014]
Stevens RD, Shoykhet M, Cadena R. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocritical care. 2015 Dec:23 Suppl 2(Suppl 2):S76-82. doi: 10.1007/s12028-015-0168-z. Epub [PubMed PMID: 26438459]
Duffy GP. Lumbar puncture in the presence of raised intracranial pressure. British medical journal. 1969 Feb 15:1(5641):407-9 [PubMed PMID: 5763958]
KOREIN J, CRAVIOTO H, LEICACH M. Reevaluation of lumbar puncture; a study of 129 patients with papilledema or intracranial hypertension. Neurology. 1959 Apr:9(4):290-7 [PubMed PMID: 13644562]
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB. Estimating the global incidence of traumatic brain injury. Journal of neurosurgery. 2018 Apr 27:130(4):1080-1097. doi: 10.3171/2017.10.JNS17352. Epub 2018 Apr 27 [PubMed PMID: 29701556]
Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001 Jun 26:56(12):1746-8 [PubMed PMID: 11425944]
Smith M. Cerebral perfusion pressure. British journal of anaesthesia. 2015 Oct:115(4):488-90. doi: 10.1093/bja/aev230. Epub 2015 Jul 18 [PubMed PMID: 26188341]
Wan WH, Ang BT, Wang E. The Cushing Response: a case for a review of its role as a physiological reflex. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2008 Mar:15(3):223-8. doi: 10.1016/j.jocn.2007.05.025. Epub 2008 Jan 7 [PubMed PMID: 18182296]
Level 3 (low-level) evidenceGrady PA, Blaumanis OR. Physiologic parameters of the Cushing reflex. Surgical neurology. 1988 Jun:29(6):454-61 [PubMed PMID: 3375974]
Level 3 (low-level) evidenceTsai YH, Lin JY, Huang YY, Wong JM. Cushing response-based warning system for intensive care of brain-injured patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2018 Dec:129(12):2602-2612. doi: 10.1016/j.clinph.2018.09.010. Epub 2018 Sep 21 [PubMed PMID: 30453271]
Pirahanchi Y, Bordoni B. Anatomy, Head and Neck: Carotid Baroreceptors. StatPearls. 2023 Jan:(): [PubMed PMID: 30725908]
Lau EO, Lo CY, Yao Y, Mak AF, Jiang L, Huang Y, Yao X. Aortic Baroreceptors Display Higher Mechanosensitivity than Carotid Baroreceptors. Frontiers in physiology. 2016:7():384. doi: 10.3389/fphys.2016.00384. Epub 2016 Aug 31 [PubMed PMID: 27630578]
Schmidt EA, Despas F, Pavy-Le Traon A, Czosnyka Z, Pickard JD, Rahmouni K, Pathak A, Senard JM. Intracranial Pressure Is a Determinant of Sympathetic Activity. Frontiers in physiology. 2018:9():11. doi: 10.3389/fphys.2018.00011. Epub 2018 Feb 8 [PubMed PMID: 29472865]
Price DJ. Is diagnostic severity grading for head injuries possible? Acta neurochirurgica. Supplementum. 1986:36():67-9 [PubMed PMID: 3467566]
Laine FJ, Shedden AI, Dunn MM, Ghatak NR. Acquired intracranial herniations: MR imaging findings. AJR. American journal of roentgenology. 1995 Oct:165(4):967-73 [PubMed PMID: 7677003]
. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. Journal of neurotrauma. 2000 Jun-Jul:17(6-7):463-9 [PubMed PMID: 10937888]
Level 1 (high-level) evidenceChesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. The Journal of trauma. 1993 Feb:34(2):216-22 [PubMed PMID: 8459458]
Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner MJ, Wilberger JW. Guidelines for the management of severe head injury. Brain Trauma Foundation. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 1996 Jun:3(2):109-27 [PubMed PMID: 9028756]
Level 1 (high-level) evidence. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Hyperventilation. Journal of neurotrauma. 2000 Jun-Jul:17(6-7):513-20 [PubMed PMID: 10937894]
Level 1 (high-level) evidenceCarney N,Totten AM,O'Reilly C,Ullman JS,Hawryluk GW,Bell MJ,Bratton SL,Chesnut R,Harris OA,Kissoon N,Rubiano AM,Shutter L,Tasker RC,Vavilala MS,Wilberger J,Wright DW,Ghajar J, Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1 [PubMed PMID: 27654000]
Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation Therapy for Control of Posttraumatic Intracranial Hypertension. Frontiers in neurology. 2017:8():250. doi: 10.3389/fneur.2017.00250. Epub 2017 Jul 17 [PubMed PMID: 28769857]
Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients: from physiology to outcome evidence. Current opinion in anaesthesiology. 2019 Oct:32(5):568-573. doi: 10.1097/ACO.0000000000000764. Epub [PubMed PMID: 31211719]
Level 3 (low-level) evidence. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Resuscitation of blood pressure and oxygenation. Journal of neurotrauma. 2000 Jun-Jul:17(6-7):471-8 [PubMed PMID: 10937889]
Level 1 (high-level) evidenceGu J, Huang H, Huang Y, Sun H, Xu H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: a meta-analysis of randomized controlled trials. Neurosurgical review. 2019 Jun:42(2):499-509. doi: 10.1007/s10143-018-0991-8. Epub 2018 Jun 15 [PubMed PMID: 29905883]
Level 1 (high-level) evidenceMiller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. Journal of neurosurgery. 1977 Oct:47(4):503-16 [PubMed PMID: 903804]
Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, Harbison JW, Lutz HA, Young HF, Becker DP. Further experience in the management of severe head injury. Journal of neurosurgery. 1981 Mar:54(3):289-99 [PubMed PMID: 7463128]
Weinberg JS, Rhines LD, Cohen ZR, Langford L, Levin VA. Posterior fossa decompression for life-threatening tonsillar herniation in patients with gliomatosis cerebri: report of three cases. Neurosurgery. 2003 Jan:52(1):216-23; discussion 223 [PubMed PMID: 12493121]
Level 3 (low-level) evidenceBullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006 Mar:58(3 Suppl):S25-46; discussion Si-iv [PubMed PMID: 16540746]
Cadena R, Shoykhet M, Ratcliff JJ. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocritical care. 2017 Sep:27(Suppl 1):82-88. doi: 10.1007/s12028-017-0454-z. Epub [PubMed PMID: 28913634]
. Trial of decompressive craniectomy for traumatic intracranial hypertension. Journal of the Intensive Care Society. 2017 Aug:18(3):236-238. doi: 10.1177/1751143716685246. Epub 2017 Jun 29 [PubMed PMID: 29118837]
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R, DECRA Trial Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. The New England journal of medicine. 2011 Apr 21:364(16):1493-502. doi: 10.1056/NEJMoa1102077. Epub 2011 Mar 25 [PubMed PMID: 21434843]
Level 1 (high-level) evidenceHutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England journal of medicine. 2016 Sep 22:375(12):1119-30. doi: 10.1056/NEJMoa1605215. Epub 2016 Sep 7 [PubMed PMID: 27602507]
Kular S, Cascella M. Chiari I Malformation. StatPearls. 2023 Jan:(): [PubMed PMID: 32119496]
Allen KJ, Leslie SW. Autonomic Dysreflexia. StatPearls. 2024 Jan:(): [PubMed PMID: 29494041]
Kowalski A, Brandis D. Shock Resuscitation. StatPearls. 2023 Jan:(): [PubMed PMID: 30521251]
Gruen P. Surgical management of head trauma. Neuroimaging clinics of North America. 2002 May:12(2):339-43 [PubMed PMID: 12391640]
Shoung HM, Sichez JP, Pertuiset B. The early prognosis of craniocerebral gunshot wounds in civilian practice as an aid to the choice of treatment. A series of 56 cases studied by the computerized tomography. Acta neurochirurgica. 1985:74(1-2):27-30 [PubMed PMID: 3976441]
Level 3 (low-level) evidenceKline LB, Morawetz RB, Swaid SN. Indirect injury of the optic nerve. Neurosurgery. 1984 Jun:14(6):756-64 [PubMed PMID: 6462414]
Vance ML. Hypopituitarism. The New England journal of medicine. 1994 Jun 9:330(23):1651-62 [PubMed PMID: 8043090]
Robinson DL. Head injuries and bicycle helmet laws. Accident; analysis and prevention. 1996 Jul:28(4):463-75 [PubMed PMID: 8870773]