Introduction
Thoracentesis is a commonly performed diagnostic and therapeutic procedure for removing fluid from the pleural space, aiding in evaluating and managing pleural effusions. By removing fluid from the pleural space, thoracentesis helps alleviate symptoms like dyspnea and provides crucial diagnostic information regarding the underlying cause of pleural fluid accumulation. There are various indications for thoracentesis, including malignant pleural effusions, infections such as pneumonia and tuberculosis
, heart failure, and other systemic diseases. When performed with appropriate technique and precautions, this minimally invasive procedure carries a low risk of complications and is vital in guiding treatment decisions for various conditions. A thorough understanding of thoracentesis, including its indications, contraindications, technique, and potential complications, is essential for clinicians managing pleural effusion cases.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Thoracentesis involves the pleural cavity, a potential space between the visceral and parietal pleurae surrounding the lungs. The pleura is a serous membrane folded onto itself, forming 2 layers: the visceral pleura, which adheres to the lungs, and the parietal pleura, attached to the chest wall. This cavity typically contains a few milliliters of pleural fluid, facilitating smooth lung movement during respiration. The pleural cavity plays a vital role in lung function, allowing chest wall movements to transmit to the lungs, which is essential during deep breathing. The pleural cavities on both sides of the chest are separate and supplied by different blood vessels—the visceral pleural blood supply comes from the bronchial circulation, and the intercostal arteries traveling along the edge of each rib supply the parietal pleura.[1]
Thoracentesis is performed when excess fluid, often due to a pathological process, accumulates in this space, causing pleural effusion. The procedure, typically performed between the sixth and eighth intercostal spaces in the midaxillary line if the patient is supine or posterior midscapular line if the patient is seated, involves removing this fluid to relieve symptoms like dyspnea and aid in diagnostic analysis. The fluid's volume, accumulation rate, cellular content, and chemical composition guide the diagnosis of underlying conditions such as heart failure or infection. Proper patient positioning, either supine or sitting, is crucial for optimal access and patient comfort during thoracentesis.[2]
Indications
The indications for thoracentesis are relatively broad and include diagnostic and therapeutic clinical management.[3] The procedural aim is to evacuate persistent small-to-moderate pleural effusions to prevent superinfection or drain symptomatic moderate-to-large effusions. The procedure is typically performed when pleural fluid is detectable on imaging, with subsequent analysis of the fluid's chemical, microbiological, and cytological properties to guide further clinical management.[4]
Diagnostic Indications for Thoracentesis
When thoracentesis is indicated for diagnosis, the Light criteria are instrumental in distinguishing between exudates and transudates. The analysis of pleural aspirate should routinely include a Gram stain, differential cell count, culture, cytology, protein levels, l-lactate dehydrogenase, and pH. Additional tests, such as tuberculosis (TB) screening, should be considered based on individual patient risk factors and regional prevalence. Typically, diagnostic thoracentesis extracts a small volume (20-30 mL) of accumulated fluid. Diagnostic indications for thoracentesis include:
- Unexplained pleural effusion: Thoracentesis is used to determine the underlying cause of pleural fluid accumulation. Fluid analysis helps differentiate between transudative and exudative effusions.
- Suspected infection: In pleural infections, such as empyema or TB, thoracentesis aids in identifying pathogens through culture and Gram staining of pleural fluid. A fluid collection believed to be infected should be drained to eliminate the source of infection and reservoirs of the disease.
- Suspected malignancy: If a malignant pleural effusion is suspected, thoracentesis facilitates cytological analysis, aiding in diagnosing metastatic or primary pleural malignancies.
- Differentiation between cardiac and noncardiac causes of pleural fluid accumulation: Thoracentesis helps distinguish effusions caused by heart failure from those due to other conditions, such as pulmonary embolism or cirrhosis.
Therapeutic Indications for Thoracentesis
A therapeutic thoracentesis is performed when the fluid volume is causing significant clinical symptoms. Typically, therapeutic thoracentesis removes a large volume (liters) of accumulated fluid. A small sample of a large-volume thoracentesis should be sent for analysis when the etiology effusion is unknown or may have changed. Therapeutic indications for thoracentesis include:
- Symptomatic relief of dyspnea: Thoracentesis is performed to relieve respiratory distress via improved lung expansion and gas exchange in patients with large pleural effusions causing lung compression.
- Pleurodesis preparation: In cases of recurrent pleural effusion, such as malignant effusions, thoracentesis may be performed before pleurodesis to drain fluid before introducing agents that prevent fluid reaccumulation.
- Drainage of empyema or hemothorax: Thoracentesis may be indicated to drain infected pleural fluid (empyema) or blood (hemothorax) to prevent infection, improve symptoms, and facilitate recovery.
- Drain placement: If fluid is anticipated to reaccumulate quickly, a drain is often left in place to facilitate fluid removal and collection. Drain placement is most commonly performed in patients with traumatic hemothorax, malignant effusion, or end-stage metabolic conditions with a systemic excessive colloid leak, such as cirrhosis or malabsorption syndromes. Drain placement may also be indicated to facilitate healing following cardiothoracic procedures or in certain inflammatory conditions.
Conditions in Which Thoracentesis May Be Diagnostic and Therapeutic
Pneumonia and parapneumonic effusion
Parapneumonic effusion, an accumulation of exudative fluid in the pleural space during acute pulmonary infection, is observed in approximately 40% of patients hospitalized with bacterial pneumonia and 20% with viral or Mycoplasma pneumonia. The presence of a parapneumonic effusion increases both morbidity and mortality risks, necessitating appropriate management to improve patient outcomes.[5]
The initial stage of parapneumonic effusion, often called the exudative stage, is characterized by the simple accumulation of fluid in the pleural cavity. During this phase, imaging typically reveals a free-flowing effusion. Therapeutic thoracentesis is recommended when the effusion exceeds minimal size, defined as a thickness more significant than 10 mm but less than half of the hemithorax.[6]
If left untreated or if the infection overwhelms the immune response, the parapneumonic effusion may progress to the fibropurulent stage. At this stage, the pleural fluid generally exhibits a low pH (<7.20), low glucose levels (<60 mg/dL), elevated lactate dehydrogenase, and may become loculated. This stage often signifies microbial invasion, although cultures may be negative in up to 40% of cases.[7] Parapneumonic effusion in the fibropurulent stage is considered 'complicated,' as antibiotic therapy alone is insufficient. Drainage through a chest tube or surgical intervention is necessary for resolution, regardless of the effusion's size. Empyema, a type of complicated pleural effusion, is identified by the presence of pus in the pleural space or positive Gram stain or culture results from the fluid. Treatment of empyema is similar to that of other complicated effusions.
In cases of community-acquired pneumonia requiring hospitalization, 5.5% to 7.2% of patients will develop a complicated pleural effusion or empyema.[8] Patients with a complicated pleural effusion or empyema have a higher mortality rate, and significantly so if drainage is delayed.[9] Chest tube placement is advised for effusions occupying more than 50% of the hemithorax. If these effusions are not adequately drained, they may progress to an organized or cortical stage, where fibroblasts infiltrate the pleural fluid, forming a thick visceral pleural peel and preventing lung expansion.[10] In such cases, video-assisted thoracoscopic surgery or a full thoracotomy with decortication may be required to allow lung reexpansion.[11]
Thoracentesis can diagnose and manage other conditions associated with pleural effusions. These conditions include, but are not limited to:
- Heart failure: Heart failure is the most common cause of transudative pleural effusions, often due to increased pressure in the pulmonary circulation.
- Malignancy: Thoracentesis can diagnose malignant pleural effusions due to primary pleural neoplasms such as mesothelioma or metastatic disease.
- Pulmonary embolism: Pulmonary emboli can lead to pleural effusions due to inflammation or infarction.
- Liver cirrhosis: Thoracentesis of hepatic hydrothorax helps manage the transudative pleural effusion accumulation secondary to portal hypertension.
- Nephrotic syndrome: Hypoproteinemia secondary to nephrotic syndrome may result in pleural effusion.
- Tuberculosis: TB pleuritis can lead to exudative effusions; extracted fluid can be sent for acid-fast staining and culture.
- Autoimmune diseases: Rheumatoid arthritis and systemic lupus erythematosus are examples of autoimmune diseases that can cause exudative pleural effusions.
- Traumatic hemothorax
- Postsurgical complications
- Chylothorax: Lymphatic fluid (chyle) may leak into the pleural space following trauma or secondary to malignancy.
Contraindications
The few contraindications to thoracentesis are important to recognize. Absolute contraindications include uncorrected bleeding disorders such as significant coagulopathy or thrombocytopenia. Active skin infection or cellulitis over the puncture site is also a procedural contraindication due to the risk of introducing infection into the pleural space. Patients who are mechanically ventilated carry a relative contraindication to thoracentesis because of the risk of pneumothorax and barotrauma. Caution should also be exercised in cases of severe hemodynamic instability, as thoracentesis may further destabilize the patient. Additionally, pleural adhesions from previous infections or surgeries can limit the success of fluid removal. While most of these contraindications can be managed with careful planning, they require thorough evaluation before proceeding with the procedure.
Equipment
Many prepackaged kits are available commercially, and while convenient, they are not essential for performing a thoracentesis safely. The equipment required for a thoracentesis typically includes:
- Sterile gloves and gown
- Antiseptic solution, such as chlorhexidine or povidone-iodine
- Sterile drapes
- Local anesthetic
- Syringes and needles for anesthetic administration and fluid withdrawal
- Thoracentesis needle or catheter; typically a 20- or 22-gauge needle or catheter with a safety valve
- Three-way stopcock to control fluid flow
- Collection bottles or vacuum-sealed containers for fluid drainage
- Sterile gauze and bandages
- Adhesive tape
- Ultrasound machine (optional)
- Chest tube set if larger volumes of fluid or chest drainage are needed
Preparation
Before initiating a thoracentesis, obtaining a medical history, performing a physical examination, and interpreting imaging studies are essential to assess the presence and nature of intrathoracic fluid, such as pleural effusion or hemothorax. Historical clues may include shortness of breath, orthopnea, chest pressure, pleuritic pain, and fatigue, indicating decreased lung volume, irritation, or compromised gas exchange. Physical examination findings often include diminished or absent breath sounds, dullness to percussion, and egophony, especially if there is significant fluid accumulation. Large effusions may cause mediastinal shifts and tracheal deviations, which are evident during examination.
Imaging studies are critical when preparing for thoracentesis. Chest radiographs are commonly performed to assess fluid volume and location. Point-of-care ultrasonography has become an early diagnostic tool, aiding in detecting small effusions and complications like pleural infection or malignancy. Ultrasonography also offers precise guidance during thoracentesis to minimize procedural complications. The effectiveness of ultrasound is significantly impacted by the interaction of the ultrasound beam with the tissue/air interface, leading to various artifacts such as simple (A-line) reverberations, "comet tail," and "ring down" (B-line) artifacts.[12] The presence of B-lines is highly specific for interstitial and alveolar fluid. These findings can rapidly alter the differential and dictate initial management when evaluating a patient with dyspnea.[13] Computed tomography of the chest can help rule out other causes of dyspnea and may indicate a complicated parapneumonic or malignant effusion.[14]
Once the clinical decision to proceed with thoracentesis is made, informed consent is obtained, and the correct side and puncture site are marked according to institutional policies. All necessary equipment should be gathered before starting the procedure. The patient should be continuously monitored, including pulse oximetry, blood pressure, and heart rate, to ensure safety throughout the process.
Technique or Treatment
Thoracentesis is typically performed with the patient positioned in either the upright seated position or, if necessary, in the supine position. The procedure is conducted on the affected side, targeting the midaxillary line for patients who are supine or the posterior midscapular line for those seated. Bedside ultrasonography is essential for identifying the most appropriate location for puncture, especially in patients with lower fluid volumes, as it aids in pinpointing fluid pockets and ensures the procedure's safety. Ultrasound-guided thoracentesis enhances accuracy by helping visualize the pleural effusion as an anechoic area bordered by the diaphragm and atelectatic lung, forming a "V" shape where the apex is ideal for needle insertion.[15] Ultrasonography improves safety by allowing real-time imaging of key landmarks such as the diaphragm and intercostal space. A linear probe with a sterile sheath cover should be used if available.
Once the site is identified, the skin is cleansed with an antiseptic solution, and the patient is prepped and draped in a sterile fashion. Local anesthesia is administered with a 25-gauge needle to create a superficial wheal, followed by a 20- or 22-gauge needle to anesthetize deeper tissues around the rib, carefully marching the needle tip above the rib margin. A larger needle or catheter attached to a syringe is then inserted perpendicular to the skin, and negative pressure is applied to the syringe during insertion to detect fluid flow and avoid damage to other structures. A small nick in the skin may facilitate catheter insertion if a catheter kit is used. Once pleural fluid is detected, the catheter is advanced into the thoracic cavity, and the desired amount of fluid is drained.
Diagnostic thoracenteses typically collect small samples, while therapeutic thoracentesis may remove larger fluid volumes via slow gravity drainage or by hand via serial syringe draws with a collection bag and a 3-way stopcock. After the procedure, the catheter is removed, and pressure is applied to the insertion site to control bleeding (see Image. Thoracentesis). Therapeutic thoracentesis typically involves large-volume drainage, and a sample should be sent for analysis if the cause of the effusion is unknown or suspected to have changed. In cases where fluid reaccumulation is anticipated, such as in trauma, malignancy, or postoperative conditions, a drain may be left in place.
Complications
Complications of thoracentesis include bleeding, pain, and infection at the point of needle entry.[16] If the approach is too high in the intercostal space, damage to the costal vasculature and nerve injury is possible. If too much fluid is removed or if the fluid is removed too rapidly (eg, using negative pressure chambers), reexpansion or postexpansion pulmonary edema may occur. Removal of significant fluid volumes may also induce vasovagal physiology. If the procedural needle or catheter is passed through diseased tissue before entering the chest cavity, that process can be extended into the chest space. For example, passing the needle through a thoracic or pleural tumor can seed the thoracic cavity, or passing the needle through a chest wall abscess or otherwise infected tissue can result in empyema. If the insertion site is too low, splenic and hepatic puncture can occur.
Except for localized pain from the actual procedure, pneumothorax is the most common complication and has been reported in 12% to 30% of procedures. Pre- and postprocedure chest radiographs are appropriate routine practices. Rare instances of retained intrapleural or intrathoracic catheter fragments have been documented, usually occurring when the catheter-over-trocar technique is employed. In these cases, attempting to reposition the catheter by advancing it back over the trocar can lead to tearing and compromise the integrity of the catheter, potentially leaving fragments behind.
Documentation of the presence and location of lung sliding before the procedure is essential and is best examined with a greater than 5 MHz vascular probe. The disappearance of lung sliding or B-lines suggests the interval development of a pneumothorax. Indications of chest tube placement to manage the pneumothorax following thoracentesis are the development of a large, progressive, or symptomatic pneumothorax. The occurrence of pneumothorax in mechanically ventilated patients should be handled with chest tube placement.[17]
Clinical Significance
Thoracentesis is a vital clinical procedure with significant diagnostic and therapeutic implications. Clinically, it is a crucial tool for evaluating and managing pleural effusions resulting from underlying conditions such as heart failure, infections, malignancies, and inflammatory diseases. Thoracentesis alleviates symptoms such as dyspnea and chest pain and improves respiratory function and overall patient comfort by removing excess fluid from the pleural space.
From a diagnostic perspective, thoracentesis provides valuable insights into the nature of the pleural effusion. Analysis of the aspirated fluid—through chemical, microbiological, and cytological tests—helps distinguish between transudative and exudative effusions and can identify specific etiologies such as infections, malignancies, or autoimmune disorders. This diagnostic capability is crucial for tailoring appropriate treatment strategies and guiding further investigations.
Therapeutically, thoracentesis offers immediate relief of symptoms caused by large or symptomatic pleural effusions, reducing the risk of complications such as respiratory distress or infection. The procedure can also assist in managing and monitoring conditions like parapneumonic effusion or empyema by facilitating drainage and reducing the risk of progression to more severe stages. Thoracentesis is an essential procedure in acute and chronic care settings. This procedure helps to diagnose and treat a range of pleural and pulmonary conditions, thus playing a critical role in patient management and improving clinical outcomes.
Enhancing Healthcare Team Outcomes
Effective thoracentesis requires a coordinated effort among various healthcare professionals to ensure patient-centered care, good outcomes, and safety. Clinicians must possess precise technical skills to perform the procedure, including selecting the appropriate site, managing complications, and interpreting fluid analysis results. Their strategy involves thoroughly understanding indications, contraindications, and procedural techniques and making critical decisions based on real-time data.
Nurses are crucial in patient preparation, monitoring during and after the procedure, medication administration, and providing education on postprocedural care. They ensure the patient is comfortable, coordinate with the procedural team, and monitor for immediate complications. Pharmacists contribute by managing patients' medications, ensuring preprocedural antibiotics are correctly administered, and addressing potential drug interactions.
Effective interprofessional communication is essential, as it allows for the seamless exchange of information about the patient's condition and procedural plan. This collaboration ensures that all team members are informed, which enhances patient safety and optimizes outcomes. Additionally, care coordination involves timely scheduling, appropriate follow-up, and integration of care plans across different specialties, ultimately improving team performance and patient satisfaction.
Media
(Click Image to Enlarge)
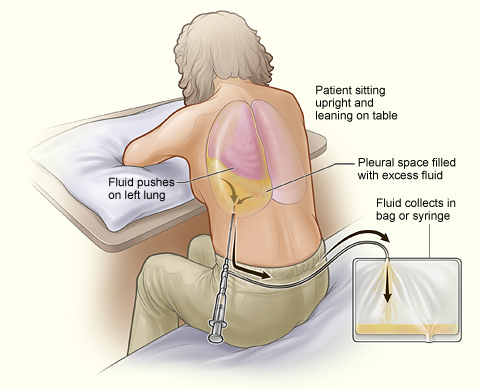
Thoracentesis. The illustration shows a person undergoing a thoracentesis, a procedure that drains excess fluid collected in the pleural space between the chest wall and lung. The person sits upright and leans on a table to allow excess fluid to drain into a bag.
National Heart, Lung, and Blood Institute (PD-US NIH)
References
Visouli AN, Zarogoulidis K, Kougioumtzi I, Huang H, Li Q, Dryllis G, Kioumis I, Pitsiou G, Machairiotis N, Katsikogiannis N, Papaiwannou A, Lampaki S, Zaric B, Branislav P, Porpodis K, Zarogoulidis P. Catamenial pneumothorax. Journal of thoracic disease. 2014 Oct:6(Suppl 4):S448-60. doi: 10.3978/j.issn.2072-1439.2014.08.49. Epub [PubMed PMID: 25337402]
Alzghoul B, Innabi A, Subramany S, Boye B, Chatterjee K, Koppurapu VS, Bartter T, Meena NK. Optimizing the Approach to Patients With Pleural Effusion and Radiologic Findings Suspect for Cancer. Journal of bronchology & interventional pulmonology. 2019 Apr:26(2):114-118. doi: 10.1097/LBR.0000000000000537. Epub [PubMed PMID: 30048417]
Terra RM, Dela Vega AJM. Treatment of malignant pleural effusion. Journal of visualized surgery. 2018:4():110. doi: 10.21037/jovs.2018.05.02. Epub 2018 May 22 [PubMed PMID: 29963399]
Sperandeo M, Quarato CMI, Squatrito R, Fuso P, Dimitri L, Simeone A, Notarangelo S, Lacedonia D. Effectiveness and Safety of Real-Time Transthoracic Ultrasound-Guided Thoracentesis. Diagnostics (Basel, Switzerland). 2022 Mar 16:12(3):. doi: 10.3390/diagnostics12030725. Epub 2022 Mar 16 [PubMed PMID: 35328278]
Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. The American journal of medicine. 1980 Oct:69(4):507-12 [PubMed PMID: 7424940]
Davies HE, Davies RJ, Davies CW, BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010 Aug:65 Suppl 2():ii41-53. doi: 10.1136/thx.2010.137000. Epub [PubMed PMID: 20696693]
Rosenstengel A. Pleural infection-current diagnosis and management. Journal of thoracic disease. 2012 Apr 1:4(2):186-93. doi: 10.3978/j.issn.2072-1439.2012.01.12. Epub [PubMed PMID: 22833824]
Falguera M, Carratalà J, Bielsa S, García-Vidal C, Ruiz-González A, Chica I, Gudiol F, Porcel JM. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. The European respiratory journal. 2011 Nov:38(5):1173-9. doi: 10.1183/09031936.00000211. Epub 2011 May 12 [PubMed PMID: 21565916]
Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest. 1991 May:99(5):1162-5 [PubMed PMID: 2019172]
Light RW. Parapneumonic effusions and empyema. Proceedings of the American Thoracic Society. 2006:3(1):75-80 [PubMed PMID: 16493154]
Sorino C, Mondoni M, Lococo F, Marchetti G, Feller-Kopman D. Optimizing the management of complicated pleural effusion: From intrapleural agents to surgery. Respiratory medicine. 2022 Jan:191():106706. doi: 10.1016/j.rmed.2021.106706. Epub 2021 Nov 26 [PubMed PMID: 34896966]
Sperandeo M, Rotondo A, Guglielmi G, Catalano D, Feragalli B, Trovato GM. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: use and limitations. La Radiologia medica. 2014 Oct:119(10):729-40. doi: 10.1007/s11547-014-0385-0. Epub 2014 Feb 5 [PubMed PMID: 24496592]
Russell FM, Ehrman RR, Barton A, Sarmiento E, Ottenhoff JE, Nti BK. B-line quantification: comparing learners novice to lung ultrasound assisted by machine artificial intelligence technology to expert review. The ultrasound journal. 2021 Jun 30:13(1):33. doi: 10.1186/s13089-021-00234-6. Epub 2021 Jun 30 [PubMed PMID: 34191132]
Shen-Wagner J, Gamble C, MacGilvray P. Pleural Effusion: Diagnostic Approach in Adults. American family physician. 2023 Nov:108(5):464-475 [PubMed PMID: 37983698]
Boccatonda A, Baldini C, Rampoldi D, Romani G, Corvino A, Cocco G, D'Ardes D, Catalano O, Vetrugno L, Schiavone C, Piscaglia F, Serra C. Ultrasound-Assisted and Ultrasound-Guided Thoracentesis: An Educational Review. Diagnostics (Basel, Switzerland). 2024 May 29:14(11):. doi: 10.3390/diagnostics14111124. Epub 2024 May 29 [PubMed PMID: 38893651]
Mohammed A, Hochfeld U, Hong S, Hosseini DK, Kim K, Omidvari K. Thoracentesis techniques: A literature review. Medicine. 2024 Jan 5:103(1):e36850. doi: 10.1097/MD.0000000000036850. Epub [PubMed PMID: 38181250]
Cantey EP, Walter JM, Corbridge T, Barsuk JH. Complications of thoracentesis: incidence, risk factors, and strategies for prevention. Current opinion in pulmonary medicine. 2016 Jul:22(4):378-85. doi: 10.1097/MCP.0000000000000285. Epub [PubMed PMID: 27093476]
Level 3 (low-level) evidence