Introduction
Thalamocortical radiations are the nerve fibers between the thalamus and the cerebral cortex. Functionally, thalamocortical radiations, also called thalamocortical fibers, relay sensory or motor information from the thalamus to distinct areas of the cerebral cortex through relay neurons. Structurally, thalamocortical radiations are parallel pathways linking specific thalamic nuclei with specific cortical areas. There are also corticothalamic fibers, which relay information back to the thalamus from the cerebral cortex. Thalamocortical radiations are involved in the control of cortical arousal and consciousness, and play a role in movement disorders such as Parkinson disease.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
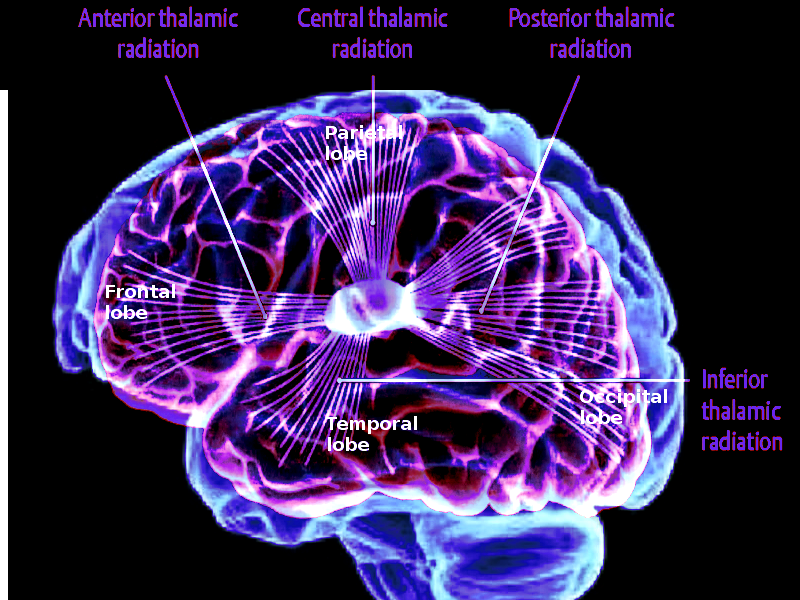
Thalamocortical radiations are nerve fibers that connect the thalamus to the cerebral cortex. The thalamus is the primary relay center of the brain, sending all sensory information besides olfaction to the cerebral cortex, where it is further processed. Thalamocortical interneurons receive sensory or motor information from the body and signal distinct thalamic nuclei to relay selected information via relay neurons in the thalamocortical radiations to the cerebral cortex.[1] Thalamocortical radiations can be separated into four distinct pathways, including the anterior thalamic radiations, posterior thalamic radiations, superior thalamic radiations, and inferior thalamic radiations. The anterior thalamic radiations connect the anterior and midline nuclear groups of the thalamus with the frontal lobe through the anterior thalamic peduncle and the anterior limb of the internal capsule. The posterior thalamic radiations connect the caudal parts of the thalamus with the parietal and occipital lobe through the posterior thalamic peduncle and posterior limb of the internal capsule. The superior thalamic radiations connect the ventral nuclear group of the thalamus with the precentral and postcentral gyrus through the superior thalamic peduncle and the posterior limb of the internal capsule. Lastly, the inferior thalamic radiations connect the thalamus with the insula, temporal lobe, and ventral portion of the frontal lobe through the inferior thalamic peduncle and sublenticular part of the internal capsule.[2]
Relay neurons in the thalamus can be separated into core cells and matrix cells. Matrix cells, or calbindin-immuno-reactive (CIR) neurons, are diffusely distributed in each of the nuclei of the dorsal thalamus. Core cells, or parvalbumin-immuno-reactive (PIR) neurons, are only found in principal sensory and motor relay nuclei, such as the pulvinar and intralaminar nuclei. The PIR neurons tend to cluster together and create dense terminating afferent fibers. While CIR neurons have dispersed projections to non-specific areas of the cerebral cortex and terminate in the superficial layers I, II, and upper III, PIR neurons project via an organized topographic pathway and terminate in localized zones in deep layers III and IV.[1]
Topographically, thalamocortical networks are organized into parallel pathways that link specific cortical areas to individual thalamic nuclei. The thalamus contains many distinct nuclei with multiple functions.[3] There are three types of thalamic nuclei: relay nuclei, association nuclei, and nonspecific nuclei. Relay nuclei include nuclei that relay primary sensations, such as the ventral posterolateral, ventral posteromedial, medial geniculate, and lateral geniculate nuclei, as well as nuclei for cerebellar feedback, such as the ventral lateral nucleus, and lastly nuclei for basal ganglia feedback, such as the ventral anterior nucleus. Association nuclei include the pulvinar, the dorsomedial nucleus or mediodorsal, and anterior nuclei. Nonspecific nuclei include the intralaminar and midline thalamic nuclei.[4]
All thalamic nuclei project primarily to the cortex; for example, visual information is received in the thalamus by the pulvinar nucleus and lateral geniculate nucleus and relayed to the visual TAuditory information is received by the medial geniculate nucleus of the thalamus receives auditory information and relays it to the auditory cortex in the temporal lobe. Other examples include the ventral anterior and ventrolateral nuclei, which relay motor information, and the ventral posterolateral and ventral posteromedial nuclei, which relay somatosensory information.[3]
Thalamocortical radiations, or fibers, have a tree-like appearance and extend from the thalamus through the internal capsule and project to the cerebral cortex. Once the information is processed in the cerebral cortex, it is relayed back to the area of original activity in the thalamus via corticothalamic fibers. These corticothalamic projections, which synapse with the thalamic reticular neurons, are more numerous than the thalamocortical projections to the cerebral cortex. This suggests that the cerebral cortex has a more significant role in processing information than the processes occurring in the thalamic interneurons.[5] While the cortico-thalamic radiations are comprised of massive projections from each cortical area to a specific dorsal thalamic nucleus, the thalamocortical projections are much smaller.[6]
Most of the thalamocortical radiations project to layer IV of the cerebral cortex, or the inner granular layer, where sensory information is then directed to other cortical layers.[7] Thalamocortical neuronal activation depends on the direct and indirect effects of the excitatory neurotransmitter glutamate, which causes excitatory post-synaptic potentials (EPSPs) at the terminal branches of the primary sensory cortex.[8]
Embryology
Thalamocortical radiations begin growing during the embryonic period and complete growth by the third trimester of gestation.[9] Thalamocortical radiations grow out from the diencephalon through the internal capsule and accumulate below specific regions of the cerebral cortex. The fibers travel through several emerging telencephalic subdivisions of the embryonic forebrain until they eventually reach their targeted cortical sites. Afferent thalamocortical fibers first pass through the ventral thalamus, then advance in the internal capsule, where they associate with pre-existing cells, and finally, travel across the striatocortical junction until they reach the cerebral cortex. The fibers traverse by associating with subplate cells and early corticofugal fibers.[10]
Blood Supply and Lymphatics
The thalamocortical fibers run between the thalamus and the cerebral cortex. The thalamus receives its blood supply from various branches of the posterior cerebral artery, including the posterior communicating artery and basilar communicating artery.[11] The cerebral cortex receives blood from the internal carotid arteries and the vertebral arteries, which connect via bilateral posterior communicating arteries.
Surgical Considerations
Thalamocortical fibers must merit consideration during any neurosurgery. As far back as 1942, the bilateral severing of the thalamocortical radiations between the medial and anterior thalamic nuclei was used functionally to provide a prefrontal lobotomy. This procedure was an attempt in psychotic patients to reduce their emotional reactions yet maintain other cognitive abilities.[12]
Clinical Significance
The current belief is that nonspecific thalamocortical connectivity, particularly involving the intralaminar nuclei, have involvement in the control of cortical arousal and consciousness. Several studies have found that the lack of consciousness of patients in a vegetative state is linked directly to a disruption of thalamocortical function and connectivity.[13] For example, one study found that loss of consciousness in a patient in a vegetative state correlated with impaired thalamocortical functional connectivity between the intralaminar nuclei and the prefrontal and anterior cingulate cortices.[14]
There is ongoing research into the relationship between thalamocortical radiations and attention and arousal. Studies have found that the thalamocortical system is involved in the epileptic loss of consciousness. In absence seizures, the ability of thalamocortical-corticothalamic feedback loop to regulate the flow of information to the cortex suffers transient loss because large numbers of corticothalamic loops are engaged in strong, low-frequency oscillations of excitatory postsynaptic potentials (EPSP)/inhibitory post-synaptic potentials (IPSP). These EPSP/IPSP sequences generate specific electroencephalographic patterns called spike-and-wave discharges (SWDs). The thought is that the enhancement of corticothalamic feedback during SWDs may inhibit the discrete feedback which normally selects distinct thalamocortical circuits for conscious perception and motor reaction. As such, the increase in SWD activity within the thalamocortical network can disrupt normal sleep-wakefulness rhythms and ultimately cause absence seizures.[15]
The thalamocortical radiations that extend to the motor cortex and comprise the motor circuit are implicated in movement disorders such as Parkinson disease. The signs and symptoms may result from abnormalities in one of the many basal ganglia thalamocortical circuits. Evidence from current literature suggests that various movement disorders result from the abnormalities of the basal ganglia thalamocortical circuit, due to the dysfunction disrupting downstream network activity in the brainstem, thalamus, and cortex.[16]
Dysfunction of frontal thalamocortical circuits may be involved in juvenile myoclonic epilepsy. While the routine MRI in patients with juvenile myoclonic epilepsy typically appears normal, subtle structural and functional deficits in the frontal thalamocortical circuits have been demonstrated.[17]
The length of anterior thalamic radiations has been found to be negatively correlated with thalamic choline levels in patients with obsessive-compulsive disorder, indicating that abnormalities in thalamocortical radiations play a role in the pathophysiology and symptomology of the disorder.[18] Additionally, anomalies in the posterior thalamic radiation and left mediodorsal thalamic nucleus may connect to patients with anorexia nervosa.[19] Anterior thalamic radiation abnormalities may share links with the negative symptoms and cognitive abnormalities in schizophrenia.[20] One study found evidence of anatomical and functional underconnectivity between the thalamus and the prefrontal, parieto-occipital, motor, and somatosensory cerebral cortices in patients with an autism spectrum disorder, indicating abnormal thalamocortical connectivity.[21] Also, volume reductions of several thalamic nuclei, including the central nuclear complex, anterior nucleus, and lateral dorsal nucleus, were found in patients with migraines.[22] Lastly, abnormalities in ascending cholinergic projections within thalamic nuclei and visual areas in patients with dementia with Lewy bodies may elucidate the visual hallucinations associated with the disease.[23]
Media
References
Jones EG. Thalamic circuitry and thalamocortical synchrony. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2002 Dec 29:357(1428):1659-73 [PubMed PMID: 12626002]
Level 3 (low-level) evidenceZhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa-Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PC, Mori S. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. NeuroImage. 2010 Oct 1:52(4):1289-301. doi: 10.1016/j.neuroimage.2010.05.049. Epub 2010 May 24 [PubMed PMID: 20570617]
Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. The American journal of psychiatry. 2012 Oct:169(10):1092-9. doi: 10.1176/appi.ajp.2012.12010056. Epub [PubMed PMID: 23032387]
Scheibel ME, Scheibel AB. Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain research. 1967 Sep:6(1):60-94 [PubMed PMID: 4964024]
Level 3 (low-level) evidenceBriggs F, Usrey WM. Emerging views of corticothalamic function. Current opinion in neurobiology. 2008 Aug:18(4):403-7. doi: 10.1016/j.conb.2008.09.002. Epub 2008 Oct 6 [PubMed PMID: 18805486]
Level 3 (low-level) evidenceLlinás RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991:44(3):521-35 [PubMed PMID: 1754050]
Level 3 (low-level) evidencePalmer SL, Noctor SC, Jablonska B, Juliano SL. Laminar specific alterations of thalamocortical projections in organotypic cultures following layer 4 disruption in ferret somatosensory cortex. The European journal of neuroscience. 2001 Apr:13(8):1559-71 [PubMed PMID: 11328350]
Level 3 (low-level) evidenceKharazia VN, Weinberg RJ. Glutamate in thalamic fibers terminating in layer IV of primary sensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994 Oct:14(10):6021-32 [PubMed PMID: 7931559]
Level 3 (low-level) evidenceKrsnik Ž, Majić V, Vasung L, Huang H, Kostović I. Growth of Thalamocortical Fibers to the Somatosensory Cortex in the Human Fetal Brain. Frontiers in neuroscience. 2017:11():233. doi: 10.3389/fnins.2017.00233. Epub 2017 Apr 27 [PubMed PMID: 28496398]
Molnár Z. Development and evolution of thalamocortical interactions. European journal of morphology. 2000 Dec:38(5):313-20 [PubMed PMID: 11151044]
Level 3 (low-level) evidenceSchmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003 Sep:34(9):2264-78 [PubMed PMID: 12933968]
Level 3 (low-level) evidenceFreeman W, Watts JW. Prefrontal Lobotomy: The Surgical Relief of Mental Pain. Bulletin of the New York Academy of Medicine. 1942 Dec:18(12):794-812 [PubMed PMID: 19312295]
Zhou J, Liu X, Song W, Yang Y, Zhao Z, Ling F, Hudetz AG, Li SJ. Specific and nonspecific thalamocortical functional connectivity in normal and vegetative states. Consciousness and cognition. 2011 Jun:20(2):257-68. doi: 10.1016/j.concog.2010.08.003. Epub 2010 Nov 13 [PubMed PMID: 21078562]
Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet (London, England). 2000 May 20:355(9217):1790-1 [PubMed PMID: 10832834]
Level 3 (low-level) evidenceKostopoulos GK. Involvement of the thalamocortical system in epileptic loss of consciousness. Epilepsia. 2001:42 Suppl 3():13-9 [PubMed PMID: 11520316]
Level 3 (low-level) evidenceDeLong MR, Wichmann T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA neurology. 2015 Nov:72(11):1354-60. doi: 10.1001/jamaneurol.2015.2397. Epub [PubMed PMID: 26409114]
Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009 May:50(5):1210-9. doi: 10.1111/j.1528-1167.2008.01952.x. Epub 2009 Jan 17 [PubMed PMID: 19183226]
Wang R, Fan Q, Zhang Z, Chen Y, Zhu Y, Li Y. Anterior thalamic radiation structural and metabolic changes in obsessive-compulsive disorder: A combined DTI-MRS study. Psychiatry research. Neuroimaging. 2018 Jul 30:277():39-44. doi: 10.1016/j.pscychresns.2018.05.004. Epub 2018 May 12 [PubMed PMID: 29807209]
Frieling H, Fischer J, Wilhelm J, Engelhorn T, Bleich S, Hillemacher T, Dörfler A, Kornhuber J, de Zwaan M, Peschel T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa--a voxel based diffusion tensor imaging (DTI) study. Journal of psychiatric research. 2012 Sep:46(9):1237-42. doi: 10.1016/j.jpsychires.2012.06.005. Epub 2012 Jul 4 [PubMed PMID: 22770509]
Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, Gado MH, Barch DM, Csernansky JG. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry research. 2010 Aug 30:183(2):144-50. doi: 10.1016/j.pscychresns.2010.04.013. Epub 2010 Jul 8 [PubMed PMID: 20619618]
Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain : a journal of neurology. 2013 Jun:136(Pt 6):1942-55. doi: 10.1093/brain/awt079. Epub [PubMed PMID: 23739917]
Level 2 (mid-level) evidenceMagon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M, Amin FM, Seifert CL, Chakravarty MM, Müller J, Sprenger T. Morphological Abnormalities of Thalamic Subnuclei in Migraine: A Multicenter MRI Study at 3 Tesla. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015 Oct 7:35(40):13800-6. doi: 10.1523/JNEUROSCI.2154-15.2015. Epub [PubMed PMID: 26446230]
Taylor JP, Firbank M, Barnett N, Pearce S, Livingstone A, Mosimann U, Eyre J, McKeith IG, O'Brien JT. Visual hallucinations in dementia with Lewy bodies: transcranial magnetic stimulation study. The British journal of psychiatry : the journal of mental science. 2011 Dec:199(6):492-500. doi: 10.1192/bjp.bp.110.090373. Epub 2011 Oct 20 [PubMed PMID: 22016436]
Level 2 (mid-level) evidence