Introduction
Testicular cancer is the most common malignancy in men aged 15 to 45 years and represents one of the most common curable malignancies when identified promptly and treated with a multimodal approach. It represents 1% of male tumors and 5% of urological malignancies.[1] The incidence of testicular cancer has been increasing over recent years, gaining increased significance due to the long impact both the disease and its treatment can have over the course of a patient's life. Testicular cancer incidence has doubled over the past 40 years.[2]
With effective management, the prognosis is excellent with >90% cure rate and >95% five-year survival rate.[3][4] Complex environmental and genetic factors are involved in the development of testicular cancer; common risk factors include cryptorchidism, family history of testicular cancer, personal history of testicular cancer in the contralateral testis, age, and ethnicity. Initial evaluation includes history and physical examination, tumor marker assessment, and scrotal ultrasound. Once a solid intratesticular tumor is identified, radical inguinal orchiectomy is performed both for diagnostic and therapeutic purposes. Tumor staging guides further management with options including active surveillance, chemotherapy, retroperitoneal lymph node dissection, and radiation therapy.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Both genetic and environmental factors have been studied in the development of testicular cancers.
Epidemiological risk factors: They include cryptorchidism, decreased spermatogenesis evidenced by sub- or infertility, disorders of sexual development, familial history of testicular tumors among first-degree relatives, the presence of a contralateral tumor or germ-cell neoplasia in-situ (GCNIS), etc.[5][6][7][8][9]
The most common environmental risk factors for testicular cancers can be summarized as below:
-
Cryptorchidism —2–4 fold increase in risk.[10] As cryptorchidism is more common on the right side, a small increase in incidence in right-sided testicular cancer is observed.[11]
- Infections - Human papillomavirus (HPV), Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Parvovirus B-19, and Human immunodeficiency virus (HIV)[14]
- High maternal estrogen levels[17]
- Carcinoma in situ (intratubular germ cell neoplasia)
- Prior history of testis cancer or extragonadal germ cell tumor
Genetic risk factors: Multiple genetic changes have been described in the etiology of testicular cancer. The isochromosome of the short arm of chromosome 12 – (i12p) – is pathognomonic of all types of adult germ cell tumors (GCTs), as well as GCNIS. Alterations in p53 have been observed in about 66% of cases of GCNIS.[18][19] Genetic polymorphisms in the PTEN tumor suppressor gene and the risk of testicular cancer (TC) have also been described.[20] Dysregulation in the pluripotent program of fetal germ cells (identified by markers, M2A, C-KIT, and OCT4/NANOG) is thought to be responsible for the development of GCNIS and germ cell neoplasia. Along with this, genome-wide association studies (GWAS) showed evidence of several single nucleotide polymorphisms (SNPs) markers known to have an association with an increased risk of developing testicular cancer, in particular at 15q21.3.[21]
However, current genetic studies have not revealed any evidence for a major single high-penetrance gene known to cause increased testicular cancer susceptibility.[22] An overlap is observed between the development of seminoma and of embryonal carcinoma, as evidenced by genome-wide expression analysis and detection of alpha-fetoprotein (AFP) mRNA in some cases of atypical seminoma.[23]
Epidemiology
The highest incidence of testicular cancer is observed in Western and Northern Europe (8.7 and 7.2 per 100,000 men, respectively).[1] The highest mortality rates are reported in western Asia, with most countries showing a decrease in mortality, likely due to the combined impact of earlier detection through self-examination and integration of multimodal treatments.[1]
In the United States, testicular cancer is most frequently diagnosed among men aged 20 to 34 (51% of all cases). 22.9 % of the cases are diagnosed in the age group of 35 to 44, 12.9% between 45 to 54, and the rest in other age groups. The mean age of diagnosis is 33 years. It is more commonly seen in White race men with an incidence rate of 7.1 per 100,000 persons, compared to 5.4 in Hispanic men and 1.7 in African American men.[24]
The overall incidence in the United States increased gradually over the last 4 decades (6.3 per 100,000 persons in 2017 compared to 3.7 per 100,000 in 1975). Testicular cancer incidence is higher in industrialized countries than in developing countries and, while higher incidences are seen in Caucasian men, the incidence of testicular cancer among non-white and immigrant men within the United States is increasing for unknown reasons.[25] Synchronous contralateral tumors are seen in 0.6% of cases and metachronous contralateral tumors are identified in 1.9% of cases.[26]
Pathophysiology
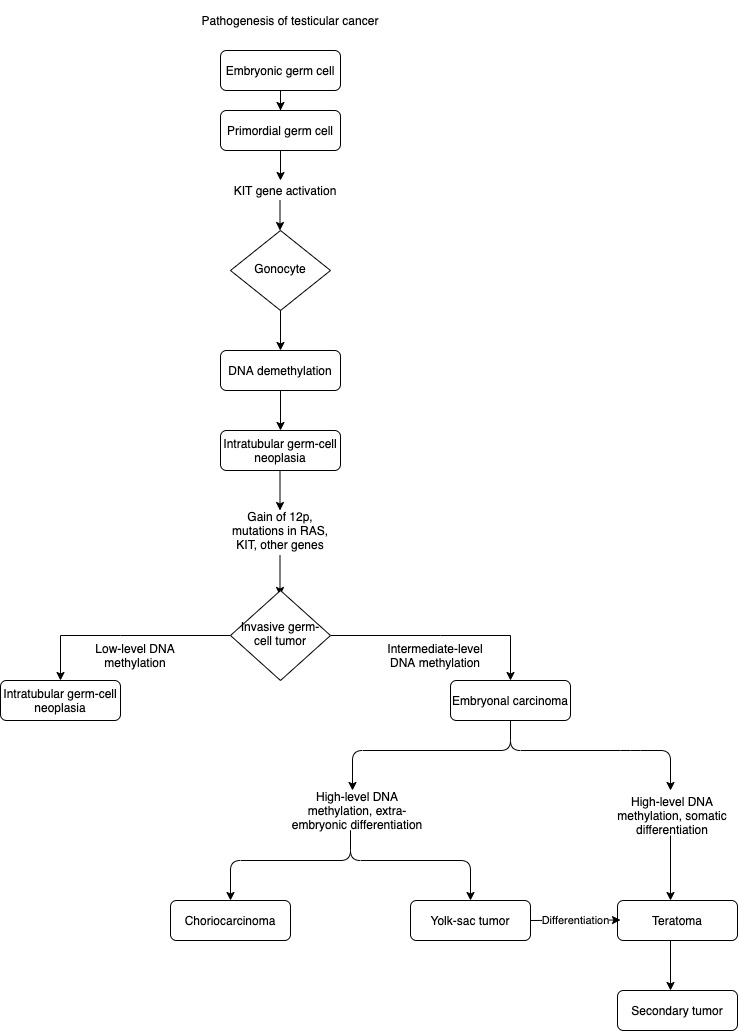
Germ-cell tumors are observed to develop secondary to a tumorigenic event in utero that leads to intratubular germ-cell neoplasia.[27] Intratubular germ-cell neoplasia is derived from gonocytes that have failed to differentiate into spermatogonia.[28] These cells do not attain invasive potential until after hormonal changes occur during puberty. Seminomas consist of transformed germ cells that are blocked in their differentiation. Embryonal carcinoma cells resemble undifferentiated stem cells, and their gene expression is similar to those of stem cells and intratubular germ-cell neoplasms.[29][30] Choriocarcinomas and yolk-sac tumors have extraembryonic differentiation, and teratomas have somatic differentiation.
Several genetic loci that confer a predisposition to testicular cancer have been identified.[31][32] The variant with the highest impact was detected at 12q21, the genes encoding the proteins involved in KITLG–KIT signaling.[33] The development of intratubular germ-cell neoplasia may involve aberrantly activated KITLG–KIT in utero, which induces arrest of embryonic germ cells at the gonocyte stage; subsequently, overexpression of embryonic transcription factors such as NANOG, sex-determining-region Y–box 17 (SOX17), and octamer-binding transcription factor 3–4 (OCT3/4, also known as POU domain, class 5, transcription factor 1 [POU5F1]) leading to suppression of apoptosis, increased proliferation, and accumulation of mutations in gonocytes.[34]
The genes related to the pathogenesis and their respective chromosomes as outlined below:[35]
- UCK1: Chromosome 1
- HPGDS: Chromosome 4
- CENPE: Chromosome 4
- TERT: Chromosome 5
- TERT/CLPTM1L: Chromosome 5
- SPRY4: Chromosome 5
- BAK-1: Chromosome 6
- MAD1L1: Chromosome 7
- DMRT1: Chromosome 9
- AFT7IP: Chromosome 12
- KITLG: Chromosome 12
- RFWD3: Chromosome 16
- TEX14: Chromosome 17
- PPM1E: Chromosome 17
Distinct gene expression through epigenetic regulation, including DNA methylation, may result in the formation of the different histologic subtypes, as outlined in the flowchart.[36]
Histopathology
Testicular cancers are defined based on their cell type. 2016 updated WHO histopathological classification characterizes testicular cancers with the following classifications:[37]
1. Germ Cell Tumors
- Germ cell neoplasia in situ(GCNIS)
2. Derived from Germ Cell Neoplasia In Situ (GCNIS)
- Seminoma
- Embryonal carcinoma
- Yolk sac tumor, post-pubertal type
- Trophoblastic tumor
- Teratoma, post-pubertal type
- Teratoma with somatic-type malignancies
- Mixed germ cell tumors
3. Germ Cell Tumors Unrelated to GCNIS
- Spermatocytic tumor
- Yolk sac tumor, pre-pubertal type
- Mixed germ cell tumor, pre-pubertal type
4. Sex Cord/Stromal Cell Tumors
- Leydig cell tumor
Malignant Leydig cell tumor
- Sertoli cell tumor
Malignant Sertoli cell tumor
Large cell calcifying Sertoli cell tumor
Intratubular large cell hyalinizing Sertoli cell neoplasia
- Granulosa cell tumor
Adult type
Juvenile type
- Thecoma/fibroma group of tumors
- Other sex cord/gonadal stromal tumors
Mixed
Unclassified
- Tumors containing both germ cell and sex cord/gonadal stromal
Gonadoblastoma
5. Miscellaneous Non-specific Stromal Cell Tumors
- Ovarian epithelial tumors
- Tumors of collecting ducts and rete testes
Adenoma
Carcinoma
- Tumors of paratesticular structures
Adenomatoid tumor
Mesothelioma(epithelioid, biphasic)
Epididymal tumors
- Cystadenoma of the epididymis
- Papillary cystadenoma
- Adenocarcinoma of the epididymis
- Mesenchymal tumors of the spermatic cord and the testicular adnexa
History and Physical
Testicular malignancy usually presents as a unilateral lump or painless swelling as an incidental finding. Less commonly, testicular cancer presents with pain, with about one-third of the patients having dull pain. Acute pain is noted in about 10% of the individuals. While testicular cancer is not associated with trauma, antecedent trauma may elicit examination or imaging of the testes and result in an ultimate diagnosis. A careful physical examination will reveal a firm intratesticular lesion. Each testis should be gently held and rolled between the fingers to identify a firm intratesticular mass and differentiate intratesticular from extratesticular lesions. It is important to completely examine the contralateral testis as 0.6% of patients have a synchronous contralateral testis tumor. Occasionally, the testis is unable to be completely palpated due to the presence of a hydrocele, and the presence of a testicular lesion should be confirmed with ultrasonography, which is an extension of the physical examination. Patients uncommonly present with symptoms or signs of metastatic disease.[38] These are summarised below:
Clinical manifestations of testis cancer from metastatic disease
-
Systemic symptoms: anorexia, malaise, weight loss
-
Pulmonary metastasis: cough or shortness of breath
-
Lymphatic metastasis: cervical or supraclavicular lymphadenopathy
-
Retroperitoneal disease: Bulky retroperitoneal disease can present as back pain or may lead to compression on the gonadal veins leading to findings of varicocele
-
Vascular obstruction or thrombosis leading to lower extremity edema
-
Nausea, vomiting, or gastrointestinal hemorrhage from retroduodenal metastases
-
Central or peripheral nervous system symptoms from the cerebral, spinal cord, or peripheral nerve root involvement
Prompt diagnosis and treatment are crucial since testicular malignancy has excellent cure rates due to high chemo-sensitivity with modern cisplatin-based chemotherapy, radio-sensitivity, and surgical excision with orchiectomy or retroperitoneal lymph node dissection.
Evaluation
Evaluation of testicular cancer begins with obtaining a thorough history and performing a detailed physical examination. History includes inquiry regarding clinical features, as discussed above. It is important to ask specifically about the history of cryptorchidism, orchiopexy, or inguinal hernia repair as an infant. A family history of testicular cancer in father or brother should be elicited. Physical examination findings of any solid intratesticular mass should be considered to be testicular cancer until proven otherwise. Examination findings related to metastatic disease, described previously, may also be identified.
Testicular imaging with trans-scrotal ultrasound is the primary imaging modality to identify testicular cancer when suspected on physical examination.[39][40][41] Ultrasound imaging, when combined with a physical examination, provides nearly 100% sensitivity in the diagnosis of testicular cancer.[42] Testicular cancer is suspected when an ultrasound reveals a hypoechoic, solid, vascularized intratesticular lesion, and different testicular cancer types show subtle morphologic differences in imaging.[43] Further evaluation should include serum tumor markers (AFP, HCG, and LDH) before any intervention, including orchiectomy. Adequate counseling should be given regarding the possibility of infertility and placement of the testicular prosthesis if desired. Sperm banking should be considered in patients with bilateral testicular pathology.[39][40]
Radical inguinal orchiectomy is both therapeutic and provides tissue for histopathological diagnosis. Trans-scrotal biopsy should not be undertaken due to the risk of local dissemination due to the manipulation of established lymphatic drainage pathways. A retrospective analysis study showed a small but statistically significant increase in the incidence of local recurrence with trans-scrotal biopsy when compared to orchiectomy (2.9% vs. 0.4%).[44]
Testicular germ cell tumors spread along well-described and predictable lymphatic channels.[45][46] For tumors arising in the right testis, the primary landing zone is the infrarenal inter-aortocaval lymph nodes, followed by paracaval lymph nodes and para-aortic lymph nodes. Tumors arising from the left testis spread primarily to the para-aortic lymph nodes, followed by inter-aortocaval lymph nodes. Retroperitoneal spread from the right side to the left side can be seen in tumors arising from the right testis, but left-to-right spread within the retroperitoneum is rarely seen unless it is associated with bulky lymph node disease.[47] Abdominopelvic cross-sectional imaging is important to identify retroperitoneal lymph node disease arising from primary testis cancer and to inform multimodal treatment.
All patients with testicular germ cell tumors should undergo abdominopelvic imaging with computed tomography (CT). Patients with elevated serum tumor markers (AFP, α-fetoprotein; β-hCG, β-subunit of human chorionic gonadotropin; LDH, lactate dehydrogenase) should be evaluated further with computed tomography (CT) of the chest, abdomen, and pelvis for staging.[39] If testicular tumor markers are within the normal range, the rate of metastasis almost outside of the retroperitoneum is very low; therefore, the addition of a chest CT-scan to cross-sectional imaging of the abdomen and pelvis is highly unlikely to alter the treatment plan, and a chest radiograph suffices when combined with abdominopelvic CT imaging. Choriocarcinoma has been shown to spread via hematogenous routes, and patients with high levels of β-hCG should undergo cross-sectional imaging of the brain to identify metastatic lesions due to choriocarcinoma hematogenously spreading to the brain.
Serum tumor markers play an important role in the evaluation and management of testicular cancer. Post-orchiectomy markers are part of the tumor staging and risk categorization of testicular cancer and are part of any evaluation of treatment response or of disease relapse. LDH is found in diverse tissues throughout the body, and LDH-1 is expressed on chromosome 12p, which is over-expressed in germ cell tumors. Higher LDH levels can correspond to higher tumor burden. AFP and β-hCG evaluations are seen in many germ cell tumors.[48] β-hCG is produced by syncytiotrophoblasts within germ cell tumors and is seen in both non-seminomatous and seminomatous germ cell tumors. Choriocarcinomas commonly express β-hCG, as do approximately 15% of seminomas. AFP derives from the yolk sac, embryonal, and some teratomas within germ cell tumors and is never elevated in pure seminoma or pure choriocarcinoma.[49]
AFP and β-hCG are expressed not only in germ cell tumors but also in other neoplasms. β-hCG levels are increased in neuroendocrine tumors, cancers of kidney, lung, head, and neck, bladder, and GI tract.[50][51] AFP levels have been found to be elevated in liver diseases.[52][53] LDH is fairly nonspecific and is observed to be elevated in multiple different conditions. Recent studies have evaluated levels of circulating microRNAs, especially miR-371a-3p, in the evaluation of germ cell tumors, though their precise role in testicular cancer management remains to be fully defined.[54][55]
Multiple new immunohistochemical markers have been studied for diagnostic purposes.[2] They include PLAP, OCT3/4 (POU5F1), NANOG, HMGA1, HMGA2, PATZ1, RNF4, Aurora-B, AP-2γ (TFAP2C), and LIN28.[56] The table represents the types of tumors and respective immunohistochemical markers.[57][58][59][60][61][60]
Note --> E: Expressed; NE: Not Expressed; E(c): Expressed with cytoplasmic localization; NO: Not observed.
Treatment / Management
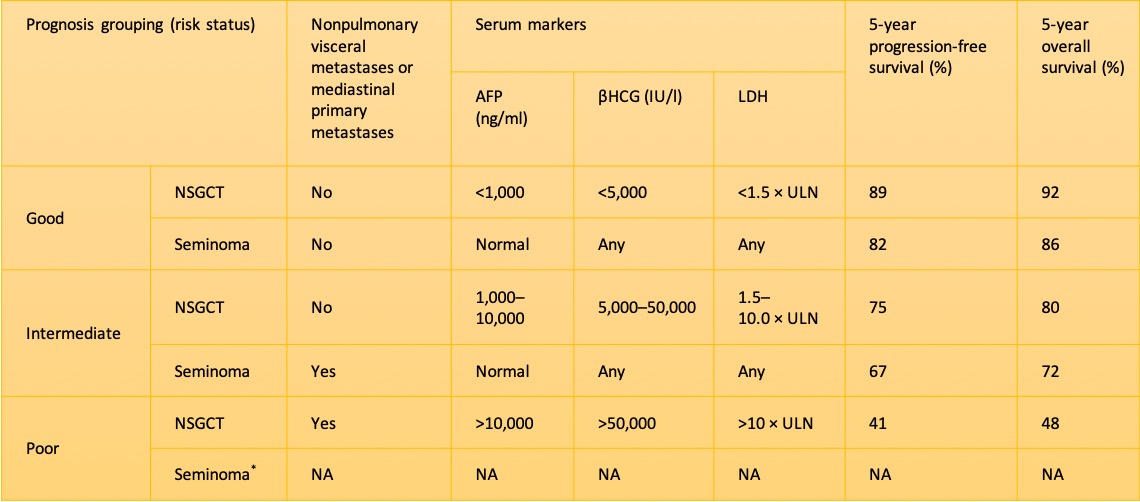
The International Germ Cell Cancer Collaborative Group (IGCCCG)-based clinical staging is used to tailor management strategies in patients with testicular malignancies. All patients are classified as Clinical Stage 0, I, II, or III after imaging and quantification of tumor markers. Stage 0 includes patients with germ cell neoplasia in-situ, stage I includes patients with tumor limited to the testis, stage II patients have lymph node involvement, and stage III patients have distant metastases.
Stage 0: If untreated, GCNIS has a risk of developing into TGCT after 7 years in 70% of cases.[62] Management of GCNIS should involve shared decision-making, and options include close surveillance with ultrasonography, orchiectomy, and radiotherapy. Though low-dose radiotherapy (at least 20 cGy) has similar cure rates as orchiectomy, scatter to contralateral testis can result in up to 40% of men treated by radiotherapy requiring testosterone replacement therapy or experiencing subfertility.[63] Contralateral GCNIS screening is recommended for patients with high-risk factors such as contralateral cryptorchid testis with testicular volume <12ml and age <40 years (30% risk of developing GCNIS). In such patients, screening for GCNIS in the contralateral testis should be offered with biopsy, and radiotherapy/orchiectomy can be given as required. However, regular ultrasonography is preferable for male individuals who desire to maintain fertility.
Stage I: Orchiectomy is the initial management strategy. The risk of relapse differs between seminoma and non-seminomatous tumors, and management differs accordingly, as discussed below.
Seminoma: Approximately 15% to 18% of patients with stage I disease will relapse after orchiectomy without adjuvant treatment.[64] While surveillance is recommended, two strategies may be considered: single-agent carboplatin chemotherapy for high-risk patients (rete-testis invasion and tumor size >4cm) or surveillance. In the absence of high volume(>4cm) and rete-testis invasion, the risk of relapse is <5%, and no adjuvant therapy is indicated. Invasion of both rete-testis and lymphovascular tissue yields a 25% risk of relapse, while the risk of relapse is only 4% if neither of them is involved.[65] Primary radiotherapy to the retroperitoneum and ipsilateral pelvis with 20 to 30 Gy has been the historic standard therapy for patients not agreeable to surveillance, and this treatment results in a 1% in-field recurrence rate, though longer-term cardiovascular side effects of such treatment now limit its common use.[66][67] (B2)
Surveillance of seminoma, however, relies heavily on cross-sectional imaging, as serum tumor markers are elevated only in approximately 15% of pure seminoma, and longer-term surveillance is required.[68] Though not routinely performed for clinical Stage I seminoma, RPLND is increasingly advocated by some due to high surgical cure rates and avoidance of late toxicities associated with chemotherapy, radiotherapy, or the cumulative effect of multiple surveillance CT scans in young men.[69]
Non-seminomatous tumors: There is a 14% to 22% risk of relapse without adjuvant treatment in tumors without lymphovascular invasion. The risk of occult metastatic disease is greater if the lymphovascular invasion or embryonal predominance is seen in the primary tumor. If the lymphatic or vascular invasion is involved, there is an approximately 50% risk of developing metastasis without adjuvant treatment.[70] The chemotherapy regimens used commonly are BEP (bleomycin, etoposide, and cisplatin) or EP (etoposide and cisplatin). Active surveillance without adjuvant therapy is proposed due to the 70%-80% cure rates seen by orchiectomy alone.[71] Such a strategy avoids long-term effects of chemotherapy and surgical recovery from primary retroperitoneal lymph node dissection, though it relies upon regular laboratory and cross-sectional imaging evaluation. Though most relapses occur within the first two years after diagnosis, longer-term surveillance is required due to the risk of late relapse. For patients who decline to undergo surveillance, one or two cycles of BEP chemotherapy can be considered.[72] (A1)
A single cycle of BEP is found to reduce the risk of relapse from 50% to 3%.[72][73] While relapse rates after primary chemotherapy are low, those patients who do recur following BEP are commonly chemoresistant. In select patients, at dedicated referral centers with experienced surgeons, nerve-sparing retroperitoneal lymph node detection(RPLND) offers cure rates comparable to chemotherapy and also results in a decreased need for surveillance cross-sectional imaging.[72] (A1)
The rationale for surgery is that modern nerve-sparing techniques can result in an excellent cure rate without diminishing fertility. Retroperitoneal lymph node dissection also treats occult metastatic teratoma, which is not sensitive to any chemotherapy regimens, and which is present in up to 25% of men with occult metastatic disease. Studies have shown that between 19% and 28% of men with clinical Stage I disease harbor pathologic Stage II disease in the retroperitoneum and that over 80% of these patients can be cured by RPLND alone, sparing chemotherapy and its side effects.[74][75] Additionally, the long-term cancer-specific survival following RPLND (with or without adjuvant chemotherapy) is nearly 100%, and the risk of difficult-to-cure late relapse is less than 2%.[76](A1)
Stage IIA and IIB: Orchiectomy is the initial management strategy. Further management depends on the histopathological type as below.
Seminoma: Radiotherapy has been the historical standard for Stage IIA seminomas, though induction chemotherapy with three cycles of BEP or four cycles of EP is increasingly used. The standard radiation field should be extended from the ipsilateral iliac region to the para-aortic region (also called as 'hockey-stick field'). The relapse rate is 5% after radiotherapy, and overall survival is approximately 100%.[77][78] IIB seminomas warrant three cycles of BEP or four cycles of EP.[79] The PRIMETEST and SEMS trials are currently investigating the role of primary RPLND in clinical Stage IIa and IIb seminoma.[80](A1)
Non-seminomatous tumors: All IIA and IIB non-seminomatous tumors are treated with IGCCCG risk group-directed chemotherapy and can be treated with three cycles of BEP, four cycles of EP, or primary RPLND.[81][82] In patients with residual retroperitoneal disease of >1cm after chemotherapy, salvage resection is recommended.[83] Nerve-sparing surgical techniques can be undertaken to preserve ejaculation and to conserve fertility. For N1 disease, surveillance is generally preferred after RPLND since the rate of relapse is <20%. For N2 or N3 disease, recurrence rates are higher, and hence chemotherapy with two cycles of BEP or EP is justified. For N2 and N3 teratomas, surveillance after RPLND is adequate, as teratoma is chemoresistant.[81][82] (A1)
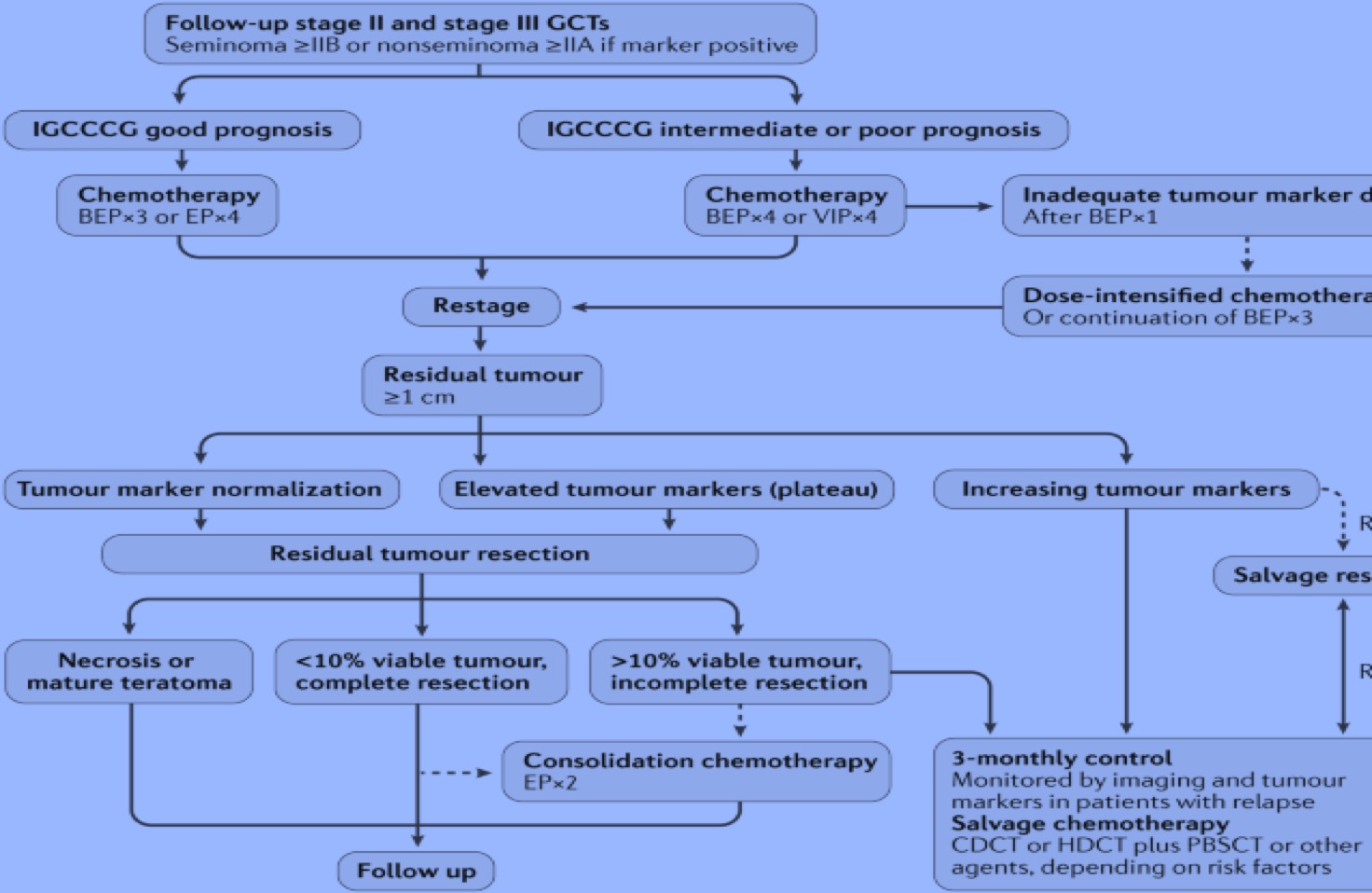
When imaging data is insufficient to determine the stage, RPLND can be performed, or surveillance with imaging six weeks later to evaluate disease status is also an acceptable alternative. Rapidly growing lesions with rising tumor marker levels should be treated with primary chemotherapy according to IGCCCG risk-group classification, as shown in the figure below. Following chemotherapy, if there are any residual lesions ≥, 1 cm, RPLND should be performed. If patients undergoing chemotherapy have growing tumors and normal or declining tumor markers, teratoma or an undifferentiated malignant tumor should be suspected, and RPLND is indicated.
Stage IIC and III: Standard treatment regimens for advanced tumors include chemotherapy with BEP, EP, or Cisplatin-based chemotherapy regimens such as VIP (etoposide, ifosfamide, cisplatin) or VeIP (vinblastine, ifosfamide, cisplatin), according to the IGCCCG risk classification. In an IGCCCG analysis, the 5-year overall survival rate for patients with intermediate prognosis was 80% and for patients with poor prognosis was 48%.[84] For patients with good prognosis according to the IGCCCG risk stratification, three cycles of BEP or four cycles of EP are recommended. For patients with intermediate or poor prognosis, four cycles of BEP are recommended. Several other studies did not show more effective or less toxic chemotherapy regimens.[85] (A1)
Tumor marker levels should be assessed at the end of the first cycle of chemotherapy. For patients with unsatisfactory tumor marker decline, dose-intensified chemotherapy should be considered. High dose chemotherapy regimens are not indicated as the initial treatment itself since trials have failed to prove a survival benefit when compared to standard doses.[81][82] Rarely, in a few patients, the levels of tumor markers decline but the tumor mass increases, indicating growing teratoma syndrome. In such cases, immediate tumor resection should be completed.(A1)
Approximately 1 to 2% of patients present with brain metastasis. The overall survival in such patients at initial diagnosis is poor (30% to 40%), but it is worse in patients in whom brain metastasis occurs as relapsed disease (2% to 5%).[81][82] Currently, an optimal management protocol for patients with brain metastasis is not defined. Chemotherapy is necessary for all patients with brain metastasis. An analysis from the Global Germ Cell Cancer Group showed that primary chemotherapy is an adequate treatment modality in patients with brain metastasis discovered at the initial diagnosis of TGCT. In patients with brain metastasis at relapse, cranial radiotherapy in combination with systemic chemotherapy improved the overall prognosis.[86] (A1)
Concurrent whole-brain radiotherapy and chemotherapy strategy is found to cause central nervous system toxicity (progressive multifocal leukoencephalopathy or cerebral atrophy), and hence should be avoided.[87] There is an increasing interest in the use of stereotactic radiosurgery to treat oligometastases or solitary brain metastasis when radiation is required. The role of neurosurgical interventions in such cases is unclear. Further trials are needed to clearly define the utility of neurosurgery in such cases.(B3)
Treatment of Residual Lesions
Seminoma
Usually, seminoma responds well to chemotherapy (approximately 95% of patients) and does not need residual tumor resection. If serum βHCG levels are increased, salvage chemotherapy is indicated.[81][82] Over half of patients with seminoma have residual tumor masses after primary chemotherapy, and studies have found that residual masses under 3cm in size after risk group-directed chemotherapy are comprised of fibrosis with rare findings of the viable disease, while residual masses over 3cm in size may harbor viable tumor in approximately a third of cases.[88][89] For tumor masses over 3cm in size after induction chemotherapy, a PET scan should be performed, and any PET-avid mass should be surgically excised, while patients with non-avid masses may be observed.[90] There is no role for radiotherapy in residual masses following induction chemotherapy. (A1)
Nonseminomatous Tumors
When residual radiographic abnormalities (classed as lesions >1 cm) are present after chemotherapy, residual tumor resection is warranted. Among residual masses over 1cm in size following chemotherapy, approximately 10% can harbor vital cancer, 40% may harbor chemotherapy-resistant teratoma, and 50% can be expected to harbor necrotic tissue and/or fibrosis.[91][92] Patients with disseminated disease, who, following chemotherapy, have serological remission and radiographic residual tumors of <1 cm can be safely observed without residual tumor resection.[93][94] (B2)
However, some centers prefer to perform RPLND to decrease the risk of late relapse, particularly in the case of teratoma and secondary somatic malignancy. Patients in whom the levels of serum tumor markers (especially AFP) plateau after chemotherapy need residual tumor resection rather than salvage chemotherapy. Even in patients with localized disease and plateauing levels of βHCG, surgery may offer a superior cure rate, and in these cases, the treatment decision should be made by specialized centers for testicular tumors.[95] After the resection of residual tumors with necrosis or the complete resection of teratomas, no further treatment is warranted.[81][82](A1)
Treatment of Relapsed or Refractory Disease
Standard treatment of patients with tumor marker-positive relapse after first-line chemotherapy, refractory disease, and tumor progression during chemotherapy, or within 3 months thereafter, is salvage chemotherapy followed by surgical resection of residual disease. Standard salvage regimens include four cycles of paclitaxel, ifosfamide, and cisplatin (TIP), four cycles of vinblastine, ifosfamide, and cisplatin (VIP), and two or three courses of carboplatin and etoposide high-dose chemotherapy (HDCT).[96][97] To date, no randomized trial has been done to evaluate the superiority of HDCT over conventional-dose chemotherapy. The TIGER trial (a randomized phase III trial) is currently ongoing comparing these two strategies.[98] (A1)
The residual disease of any size should be resected since resection is known to be superior to salvage therapy in preventing relapse. Unresectable disease warrants salvage chemotherapy. Late relapse (defined as disease recurrence >2 years after completion of first-line therapy) tumors are more likely to be chemoresistant, and surgery is the first choice of treatment in patients with resectable disease, irrespective of tumor marker levels.[99] In the case of unresectable tumors, salvage chemotherapy should be undertaken.[100][101] TGCTs that progressed after cisplatin-based combination chemotherapy may be treated with HDCT or peripheral blood stem cell transplantation (PBSCT). The 2-year progression-free and overall survival values were found to be 60% and 66% with HDCT and PBSCT, respectively.[102](B2)
Differential Diagnosis
A hard intratesticular mass is a diagnostic of testicular cancer unless proven otherwise. However, some other diagnoses to consider while evaluating a testicular mass include:
- Epididymo-orchitis
- Hematoma
- Inguinal hernia
- Hydrocele
- Spermatocele or epididymal head cyst
- Varicocele
- Lymphoma (the most common finding in bilateral testis lesions in older men)
- Metastasis from other cancers (eg, lung cancer, melanoma, prostate cancer)
- Syphilitic gumma
- Tuberculoma
Ultrasonography helps further narrow down the diagnosis and radical inguinal orchiectomy is the definitive modality for diagnosis.
Surgical Oncology
When a diagnosis of testicular cancer is suspected based on physical examination and/or ultrasound findings, radical inguinal orchiectomy is performed, which removes the testicle, epididymis, and spermatic cord up to the level of the internal inguinal ring. In this procedure, these structures are delivered through an inguinal incision made along Langer's lines in the groin. If the mass is too large to pass through a standard 3 to 5 cm inguinal incision, the incision can be carried inferiorly to the anterior scrotum to allow for removal of the testis in its tunics along with the spermatic cord. Trans-scrotal orchiectomy or biopsy is contraindicated, as doing so alters the lymphatic drainage patterns and impacts further management. Partial orchiectomy is contraindicated in the presence of normal contralateral testis and can be considered in small polar lesions in solitary testicles only after biopsy of the adjoining testicles are confirmed on frozen sectioning to be clear of GCNIS or malignancy. Such a procedure should be performed through an inguinal incision and should not be routinely considered.
Predictable lymph node drainage patterns contribute to high success rates of retroperitoneal lymph node dissection. A full RPLND removes all lymphatic tissue within a template whose superior border is the renal vessels and whose lateral borders are the ureters and with an inferior border of where the ureter crosses the iliac vessels. Lymphatic tissue is removed from the great vessels using the "split and roll" technique, whereby para-aortic, pre-aortic, interaortocaval, para-caval, and pre-caval node packets are removed. The ipsilateral gonadal vein is removed and the stump of the spermatic cord is removed. The postganglionic sympathetic nerve fibers can be prospectively identified and spared in nerve-sparing RPLNDs.[103]
Radiation Oncology
Though radiotherapy to the retroperitoneum and ipsilateral pelvic lymph nodes has historically been the standard treatment for early-stage seminoma, long-term deleterious impacts on cardiovascular health and secondary malignancies have limited its use in many contemporary series.[104][105] Short-term complications of gastrointestinal upset, nausea, and fatigue are typically managed conservatively.[106] Typical dosing regimens apply 20 to 30 Gy over 10 to 15 fractions in a dog-leg configuration.[107]
Pertinent Studies and Ongoing Trials
The SEMS and PRIMETEST trials are ongoing to evaluate the role of RPLND in seminoma. The TIGER trial is a phase III international trial to determine the optimal first salvage treatment in relapsed or refractory germ cell tumors. In this trial, Paclitaxel, Ifosfamide, cisplatin (TIP) is compared with high-dose chemotherapy with paclitaxel followed by carboplatin and etoposide TICE in relapsed germ cell tumors.
Treatment Planning
Treatment of testicular cancer requires cooperative evaluation by urologists, medical oncologists, and radiation oncologists. Expert radiologic evaluation during surveillance is mandatory to detect recurrent disease. Careful pathologic evaluation is needed to describe different components of seminomatous and non-seminomatous germ cell tumors or GCNIS within specimens. Multidisciplinary discussions and planning result in the optimization of treatment, which results in an excellent prognosis for testicular cancer patients within all risk categories.
Staging
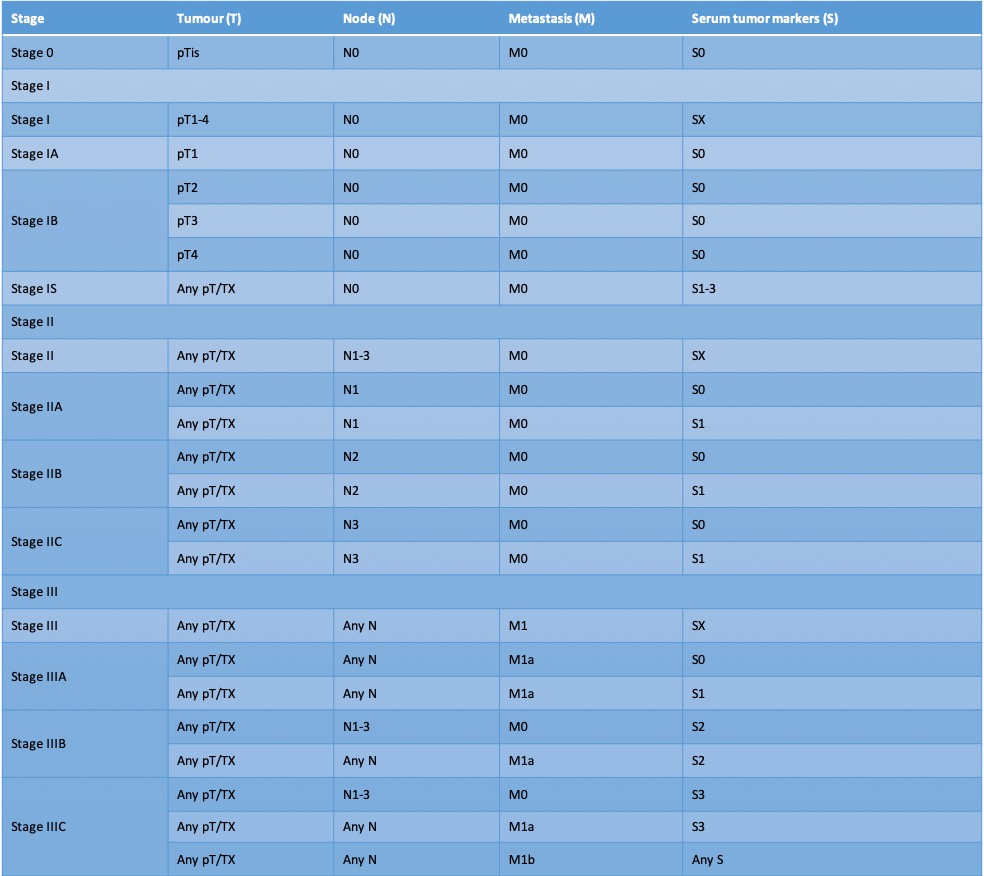
Staging of testicular germ cell tumors is commonly performed using the American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging; it also includes a serum tumor marker (S) classification.[108] Notably, a patient is staged only once and this staging is determined only after
Tumor (T) Classification
- pTX: primary tumor cannot be assessed
- pT0: No evidence of primary tumors
- pTis: germ cell neoplasia in situ
- pT1: tumor limited to the testis (including rete testis invasion) without lymphovascular invasion
- pT1a: tumor <3 cm in size
- pT1b: tumor ≥3 cm in size
- pT2: tumor limited to the testis (including rete testis invasion) with lymphovascular invasion, or tumor invading hilar soft tissue or epididymis or penetrating visceral mesothelial layer covering the external surface of tunica albuginea with or without lymphovascular invasion
- pT3: tumor invades spermatic cord with or without lymphovascular invasion
- pT4: tumor invades scrotum with or without lymphovascular invasion
Clinical Node (N) Classification (imaging-based)
- cNX: regional lymph nodes cannot be assessed
- cN0: No clinically enlarged regional lymph nodes
- cN1: Imaging reveals one or more enlarged lymph nodes with no node larger than 2cm in greatest dimension
- cN2: Imaging reveals at least one enlarged lymph node larger than 2cm in greatest dimension but less than 5cm in greatest dimension
- cN3: Imaging reveals at least one enlarged lymph node larger than 5cm in greatest dimension
Pathologic Node (N) Classification (obtained after retroperitoneal lymph node dissection)
- pNX: regional lymph nodes cannot be assessed
- pN0: No regional lymph node metastasis
- pN1: metastasis with a lymph node mass ≤2 cm in the greatest dimension and ≤5 nodes positive (none >2 cm in the greatest dimension)
- pN2: metastasis with a lymph node mass >2 cm but not >5 cm in the greatest dimension; or >5 nodes positive (none >5 cm); or evidence of extra-nodal extension of tumor
- pN3: metastasis with a lymph node mass >5 cm in the greatest dimension
Metastasis (M) Classification
- M0: no distant metastases
- M1a: nonregional nodal or lung metastases
- M1b: distant metastases other than nonregional nodal or lung
TNM Descriptors
p indicates pathological classification. For identification of special cases of TNM or pTNM classifications, the m suffix and y and r prefixes are used. The m suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pT(m)NM. The y prefix indicates those patients in which classification is performed during or following initial multimodality therapy. The r prefix indicates a recurrent tumor when staged after a documented disease-free interval: rTNM.
Serum Markers (S)
- SX: marker studies not available or not performed
- S0: marker study levels within normal limits
- S1: LDH <1.5 × ULN and HCG <5,000 mIU/ml and AFP <1,000 ng/ml
- S2: LDH 1.5–10.0 × ULN or HCG 5,000–50,000 mIU/ml or AFP 1,000 to 10,000 ng/ml
- S3: LDH >10.0 × ULN or HCG >50,000 mIU/ml or AFP >10,000 ng/ml
The S stage is based on post-orchiectomy tumor markers. For patients undergoing chemotherapy, the S stage should be based on the tumor marker levels on the first day of the first cycle of chemotherapy.[109]
The tables outline various clinical stages.
Prognosis
Prognosis is majorly determined by the histology, extent of distant tumor spread, and extent of tumor marker elevations. For men with disseminated seminomas, the main adverse prognostic variable is the presence of metastases to visceral organs other than the lungs. A tumor that originated in the mediastinum has a worse prognosis when compared to a tumor that originated within the testicle. Nonetheless, even patients with widespread metastases at presentation, including those with brain metastases, may have a curable disease and should be treated with this intent.[110]
Several studies have suggested that a larger tumor size (at least 3 to 4 cm) is predictive of recurrence for seminomas.[111][65] Invasion of rete testis and lymphovascular invasion have also been shown to predict the recurrence of TGCTs.[64][112][65][113][114] A new biomarker, CXC-chemokine ligand 12 (CXCL12), has provisionally been shown to independently predict recurrence in non-seminomatous germ cell tumors.[115]
Complications
Complications due to testicular malignancy can be broadly classified into two groups:
Complications secondary to the disease itself:
Complications secondary to the treatment:
- Hypogonadism, leading to depression, sexual problems, and decreased physical well-being[119][120][121]
- Peripheral neuropathy (cisplatin use)[122][123]
- Hearing loss (cisplatin use)[122]
- Tinnitus (cisplatin use)
- Raynaud phenomenon (cisplatin use)
- Secondary malignancies[124][125]
- Cardiovascular disease[126][127]
- Infertility[128]
- Infections[129]
- Surgical complications(antegrade ejaculation failure, small bowel obstruction, etc.)[130][131]
Postoperative and Rehabilitation Care
Following radical inguinal orchiectomy, patients should avoid heavy lifting and high-impact activities for four weeks and should wear supportive underwear to prevent scrotal swelling or hematoma. Retroperitoneal lymph node dissection is major intra-abdominal surgery, after which dedicated postoperative rehabilitation should be undertaken.
Consultations
Treatment of testicular cancer commonly involves primary care physicians, urologists, medical oncologists, and radiation oncologists. Fertility services may be required for sperm banking.
Deterrence and Patient Education
Testicles are the male reproductive glands located within the scrotum. Testicular cancer is cancer that develops from the cells in the testicles. Testicular cancer is the most common cancer in young men and is one of the most curable of all cancers. More than 95 percent of all men diagnosed with testicular cancer survive their disease. It most commonly presents a painless hard mass within the scrotum, in the testicles, mostly on only one side, and uncommonly on both sides. When cancer spreads to other sites such as the lungs, brain, abdomen, neck, etc., symptoms including nausea, vomiting, stomach upset, cough, shortness of breath, weakness, sensory disturbances, abdominal pain, lumps in the neck/groin areas, and back pain, can occur. Prompt evaluation is important to ensure early diagnosis and treatment and decrease the burden of treatment in advanced disease—medical evaluation, physical examination, and ultrasound of the testis. Serum tumor markers and cross-sectional imaging follow. When suspicion for metastatic disease is present, additional imaging with computed tomography (CT) of the chest and abdomen may be done for staging.
Both definitive diagnosis and initial management can be achieved by removing the mass along with the testicle. Further surgery or radiotherapy or chemotherapy will be based on the disease's stage and response to the initial management. In summary, it is one of the most curable cancers with excellent survival rates; having awareness about the disease and seeking prompt healthcare evaluation is of utmost importance.
Pearls and Other Issues
No significant difference in clinical outcomes was observed with screening for testicular cancer in men with a family history of testicular cancer. Routine screening (with ultrasonography) should not be performed in men with no palpable testicular mass. The risk of testicular cancer can be lowered by prepubertal orchiopexy in men with cryptorchidism. In such individuals, a twofold to a sixfold higher risk of developing testicular cancer has been observed in men who undergo orchiopexy after age 12 when compared to orchiopexy before age 12.[132] In men with testicular cancer, adequate counseling should be given regarding the possibility of infertility and testicular prosthesis. Sperm banking should also be considered.
Enhancing Healthcare Team Outcomes
A collaborative team approach is essential in the evaluation and management of testicular cancer. Excellent cure rates in testicular cancer are a testament to multimodal evaluation and treatment. Testicular cancer is exquisitely sensitive to platinum-based chemotherapy and careful surgical management. The chain of care begins with the patient himself (noticing a palpable mass and seeking prompt medical evaluation), followed by prompt initiation of investigations by the primary care physician, followed by appropriate referrals to radiology, medical oncology, urology, and radiation oncology. Other services making a significant impact in the management of testicular cancer, both pre-treatment, and post-treatment, include physical therapy, pain medicine, pharmacists, nursing staff, and fertility counselors. Effective communication and utilization of expert skills with clearly defined responsibilities and care coordination amongst all the above individuals or services are required to enhance patient-centered care and to improve clinical outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Park JS, Kim J, Elghiaty A, Ham WS. Recent global trends in testicular cancer incidence and mortality. Medicine. 2018 Sep:97(37):e12390. doi: 10.1097/MD.0000000000012390. Epub [PubMed PMID: 30213007]
Boccellino M, Vanacore D, Zappavigna S, Cavaliere C, Rossetti S, D'Aniello C, Chieffi P, Amler E, Buonerba C, Di Lorenzo G, Di Franco R, Izzo A, Piscitelli R, Iovane G, Muto P, Botti G, Perdonà S, Caraglia M, Facchini G. Testicular cancer from diagnosis to epigenetic factors. Oncotarget. 2017 Nov 28:8(61):104654-104663. doi: 10.18632/oncotarget.20992. Epub 2017 Sep 18 [PubMed PMID: 29262668]
Smith ZL, Werntz RP, Eggener SE. Testicular Cancer: Epidemiology, Diagnosis, and Management. The Medical clinics of North America. 2018 Mar:102(2):251-264. doi: 10.1016/j.mcna.2017.10.003. Epub 2017 Dec 21 [PubMed PMID: 29406056]
Baird DC, Meyers GJ, Hu JS. Testicular Cancer: Diagnosis and Treatment. American family physician. 2018 Feb 15:97(4):261-268 [PubMed PMID: 29671528]
Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. International journal of andrology. 2010 Apr:33(2):298-303. doi: 10.1111/j.1365-2605.2009.01050.x. Epub 2010 Feb 4 [PubMed PMID: 20132348]
Level 3 (low-level) evidenceGrasso C, Zugna D, Fiano V, Robles Rodriguez N, Maule M, Gillio-Tos A, Ciuffreda L, Lista P, Segnan N, Merletti F, Richiardi L. Subfertility and Risk of Testicular Cancer in the EPSAM Case-Control Study. PloS one. 2016:11(12):e0169174. doi: 10.1371/journal.pone.0169174. Epub 2016 Dec 30 [PubMed PMID: 28036409]
Level 2 (mid-level) evidenceHughes IA, Houk C, Ahmed SF, Lee PA, Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group. Consensus statement on management of intersex disorders. Journal of pediatric urology. 2006 Jun:2(3):148-62. doi: 10.1016/j.jpurol.2006.03.004. Epub 2006 May 23 [PubMed PMID: 18947601]
Level 3 (low-level) evidencePeng X, Zeng X, Peng S, Deng D, Zhang J. The association risk of male subfertility and testicular cancer: a systematic review. PloS one. 2009:4(5):e5591. doi: 10.1371/journal.pone.0005591. Epub 2009 May 18 [PubMed PMID: 19440348]
Level 1 (high-level) evidenceKharazmi E, Hemminki K, Pukkala E, Sundquist K, Tryggvadottir L, Tretli S, Olsen JH, Fallah M. Cancer Risk in Relatives of Testicular Cancer Patients by Histology Type and Age at Diagnosis: A Joint Study from Five Nordic Countries. European urology. 2015 Aug:68(2):283-9. doi: 10.1016/j.eururo.2014.12.031. Epub 2015 Apr 23 [PubMed PMID: 25913387]
Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Frontiers in endocrinology. 2013:4():32. doi: 10.3389/fendo.2013.00032. Epub 2013 Mar 20 [PubMed PMID: 23519268]
Leslie SW, Sajjad H, Villanueva CA. Cryptorchidism. StatPearls. 2025 Jan:(): [PubMed PMID: 29261861]
Del Risco Kollerud R, Ruud E, Haugnes HS, Cannon-Albright LA, Thoresen M, Nafstad P, Vlatkovic L, Blaasaas KG, Næss Ø, Claussen B. Family history of cancer and risk of paediatric and young adult's testicular cancer: A Norwegian cohort study. British journal of cancer. 2019 May:120(10):1007-1014. doi: 10.1038/s41416-019-0445-2. Epub 2019 Apr 10 [PubMed PMID: 30967648]
Hemminki K, Chen B. Familial risks in testicular cancer as aetiological clues. International journal of andrology. 2006 Feb:29(1):205-10 [PubMed PMID: 16466541]
Level 2 (mid-level) evidenceGarolla A, Vitagliano A, Muscianisi F, Valente U, Ghezzi M, Andrisani A, Ambrosini G, Foresta C. Role of Viral Infections in Testicular Cancer Etiology: Evidence From a Systematic Review and Meta-Analysis. Frontiers in endocrinology. 2019:10():355. doi: 10.3389/fendo.2019.00355. Epub 2019 Jun 12 [PubMed PMID: 31263452]
Level 1 (high-level) evidenceCoupland CA, Chilvers CE, Davey G, Pike MC, Oliver RT, Forman D. Risk factors for testicular germ cell tumours by histological tumour type. United Kingdom Testicular Cancer Study Group. British journal of cancer. 1999 Aug:80(11):1859-63 [PubMed PMID: 10468310]
Level 2 (mid-level) evidenceHaughey BP, Graham S, Brasure J, Zielezny M, Sufrin G, Burnett WS. The epidemiology of testicular cancer in upstate New York. American journal of epidemiology. 1989 Jul:130(1):25-36 [PubMed PMID: 2568087]
Level 2 (mid-level) evidenceDepue RH, Pike MC, Henderson BE. Estrogen exposure during gestation and risk of testicular cancer. Journal of the National Cancer Institute. 1983 Dec:71(6):1151-5 [PubMed PMID: 6140323]
Kuczyk MA, Serth J, Bokemeyer C, Jonassen J, Machtens S, Werner M, Jonas U. Alterations of the p53 tumor suppressor gene in carcinoma in situ of the testis. Cancer. 1996 Nov 1:78(9):1958-66 [PubMed PMID: 8909317]
Bosl GJ, Motzer RJ. Testicular germ-cell cancer. The New England journal of medicine. 1997 Jul 24:337(4):242-53 [PubMed PMID: 9227931]
Andreassen KE, Kristiansen W, Karlsson R, Aschim EL, Dahl O, Fosså SD, Adami HO, Wiklund F, Haugen TB, Grotmol T. Genetic variation in AKT1, PTEN and the 8q24 locus, and the risk of testicular germ cell tumor. Human reproduction (Oxford, England). 2013 Jul:28(7):1995-2002. doi: 10.1093/humrep/det127. Epub 2013 May 2 [PubMed PMID: 23639623]
Loveday C, Litchfield K, Levy M, Holroyd A, Broderick P, Kote-Jarai Z, Dunning AM, Muir K, Peto J, Eeles R, Easton DF, Dudakia D, Orr N, Pashayan N, Reid A, Huddart RA, Houlston RS, Turnbull C. Validation of loci at 2q14.2 and 15q21.3 as risk factors for testicular cancer. Oncotarget. 2018 Feb 27:9(16):12630-12638. doi: 10.18632/oncotarget.23117. Epub 2017 Dec 7 [PubMed PMID: 29560096]
Level 1 (high-level) evidenceLitchfield K, Loveday C, Levy M, Dudakia D, Rapley E, Nsengimana J, Bishop DT, Reid A, Huddart R, Broderick P, Houlston RS, Turnbull C. Large-scale Sequencing of Testicular Germ Cell Tumour (TGCT) Cases Excludes Major TGCT Predisposition Gene. European urology. 2018 Jun:73(6):828-831. doi: 10.1016/j.eururo.2018.01.021. Epub 2018 Feb 9 [PubMed PMID: 29433971]
Level 3 (low-level) evidenceLooijenga LH, Gillis AJ, Stoop H, Hersmus R, Oosterhuis JW. Relevance of microRNAs in normal and malignant development, including human testicular germ cell tumours. International journal of andrology. 2007 Aug:30(4):304-14; discussion 314-5 [PubMed PMID: 17573854]
Gajendran VK,Nguyen M,Ellison LM, Testicular cancer patterns in African-American men. Urology. 2005 Sep; [PubMed PMID: 16140086]
Ehrlich Y, Margel D, Lubin MA, Baniel J. Advances in the treatment of testicular cancer. Translational andrology and urology. 2015 Jun:4(3):381-90. doi: 10.3978/j.issn.2223-4683.2015.06.02. Epub [PubMed PMID: 26816836]
Level 3 (low-level) evidenceFosså SD, Chen J, Schonfeld SJ, McGlynn KA, McMaster ML, Gail MH, Travis LB. Risk of contralateral testicular cancer: a population-based study of 29,515 U.S. men. Journal of the National Cancer Institute. 2005 Jul 20:97(14):1056-66 [PubMed PMID: 16030303]
Chieffi P, Chieffi S. Molecular biomarkers as potential targets for therapeutic strategies in human testicular germ cell tumors: an overview. Journal of cellular physiology. 2013 Aug:228(8):1641-6. doi: 10.1002/jcp.24328. Epub [PubMed PMID: 23359388]
Level 3 (low-level) evidenceRajpert-De Meyts E, Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Human reproduction update. 2006 May-Jun; [PubMed PMID: 16540528]
Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003 Nov 11:100(23):13350-5 [PubMed PMID: 14595015]
Almstrup K, Hoei-Hansen CE, Nielsen JE, Wirkner U, Ansorge W, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Genome-wide gene expression profiling of testicular carcinoma in situ progression into overt tumours. British journal of cancer. 2005 May 23:92(10):1934-41 [PubMed PMID: 15856041]
Kanetsky PA, Mitra N, Vardhanabhuti S, Li M, Vaughn DJ, Letrero R, Ciosek SL, Doody DR, Smith LM, Weaver J, Albano A, Chen C, Starr JR, Rader DJ, Godwin AK, Reilly MP, Hakonarson H, Schwartz SM, Nathanson KL. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nature genetics. 2009 Jul:41(7):811-5. doi: 10.1038/ng.393. Epub 2009 May 31 [PubMed PMID: 19483682]
Level 2 (mid-level) evidenceTurnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, Ricketts M, Linger R, Nsengimana J, Deloukas P, Huddart RA, Bishop DT, Easton DF, Stratton MR, Rahman N, UK Testicular Cancer Collaboration. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nature genetics. 2010 Jul:42(7):604-7. doi: 10.1038/ng.607. Epub 2010 Jun 13 [PubMed PMID: 20543847]
Rapley EA, Turnbull C, Al Olama AA, Dermitzakis ET, Linger R, Huddart RA, Renwick A, Hughes D, Hines S, Seal S, Morrison J, Nsengimana J, Deloukas P, UK Testicular Cancer Collaboration, Rahman N, Bishop DT, Easton DF, Stratton MR. A genome-wide association study of testicular germ cell tumor. Nature genetics. 2009 Jul:41(7):807-10. doi: 10.1038/ng.394. Epub 2009 May 31 [PubMed PMID: 19483681]
Level 2 (mid-level) evidenceSheikine Y, Genega E, Melamed J, Lee P, Reuter VE, Ye H. Molecular genetics of testicular germ cell tumors. American journal of cancer research. 2012:2(2):153-67 [PubMed PMID: 22432056]
Elzinga-Tinke JE, Dohle GR, Looijenga LH. Etiology and early pathogenesis of malignant testicular germ cell tumors: towards possibilities for preinvasive diagnosis. Asian journal of andrology. 2015 May-Jun:17(3):381-93. doi: 10.4103/1008-682X.148079. Epub [PubMed PMID: 25791729]
Koul S, Houldsworth J, Mansukhani MM, Donadio A, McKiernan JM, Reuter VE, Bosl GJ, Chaganti RS, Murty VV. Characteristic promoter hypermethylation signatures in male germ cell tumors. Molecular cancer. 2002 Nov 28:1():8 [PubMed PMID: 12495446]
Williamson SR, Delahunt B, Magi-Galluzzi C, Algaba F, Egevad L, Ulbright TM, Tickoo SK, Srigley JR, Epstein JI, Berney DM, Members of the ISUP Testicular Tumour Panel. The World Health Organization 2016 classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology. 2017 Feb:70(3):335-346. doi: 10.1111/his.13102. Epub 2016 Dec 14 [PubMed PMID: 27747907]
Bosl GJ, Vogelzang NJ, Goldman A, Fraley EE, Lange PH, Levitt SH, Kennedy BJ. Impact of delay in diagnosis on clinical stage of testicular cancer. Lancet (London, England). 1981 Oct 31:2(8253):970-3 [PubMed PMID: 6117736]
Stephenson A, Eggener SE, Bass EB, Chelnick DM, Daneshmand S, Feldman D, Gilligan T, Karam JA, Leibovich B, Liauw SL, Masterson TA, Meeks JJ, Pierorazio PM, Sharma R, Sheinfeld J. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. The Journal of urology. 2019 Aug:202(2):272-281. doi: 10.1097/JU.0000000000000318. Epub 2019 Jul 8 [PubMed PMID: 31059667]
Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, Emamekhoo H, Feldman DR, Geynisman DM, Hancock SL, LaGrange C, Levine EG, Longo T, Lowrance W, McGregor B, Monk P, Picus J, Pierorazio P, Rais-Bahrami S, Saylor P, Sircar K, Smith DC, Tzou K, Vaena D, Vaughn D, Yamoah K, Yamzon J, Johnson-Chilla A, Keller J, Pluchino LA. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2019 Dec:17(12):1529-1554. doi: 10.6004/jnccn.2019.0058. Epub [PubMed PMID: 31805523]
Level 1 (high-level) evidenceWood L, Kollmannsberger C, Jewett M, Chung P, Hotte S, O'Malley M, Sweet J, Anson-Cartwright L, Winquist E, North S, Tyldesley S, Sturgeon J, Gospodarowicz M, Segal R, Cheng T, Venner P, Moore M, Albers P, Huddart R, Nichols C, Warde P. Canadian consensus guidelines for the management of testicular germ cell cancer. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2010 Apr:4(2):e19-38 [PubMed PMID: 20368885]
Level 3 (low-level) evidenceKreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. AJR. American journal of roentgenology. 2013 Jun:200(6):1215-25. doi: 10.2214/AJR.12.10319. Epub [PubMed PMID: 23701056]
Necas M, Muthupalaniappaan M, Barnard C. Ultrasound morphological patterns of testicular tumours, correlation with histopathology. Journal of medical radiation sciences. 2021 Mar:68(1):21-27. doi: 10.1002/jmrs.426. Epub 2020 Sep 1 [PubMed PMID: 32869524]
Capelouto CC, Clark PE, Ransil BJ, Loughlin KR. A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? The Journal of urology. 1995 Mar:153(3 Pt 2):981-5 [PubMed PMID: 7853587]
Level 1 (high-level) evidenceDonohue JP. Evolution of retroperitoneal lymphadenectomy (RPLND) in the management of non-seminomatous testicular cancer (NSGCT). Urologic oncology. 2003 Mar-Apr:21(2):129-32 [PubMed PMID: 12856641]
Sheinfeld J. Mapping studies and modified templates in nonseminomatous germ cell tumors. Nature clinical practice. Urology. 2007 Feb:4(2):60-1 [PubMed PMID: 17287863]
Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. The Journal of urology. 1982 Aug:128(2):315-20 [PubMed PMID: 7109099]
von Eyben FE. Laboratory markers and germ cell tumors. Critical reviews in clinical laboratory sciences. 2003 Aug:40(4):377-427 [PubMed PMID: 14582602]
Milose JC, Filson CP, Weizer AZ, Hafez KS, Montgomery JS. Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open access journal of urology. 2011 Dec 30:4():1-8. doi: 10.2147/OAJU.S15063. Epub 2011 Dec 30 [PubMed PMID: 24198649]
Hotakainen K, Ljungberg B, Paju A, Rasmuson T, Alfthan H, Stenman UH. The free beta-subunit of human chorionic gonadotropin as a prognostic factor in renal cell carcinoma. British journal of cancer. 2002 Jan 21:86(2):185-9 [PubMed PMID: 11870503]
Level 2 (mid-level) evidenceStenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clinical biochemistry. 2004 Jul:37(7):549-61 [PubMed PMID: 15234236]
Staples J. Alphafetoprotein, cancer, and benign conditions. Lancet (London, England). 1986 Nov 29:2(8518):1277 [PubMed PMID: 2431238]
Level 3 (low-level) evidenceDeshpande N, Chavan R, Bale G, Avanthi US, Aslam M, Ramchandani M, Reddy DN, Ravikanth VV. Hereditary Persistence of Alpha-Fetoprotein Is Associated with the -119G}A Polymorphism in AFP Gene. ACG case reports journal. 2017:4():e33. doi: 10.14309/crj.2017.33. Epub 2017 Mar 1 [PubMed PMID: 28286798]
Level 3 (low-level) evidenceBadia RR, Abe D, Wong D, Singla N, Savelyeva A, Chertack N, Woldu SL, Lotan Y, Mauck R, Ouyang D, Meng X, Lewis CM, Majmudar K, Jia L, Kapur P, Xu L, Frazier AL, Margulis V, Strand DW, Coleman N, Murray MJ, Amatruda JF, Lafin JT, Bagrodia A. Real-World Application of Pre-Orchiectomy miR-371a-3p Test in Testicular Germ Cell Tumor Management. The Journal of urology. 2021 Jan:205(1):137-144. doi: 10.1097/JU.0000000000001337. Epub 2020 Aug 28 [PubMed PMID: 32856980]
Spiekermann M, Belge G, Winter N, Ikogho R, Balks T, Bullerdiek J, Dieckmann KP. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology. 2015 Jan:3(1):78-84. doi: 10.1111/j.2047-2927.2014.00269.x. Epub 2014 Sep 4 [PubMed PMID: 25187505]
Level 2 (mid-level) evidenceEsposito F, Boscia F, Franco R, Tornincasa M, Fusco A, Kitazawa S, Looijenga LH, Chieffi P. Down-regulation of oestrogen receptor-β associates with transcriptional co-regulator PATZ1 delocalization in human testicular seminomas. The Journal of pathology. 2011 May:224(1):110-20. doi: 10.1002/path.2846. Epub 2011 Mar 7 [PubMed PMID: 21381029]
Chieffi P. Aurora B: A new promising therapeutic target in cancer. Intractable & rare diseases research. 2018 May:7(2):141-144. doi: 10.5582/irdr.2018.01018. Epub [PubMed PMID: 29862159]
Portella G, Passaro C, Chieffi P. Aurora B: a new prognostic marker and therapeutic target in cancer. Current medicinal chemistry. 2011:18(4):482-96 [PubMed PMID: 21143115]
Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005 Nov 15:104(10):2092-8 [PubMed PMID: 16206293]
De Martino M, Esposito F, Pellecchia S, Cortez Cardoso Penha R, Botti G, Fusco A, Chieffi P. HMGA1-Regulating microRNAs Let-7a and miR-26a are Downregulated in Human Seminomas. International journal of molecular sciences. 2020 Apr 24:21(8):. doi: 10.3390/ijms21083014. Epub 2020 Apr 24 [PubMed PMID: 32344629]
Franco R, Esposito F, Fedele M, Liguori G, Pierantoni GM, Botti G, Tramontano D, Fusco A, Chieffi P. Detection of high-mobility group proteins A1 and A2 represents a valid diagnostic marker in post-pubertal testicular germ cell tumours. The Journal of pathology. 2008 Jan:214(1):58-64 [PubMed PMID: 17935122]
Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008 Feb 13:299(6):672-84. doi: 10.1001/jama.299.6.672. Epub [PubMed PMID: 18270356]
Paffenholz P, Pfister D, Heidenreich A. Testis-preserving strategies in testicular germ cell tumors and germ cell neoplasia in situ. Translational andrology and urology. 2020 Jan:9(Suppl 1):S24-S30. doi: 10.21037/tau.2019.07.22. Epub [PubMed PMID: 32055482]
Mortensen MS, Lauritsen J, Gundgaard MG, Agerbæk M, Holm NV, Christensen IJ, von der Maase H, Daugaard G. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. European urology. 2014 Dec:66(6):1172-8. doi: 10.1016/j.eururo.2014.07.001. Epub 2014 Jul 23 [PubMed PMID: 25064686]
Level 2 (mid-level) evidenceAparicio J, Maroto P, García Del Muro X, Sánchez-Muñoz A, Gumà J, Margelí M, Sáenz A, Sagastibelza N, Castellano D, Arranz JA, Hervás D, Bastús R, Fernández-Aramburo A, Sastre J, Terrasa J, López-Brea M, Dorca J, Almenar D, Carles J, Hernández A, Germà JR. Prognostic factors for relapse in stage I seminoma: a new nomogram derived from three consecutive, risk-adapted studies from the Spanish Germ Cell Cancer Group (SGCCG). Annals of oncology : official journal of the European Society for Medical Oncology. 2014 Nov:25(11):2173-2178. doi: 10.1093/annonc/mdu437. Epub 2014 Sep 10 [PubMed PMID: 25210015]
Tandstad T, Smaaland R, Solberg A, Bremnes RM, Langberg CW, Laurell A, Stierner UK, Ståhl O, Cavallin-Ståhl EK, Klepp OH, Dahl O, Cohn-Cedermark G. Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish norwegian testicular cancer study group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Feb 20:29(6):719-25. doi: 10.1200/JCO.2010.30.1044. Epub 2011 Jan 4 [PubMed PMID: 21205748]
Level 2 (mid-level) evidenceBeard CJ, Travis LB, Chen MH, Arvold ND, Nguyen PL, Martin NE, Kuban DA, Ng AK, Hoffman KE. Outcomes in stage I testicular seminoma: a population-based study of 9193 patients. Cancer. 2013 Aug 1:119(15):2771-7. doi: 10.1002/cncr.28086. Epub 2013 Apr 30 [PubMed PMID: 23633409]
Chung P, Parker C, Panzarella T, Gospodarowicz MK, Jewett S, Milosevic MF, Catton CN, Bayley AJ, Tew-George B, Moore M, Sturgeon JF, Warde P. Surveillance in stage I testicular seminoma - risk of late relapse. The Canadian journal of urology. 2002 Oct:9(5):1637-40 [PubMed PMID: 12431325]
Tabakin AL, Shinder BM, Kim S, Rivera-Nunez Z, Polotti CF, Modi PK, Sterling JA, Farber NJ, Radadia KD, Parikh RR, Kim IY, Saraiya B, Mayer TM, Singer EA, Jang TL. Retroperitoneal Lymph Node Dissection as Primary Treatment for Men With Testicular Seminoma: Utilization and Survival Analysis Using the National Cancer Data Base, 2004-2014. Clinical genitourinary cancer. 2020 Apr:18(2):e194-e201. doi: 10.1016/j.clgc.2019.10.018. Epub 2019 Nov 6 [PubMed PMID: 31818649]
Daugaard G, Gundgaard MG, Mortensen MS, Agerbæk M, Holm NV, Rørth M, von der Maase H, Christensen IJ, Lauritsen J. Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 1:32(34):3817-23. doi: 10.1200/JCO.2013.53.5831. Epub 2014 Sep 29 [PubMed PMID: 25267754]
Level 2 (mid-level) evidenceKollmannsberger C, Tandstad T, Bedard PL, Cohn-Cedermark G, Chung PW, Jewett MA, Powles T, Warde PR, Daneshmand S, Protheroe A, Tyldesley S, Black PC, Chi K, So AI, Moore MJ, Nichols CR. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Jan 1:33(1):51-7. doi: 10.1200/JCO.2014.56.2116. Epub 2014 Aug 18 [PubMed PMID: 25135991]
Level 2 (mid-level) evidenceAlbers P, Siener R, Krege S, Schmelz HU, Dieckmann KP, Heidenreich A, Kwasny P, Pechoel M, Lehmann J, Kliesch S, Köhrmann KU, Fimmers R, Weissbach L, Loy V, Wittekind C, Hartmann M, German Testicular Cancer Study Group. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jun 20:26(18):2966-72. doi: 10.1200/JCO.2007.12.0899. Epub 2008 May 5 [PubMed PMID: 18458040]
Level 1 (high-level) evidenceTandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, Langberg C, Bremnes RM, Laurell A, Wijkstrøm H, Klepp O. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 May 1:27(13):2122-8. doi: 10.1200/JCO.2008.18.8953. Epub 2009 Mar 23 [PubMed PMID: 19307506]
Stephenson AJ, Bosl GJ, Bajorin DF, Stasi J, Motzer RJ, Sheinfeld J. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. The Journal of urology. 2005 Aug:174(2):557-60; discussion 560 [PubMed PMID: 16006891]
Stephenson AJ, Bosl GJ, Motzer RJ, Kattan MW, Stasi J, Bajorin DF, Sheinfeld J. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Apr 20:23(12):2781-8 [PubMed PMID: 15837993]
Level 2 (mid-level) evidenceStephenson AJ, Bosl GJ, Motzer RJ, Bajorin DF, Stasi JP, Sheinfeld J. Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Dec 10:25(35):5597-602 [PubMed PMID: 18065732]
Level 2 (mid-level) evidenceClassen J, Schmidberger H, Meisner C, Souchon R, Sautter-Bihl ML, Sauer R, Weinknecht S, Köhrmann KU, Bamberg M. Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Mar 15:21(6):1101-6 [PubMed PMID: 12637477]
Level 1 (high-level) evidenceSchmidberger H, Bamberg M, Meisner C, Classen J, Winkler C, Hartmann M, Templin R, Wiegel T, Dornoff W, Ross D, Thiel HJ, Martini C, Haase W. Radiotherapy in stage IIA and IIB testicular seminoma with reduced portals: a prospective multicenter study. International journal of radiation oncology, biology, physics. 1997 Sep 1:39(2):321-6 [PubMed PMID: 9308934]
Level 2 (mid-level) evidenceGarcia-del-Muro X, Maroto P, Gumà J, Sastre J, López Brea M, Arranz JA, Lainez N, Soto de Prado D, Aparicio J, Piulats JM, Pérez X, Germá-Lluch JR. Chemotherapy as an alternative to radiotherapy in the treatment of stage IIA and IIB testicular seminoma: a Spanish Germ Cell Cancer Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Nov 20:26(33):5416-21. doi: 10.1200/JCO.2007.15.9103. Epub 2008 Oct 20 [PubMed PMID: 18936476]
Heidenreich A, Paffenholz P, Nestler T, Pfister D, Daneshmand S. Role of primary retroperitoneal lymph node dissection in stage I and low-volume metastatic germ cell tumors. Current opinion in urology. 2020 Mar:30(2):251-257. doi: 10.1097/MOU.0000000000000736. Epub [PubMed PMID: 31972635]
Level 3 (low-level) evidenceAlbers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J, European Association of Urology. Guidelines on Testicular Cancer: 2015 Update. European urology. 2015 Dec:68(6):1054-68. doi: 10.1016/j.eururo.2015.07.044. Epub 2015 Aug 18 [PubMed PMID: 26297604]
Oldenburg J, Fosså SD, Nuver J, Heidenreich A, Schmoll HJ, Bokemeyer C, Horwich A, Beyer J, Kataja V, ESMO Guidelines Working Group. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2013 Oct:24 Suppl 6():vi125-32. doi: 10.1093/annonc/mdt304. Epub [PubMed PMID: 24078656]
Level 1 (high-level) evidenceAlbers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, Schulze H, Heidenreich A, de Riese W, Loy V, Bierhoff E, Wittekind C, Fimmers R, Hartmann M, German Testicular Cancer Study Group. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Apr 15:21(8):1505-12 [PubMed PMID: 12697874]
. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997 Feb:15(2):594-603 [PubMed PMID: 9053482]
Level 2 (mid-level) evidenceCuline S, Kramar A, Théodore C, Geoffrois L, Chevreau C, Biron P, Nguyen BB, Héron JF, Kerbrat P, Caty A, Delva R, Fargeot P, Fizazi K, Bouzy J, Droz JP, Genito-Urinary Group of the French Federation of Cancer Centers Trial T93MP. Randomized trial comparing bleomycin/etoposide/cisplatin with alternating cisplatin/cyclophosphamide/doxorubicin and vinblastine/bleomycin regimens of chemotherapy for patients with intermediate- and poor-risk metastatic nonseminomatous germ cell tumors: Genito-Urinary Group of the French Federation of Cancer Centers Trial T93MP. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jan 20:26(3):421-7. doi: 10.1200/JCO.2007.13.8461. Epub [PubMed PMID: 18202419]
Level 1 (high-level) evidenceFeldman DR, Lorch A, Kramar A, Albany C, Einhorn LH, Giannatempo P, Necchi A, Flechon A, Boyle H, Chung P, Huddart RA, Bokemeyer C, Tryakin A, Sava T, Winquist EW, De Giorgi U, Aparicio J, Sweeney CJ, Cohn Cedermark G, Beyer J, Powles T. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 Feb 1:34(4):345-51. doi: 10.1200/JCO.2015.62.7000. Epub 2015 Oct 12 [PubMed PMID: 26460295]
Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. International journal of radiation oncology, biology, physics. 2008 Apr 1:70(5):1361-4. doi: 10.1016/j.ijrobp.2007.11.005. Epub [PubMed PMID: 18374223]
Level 3 (low-level) evidenceHerr HW, Sheinfeld J, Puc HS, Heelan R, Bajorin DF, Mencel P, Bosl GJ, Motzer RJ. Surgery for a post-chemotherapy residual mass in seminoma. The Journal of urology. 1997 Mar:157(3):860-2 [PubMed PMID: 9072586]
Sheinfeld J, Bajorin D. Management of the postchemotherapy residual mass. The Urologic clinics of North America. 1993 Feb:20(1):133-43 [PubMed PMID: 8381994]
Albany C, Kesler K, Cary C. Management of Residual Mass in Germ Cell Tumors After Chemotherapy. Current oncology reports. 2019 Jan 21:21(1):5. doi: 10.1007/s11912-019-0758-6. Epub 2019 Jan 21 [PubMed PMID: 30666469]
Cheng L, Zhang S, Eble JN, Beck SD, Foster RS, Wang M, Ulbright TM. Molecular genetic evidence supporting the neoplastic nature of fibrous stroma in testicular teratoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012 Oct:25(10):1432-8. doi: 10.1038/modpathol.2012.99. Epub 2012 Jun 8 [PubMed PMID: 22684226]
Brandli DW, Ulbright TM, Foster RS, Cummings OW, Zhang S, Sweeney CJ, Eble JN, Cheng L. Stroma adjacent to metastatic mature teratoma after chemotherapy for testicular germ cell tumors is derived from the same progenitor cells as the teratoma. Cancer research. 2003 Sep 15:63(18):6063-8 [PubMed PMID: 14522936]
Ehrlich Y, Brames MJ, Beck SD, Foster RS, Einhorn LH. Long-term follow-up of Cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Feb 1:28(4):531-6. doi: 10.1200/JCO.2009.23.0714. Epub 2009 Dec 21 [PubMed PMID: 20026808]
Level 2 (mid-level) evidenceKollmannsberger C, Daneshmand S, So A, Chi KN, Murray N, Moore C, Hayes-Lattin B, Nichols C. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Feb 1:28(4):537-42. doi: 10.1200/JCO.2009.23.0755. Epub 2009 Dec 21 [PubMed PMID: 20026807]
Level 2 (mid-level) evidenceLakes J, Lusch A, Nini A, Albers P. Retroperitoneal lymph node dissection in the setting of elevated markers. Current opinion in urology. 2018 Sep:28(5):435-439. doi: 10.1097/MOU.0000000000000535. Epub [PubMed PMID: 30004909]
Level 3 (low-level) evidenceLoehrer PJ Sr, Einhorn LH, Williams SD. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1986 Apr:4(4):528-36 [PubMed PMID: 3633952]
Nichols CR, Catalano PJ, Crawford ED, Vogelzang NJ, Einhorn LH, Loehrer PJ. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998 Apr:16(4):1287-93 [PubMed PMID: 9552027]
Level 1 (high-level) evidence. Ongoing Clinical Trials in Testicular Cancer: The TIGER Trial. Oncology research and treatment. 2016:39(9):553-6. doi: 10.1159/000448868. Epub 2016 Sep 8 [PubMed PMID: 27614956]
Sharp DS, Carver BS, Eggener SE, Kondagunta GV, Motzer RJ, Bosl GJ, Sheinfeld J. Clinical outcome and predictors of survival in late relapse of germ cell tumor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Dec 1:26(34):5524-9. doi: 10.1200/JCO.2007.15.7453. Epub 2008 Oct 20 [PubMed PMID: 18936477]
Level 2 (mid-level) evidenceOing C, Seidel C, von Amsberg G, Oechsle K, Bokemeyer C. Pharmacotherapeutic treatment of germ cell tumors: standard of care and recent developments. Expert opinion on pharmacotherapy. 2016:17(4):545-60. doi: 10.1517/14656566.2016.1127357. Epub 2015 Dec 31 [PubMed PMID: 26630452]
Level 3 (low-level) evidenceKondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J, Bosl GJ, Motzer RJ. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Sep 20:23(27):6549-55 [PubMed PMID: 16170162]
Adra N, Abonour R, Althouse SK, Albany C, Hanna NH, Einhorn LH. High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Apr 1:35(10):1096-1102. doi: 10.1200/JCO.2016.69.5395. Epub 2016 Nov 21 [PubMed PMID: 27870561]
Grogg JB, Schneider K, Bode PK, Kranzbühler B, Eberli D, Sulser T, Beyer J, Lorch A, Hermanns T, Fankhauser CD. Risk factors and treatment outcomes of 239 patients with testicular granulosa cell tumors: a systematic review of published case series data. Journal of cancer research and clinical oncology. 2020 Nov:146(11):2829-2841. doi: 10.1007/s00432-020-03326-3. Epub 2020 Jul 27 [PubMed PMID: 32719989]
Level 2 (mid-level) evidenceAydin AM, Zemp L, Cheriyan SK, Sexton WJ, Johnstone PAS. Contemporary management of early stage testicular seminoma. Translational andrology and urology. 2020 Jan:9(Suppl 1):S36-S44. doi: 10.21037/tau.2019.09.32. Epub [PubMed PMID: 32055484]
Kvammen Ø, Myklebust TÅ, Solberg A, Møller B, Klepp OH, Fosså SD, Tandstad T. Causes of inferior relative survival after testicular germ cell tumor diagnosed 1953-2015: A population-based prospective cohort study. PloS one. 2019:14(12):e0225942. doi: 10.1371/journal.pone.0225942. Epub 2019 Dec 18 [PubMed PMID: 31851716]
Aass N, Fosså SD, Høst H. Acute and subacute side effects due to infra-diaphragmatic radiotherapy for testicular cancer: a prospective study. International journal of radiation oncology, biology, physics. 1992:22(5):1057-64 [PubMed PMID: 1555953]
Fosså SD, Horwich A, Russell JM, Roberts JT, Cullen MH, Hodson NJ, Jones WG, Yosef H, Duchesne GM, Owen JR, Grosch EJ, Chetiyawardana AD, Reed NS, Widmer B, Stenning SP. Optimal planning target volume for stage I testicular seminoma: A Medical Research Council randomized trial. Medical Research Council Testicular Tumor Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 Apr:17(4):1146 [PubMed PMID: 10561173]
Level 1 (high-level) evidenceAdra N, Einhorn LH. Testicular cancer update. Clinical advances in hematology & oncology : H&O. 2017 May:15(5):386-396 [PubMed PMID: 28591093]
Level 3 (low-level) evidenceGilligan TD, Seidenfeld J, Basch EM, Einhorn LH, Fancher T, Smith DC, Stephenson AJ, Vaughn DJ, Cosby R, Hayes DF, American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Jul 10:28(20):3388-404. doi: 10.1200/JCO.2009.26.4481. Epub 2010 Jun 7 [PubMed PMID: 20530278]
Level 1 (high-level) evidenceKrege S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C, Cavallin-Ståhl E, Classen J, Clemm C, Cohn-Cedermark G, Culine S, Daugaard G, De Mulder PH, De Santis M, de Wit M, de Wit R, Derigs HG, Dieckmann KP, Dieing A, Droz JP, Fenner M, Fizazi K, Flechon A, Fosså SD, del Muro XG, Gauler T, Geczi L, Gerl A, Germa-Lluch JR, Gillessen S, Hartmann JT, Hartmann M, Heidenreich A, Hoeltl W, Horwich A, Huddart R, Jewett M, Joffe J, Jones WG, Kisbenedek L, Klepp O, Kliesch S, Koehrmann KU, Kollmannsberger C, Kuczyk M, Laguna P, Galvis OL, Loy V, Mason MD, Mead GM, Mueller R, Nichols C, Nicolai N, Oliver T, Ondrus D, Oosterhof GO, Paz-Ares L, Pizzocaro G, Pont J, Pottek T, Powles T, Rick O, Rosti G, Salvioni R, Scheiderbauer J, Schmelz HU, Schmidberger H, Schmoll HJ, Schrader M, Sedlmayer F, Skakkebaek NE, Sohaib A, Tjulandin S, Warde P, Weinknecht S, Weissbach L, Wittekind C, Winter E, Wood L, von der Maase H. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): part II. European urology. 2008 Mar:53(3):497-513. doi: 10.1016/j.eururo.2007.12.025. Epub 2007 Dec 26 [PubMed PMID: 18191015]
Level 3 (low-level) evidenceWarde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Nov 15:20(22):4448-52 [PubMed PMID: 12431967]
Level 1 (high-level) evidenceChung P, Daugaard G, Tyldesley S, Atenafu EG, Panzarella T, Kollmannsberger C, Warde P. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer medicine. 2015 Jan:4(1):155-60. doi: 10.1002/cam4.324. Epub 2014 Sep 19 [PubMed PMID: 25236854]
Dunphy CH, Ayala AG, Swanson DA, Ro JY, Logothetis C. Clinical stage I nonseminomatous and mixed germ cell tumors of the testis. A clinicopathologic study of 93 patients on a surveillance protocol after orchiectomy alone. Cancer. 1988 Sep 15:62(6):1202-6 [PubMed PMID: 2842034]
Daugaard G, Gundgaard MG, Mortensen MS, Agerbæk M, Holm NV, Rørth MR, von der Maase H, Jarle Christensen I, Lauritsen J. Surgery After Relapse in Stage I Nonseminomatous Testicular Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Jul 10:33(20):2322. doi: 10.1200/JCO.2014.60.2177. Epub 2015 Jun 1 [PubMed PMID: 26033809]
Gilbert DC, Al-Saadi R, Thway K, Chandler I, Berney D, Gabe R, Stenning SP, Sweet J, Huddart R, Shipley JM. Defining a New Prognostic Index for Stage I Nonseminomatous Germ Cell Tumors Using CXCL12 Expression and Proportion of Embryonal Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 Mar 1:22(5):1265-73. doi: 10.1158/1078-0432.CCR-15-1186. Epub 2015 Oct 9 [PubMed PMID: 26453693]
Orre IJ, Fosså SD, Murison R, Bremnes R, Dahl O, Klepp O, Loge JH, Wist E, Dahl AA. Chronic cancer-related fatigue in long-term survivors of testicular cancer. Journal of psychosomatic research. 2008 Apr:64(4):363-71. doi: 10.1016/j.jpsychores.2008.01.002. Epub [PubMed PMID: 18374735]
Level 2 (mid-level) evidenceMykletun A, Dahl AA, Haaland CF, Bremnes R, Dahl O, Klepp O, Wist E, Fosså SD. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 May 1:23(13):3061-8 [PubMed PMID: 15860864]
Level 2 (mid-level) evidenceWeijl NI, Rutten MF, Zwinderman AH, Keizer HJ, Nooy MA, Rosendaal FR, Cleton FJ, Osanto S. Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000 May:18(10):2169-78 [PubMed PMID: 10811682]
Level 2 (mid-level) evidenceWiechno P, Demkow T, Kubiak K, Sadowska M, Kamińska J. The quality of life and hormonal disturbances in testicular cancer survivors in Cisplatin era. European urology. 2007 Nov:52(5):1448-54 [PubMed PMID: 17544206]
Level 2 (mid-level) evidenceFung C, Fossa SD, Williams A, Travis LB. Long-term Morbidity of Testicular Cancer Treatment. The Urologic clinics of North America. 2015 Aug:42(3):393-408. doi: 10.1016/j.ucl.2015.05.002. Epub 2015 Jun 10 [PubMed PMID: 26216826]
Zaid MA, Gathirua-Mwangi WG, Fung C, Monahan PO, El-Charif O, Williams AM, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Cook R, Althouse SK, Ardeshir-Rouhani-Fard S, Dinh PC, Sesso HD, Einhorn LH, Fossa SD, Travis LB, Platinum Study Group. Clinical and Genetic Risk Factors for Adverse Metabolic Outcomes in North American Testicular Cancer Survivors. Journal of the National Comprehensive Cancer Network : JNCCN. 2018 Mar:16(3):257-265. doi: 10.6004/jnccn.2017.7046. Epub [PubMed PMID: 29523664]
Fung C, Sesso HD, Williams AM, Kerns SL, Monahan P, Abu Zaid M, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Kollmannsberger CK, Cook R, Althouse S, Ardeshir-Rouhani-Fard S, Lipshultz SE, Einhorn LH, Fossa SD, Travis LB, Platinum Study Group. Multi-Institutional Assessment of Adverse Health Outcomes Among North American Testicular Cancer Survivors After Modern Cisplatin-Based Chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Apr 10:35(11):1211-1222. doi: 10.1200/JCO.2016.70.3108. Epub 2017 Feb 27 [PubMed PMID: 28240972]
Gilligan T. Quality of life among testis cancer survivors. Urologic oncology. 2015 Sep:33(9):413-9. doi: 10.1016/j.urolonc.2015.05.018. Epub 2015 Jun 15 [PubMed PMID: 26087970]
Level 2 (mid-level) evidenceKier MG, Hansen MK, Lauritsen J, Mortensen MS, Bandak M, Agerbaek M, Holm NV, Dalton SO, Andersen KK, Johansen C, Daugaard G. Second Malignant Neoplasms and Cause of Death in Patients With Germ Cell Cancer: A Danish Nationwide Cohort Study. JAMA oncology. 2016 Dec 1:2(12):1624-1627. doi: 10.1001/jamaoncol.2016.3651. Epub [PubMed PMID: 27711914]
Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. Journal of the National Cancer Institute. 2005 Sep 21:97(18):1354-65 [PubMed PMID: 16174857]
Fosså SD, Gilbert E, Dores GM, Chen J, McGlynn KA, Schonfeld S, Storm H, Hall P, Holowaty E, Andersen A, Joensuu H, Andersson M, Kaijser M, Gospodarowicz M, Cohen R, Pukkala E, Travis LB. Noncancer causes of death in survivors of testicular cancer. Journal of the National Cancer Institute. 2007 Apr 4:99(7):533-44 [PubMed PMID: 17405998]
Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Oct 1:33(28):3105-15. doi: 10.1200/JCO.2014.60.3654. Epub 2015 Aug 3 [PubMed PMID: 26240226]
Mehta A, Sigman M. Management of the dry ejaculate: a systematic review of aspermia and retrograde ejaculation. Fertility and sterility. 2015 Nov:104(5):1074-81. doi: 10.1016/j.fertnstert.2015.09.024. Epub 2015 Oct 1 [PubMed PMID: 26432530]
Level 1 (high-level) evidenceCullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, Stuart N, Rea D, Bower M, Fernando I, Huddart R, Gollins S, Stanley A, Simple Investigation in Neutropenic Individuals of the Frequency of Infection after Chemotherapy +/- Antibiotic in a Number of Tumours (SIGNIFICANT) Trial Group. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. The New England journal of medicine. 2005 Sep 8:353(10):988-98 [PubMed PMID: 16148284]
Level 1 (high-level) evidenceBaniel J, Sella A. Complications of retroperitoneal lymph node dissection in testicular cancer: primary and post-chemotherapy. Seminars in surgical oncology. 1999 Dec:17(4):263-7 [PubMed PMID: 10588855]
Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. European urology. 2008 Feb:53(2):260-72 [PubMed PMID: 18045770]
Wood HM, Elder JS. Cryptorchidism and testicular cancer: separating fact from fiction. The Journal of urology. 2009 Feb:181(2):452-61. doi: 10.1016/j.juro.2008.10.074. Epub 2008 Dec 13 [PubMed PMID: 19084853]
[Terminally ill patients and medications]. Sykepleien. 1979 Apr 5; [PubMed PMID: 36679]