Introduction
The human brain comprises approximately 86 billion neurons that “talk” to each other using a combination of electrical and chemical (electrochemical) signals. The places where neurons connect and communicate with each other are called synapses. Each neuron has anywhere between a few to hundreds of thousands of synaptic connections, which can be with itself, neighboring neurons, or neurons in other brain regions. A synapse is made up of a presynaptic and postsynaptic terminal. The presynaptic terminal is at the end of an axon, where the electrical signal (the action potential) is converted into a chemical signal (neurotransmitter release). The postsynaptic terminal membrane is less than 50 nanometers away and contains specialized receptors. The neurotransmitter rapidly (in microseconds) diffuses across the synaptic cleft and binds to specific receptors. The type of neurotransmitter released from the presynaptic terminal and the specific receptors on the corresponding postsynaptic terminal are critical in determining the quality and intensity of information transmitted by neurons. The postsynaptic neuron integrates all the signals it receives to determine what it does next, for example, to fire an action potential of its own or not.[1][2]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
Synapses involve many cellular structures, including:
- Neurons consist of a cell body, axons, and dendrites.
- Cell Body contains the nucleus and is the site of metabolic activity. Most neurotransmitters that are eventually released at the synapse are synthesized here.
- Dendrites are small projections from the cell body that serve a receptive role in the neuron's physiology. They receive incoming signals from other neurons and relay them to the cell body, where the signals are integrated, and a response is initiated.
- Axons are generally the outflow tracts of the neuron. It is a cylindrical tube covered by the axolemma and supported by neurofilaments and microtubules. The microtubules help to transport the neurotransmitters from the cell body down to the pre-synaptic terminal, where they are released.
- Synapse is the transmission site from the pre-synaptic to the post-synaptic neuron. The structures found on either side of the synapse vary depending on the type of synapse:
- Axodendritic is a connection formed between the axon of 1 neuron and the dendrite of another. These tend to be excitatory synapses.
- Axosomatic is a direct connection between the axon of 1 neuron and another neuron's cell body. These tend to be inhibitory synapses.
- Axoaxonic is a connection between the terminal of 1 axon and another axon. These synapses serve a regulatory role; the afferent axon modulates the release of neurotransmitters from the efferent axon.
The above discussion focuses on chemical synapses, which involve the release of a chemical neurotransmitter between the 2 neurons. This is the most common type of synapse in the mammalian central nervous system (CNS). However, it is important to note that there are electrical synapses, where electrical current (or signals) pass directly from 1 neuron to another through gap junctions. The differences between the 2 are expanded on in the mechanism section.[3][4]
Development
Two neurons form the neurological synapse, or sometimes a neuron and an anatomical structure (see Figure. Anatomy of Neurons). This topic focuses on 2 neurons composing the synapse. Neurons initially develop from the embryonic neural tube, which has 3 layers:
- The ventricular zone surrounds the central canal of the tube. This tube eventually becomes the ependyma.
- The intermediate zone is formed by dividing cells of the ventricular zone. It stretches from the outermost portion of the ventricular zone to the outermost layer of the neural tube, known as the pial layer.
- Extensions of the nerve cells of the intermediate zone form the marginal zone.
Once myelinated, the intermediate zone forms gray matter, while the nerve processes that make up the marginal zone become white matter. The neurons must then differentiate from their precursors. The order in which they do this is based on their size, with the largest neurons (motor neurons) differentiating first. Around the time of birth, the smaller neurons (sensory neurons) develop, along with glial cells. Glial cells aid in the differentiation of the neurons and facilitate their growth in the direction of their target locations. Later, glial cells participate in the reuptake of excess neurotransmitters in the synaptic cleft.
Mechanism
Synapses
As previously mentioned, there are 2 major types of synapses: electrical and chemical. In mammals, the majority of synapses are chemical. Chemical synapses can be differentiated from electrical synapses by a few distinguishing criteria: they use neurotransmitters to relay the signal and vesicles are used to store and transport the neurotransmitter from the cell body to the terminal; furthermore, the pre-synaptic terminal have a very active membrane and the post-synaptic membrane consists of a thick cell membrane made up of many receptors. In between these 2 membranes is a very distinct cleft (easily visualized with electron microscopy). The chemical neurotransmitter released must diffuse across this cleft to elicit a response in the receptive neuron. Because of this, the synaptic delay, defined as the time it takes for current in the pre-synaptic neuron to be transmitted to the post-synaptic neuron, is approximately 0.5 to 1.0 ms. This differs from the electrical synapse, which typically consists of 2 membranes located much closer to each other than in a chemical synapse. These membranes possess channels formed by proteins known as connexins, which allow the direct passage of current from 1 neuron to the next and do not rely on neurotransmitters. The synaptic delay is significantly shorter in electrical synapses versus chemical synapses.
The rest of the discussion focuses on chemical synapses, which are very diverse. They vary not only in shape and structure but also in the chemical that is transmitted. Synapses can be excitatory or inhibitory and use a variety of chemical molecules and proteins, as discussed below.
Multiple types of neurotransmitters used in synaptic communication include, but are not limited to:
- Acetylcholine (ACh): One of the most important neurotransmitters found in multiple synapses in the body, including, but not limited to, the neuromuscular junction, autonomic ganglia, caudate nucleus, and the limbic system. Generally, ACh is an excitatory neurotransmitter at the neuromuscular junction and in the autonomic ganglia. In the brain, Ach is synthesized in the basal nucleus of Meynert.
- Norepinephrine (NE): The most important molecule in the sympathetic nervous system signaling, except for the sweat glands. NE is mainly found in the locus coeruleus and lateral tegmental nuclei in the brain.
- Dopamine (DA): Dopamine signaling is generally inhibitory. There are 3 major dopaminergic pathways in the brain, the nigrostriatal, mesolimbic, and mesocortical, each serving different roles. One of the most well-known disease states involving dopamine is Parkinson's disease, where there is degeneration of dopaminergic neurons in the substantia nigra.
- Serotonin (5-HT): Produced from tryptophan using tryptophan hydroxylase, which is mostly found in the brain (raphe nucleus) and the gastrointestinal (GI) tract. Serotonin is mostly known for its role as a regulatory neurotransmitter and is implicated in various mood states and diseases.
- Common neurotransmitters include other catecholamines, gamma-aminobutyric acid (GABA), glycine, and glutamic acid.
The easiest approach to understanding synaptic transmission is to think of it as a stepwise process, beginning with synthesizing the neurotransmitter and ending with its release.
- Synthesis: The neurotransmitter is synthesized in the cell body, where it is transmitted down the microtubules of the axon to the presynaptic terminal. Alternatively, it is synthesized directly in the presynaptic terminal from recycled neurotransmitters. The neurotransmitter is then stored in presynaptic vesicles until its release.
- Release: The neurotransmitter is released in a regulated fashion from the pre-synaptic neuron into the synaptic cleft.
- Receptor activation: The neurotransmitter binds to post-synaptic receptors and produces a response in the post-synaptic neuron.
- Signal termination: Some mechanism must terminate the signal, normally by eliminating excess neurotransmitters from the synaptic cleft.
Synthesis
Neurotransmitters are synthesized differently depending on their type. They can be small-molecule chemicals, such as dopamine and serotonin, or small neuropeptides, such as enkephalin.
- Neuropeptides are synthesized in the cell body using the typical protein synthesis and translation pathways (rough endoplasmic reticulum and Golgi apparatus). They are then packaged into large, dense-core vesicles along with a protease. These vesicles are rapidly transported down the axon using microtubular proteins such as kinesin. They are ready to be released when they arrive at the pre-synaptic terminal.
- Small-molecule neurotransmitters are synthesized in the cell body and transported down the axon in small, clear core vesicles. Upon arriving at the pre-synaptic terminal, enzymes modify the small-molecule neurotransmitter, which can then be released from the vesicles into the cleft.
Release
Now that the neurotransmitters are stored in the vesicles in the pre-synaptic terminal, they must be released into the cleft. Along the membrane of the vesicle and the presynaptic membrane are proteins known as SNARE proteins; these proteins are essential in binding the vesicles to the membrane and releasing their contents. The membrane depolarizes as the action potential propagates down the pre-synaptic neuron. Once the action potential arrives at the pre-synaptic terminal, the depolarization of the membrane allows the voltage-dependent calcium channels to open, allowing the rapid influx of calcium into the pre-synaptic terminal. The influx of calcium causes the SNARE proteins to activate and change conformation, allowing the fusion of vesicles to the membrane and releasing their contents. The neurotransmitter spills into the synaptic cleft, and the vesicle membrane is recovered via endocytosis.
Receptor Activation
Once the neurotransmitter binds to the post-synaptic neuron, it can generally activate 1 of 2 types of receptors: a ligand-gated ion channel or a G-protein receptor.
- Ligand-Gated Ion Channel: When the neurotransmitter binds to this receptor, the attached ion channel has a direct opening or closing. In other words, the neurotransmitter acts directly on the target ion channel. This receptor type is described as “fast” because it generally only takes a few milliseconds to produce a response and is terminated very quickly. These receptors can be excitatory or inhibitory, depending on which neurotransmitter is binding to the receptor.
- G-Protein Coupled Receptors: These receptors produce a response (opening or closing an ion channel) by activating a signaling cascade involving secondary messengers. The most common secondary messengers are cyclic adenosine monophosphate (cAMP), inositol triphosphate (IP3), and diacylglycerol (DAG). When the neurotransmitter binds to the receptor, it activates the G-protein, which binds to guanosine triphosphate (GTP) and is activated. This activates the secondary messenger cascade, eventually leading to the phosphorylation of ion channels. Due to multiple steps having to take place to generate the final response, this pathway is generally described as “slow,” and the effects last longer (seconds to minutes).
Signal Termination
Inactivation of the signal must involve clearing the neurotransmitter from the synapse in at least 1 of 3 ways:
- Re-uptake: Re-uptake can either be pre-synaptic or by glial cells. One important point to remember about reuptake is that only small-molecule chemical neurotransmitters can be taken back up. Neuropeptides cannot participate in reuptake; they must be eliminated by other means, such as degradation.
- In pre-synaptic reuptake, the pre-synaptic neuron uses either endocytosis or specific transporters to remove the neurotransmitter from the synapse. This mechanism has the advantage of being recyclable, which prevents the neuron from having to re-synthesize the neurotransmitter every cycle of release.
- In some cases, like with glutamate, a glial cell is involved in the reuptake. Glutamate is toxic to the cell, so it is stored inside the neuron as glutamine. When glutamate is released into the synapse, it be taken up by the glial cell using a specific transporter, converted into glutamine via glutaminase, and then returned to the neuron to be recycled.
- Enzymatic Destruction: The neurotransmitter can be destroyed directly in the cleft or pre-synaptic terminal using certain enzymes. Two major enzymes are involved in the destruction of the neurotransmitter:
- Monoamine Oxidases (MAO): These enzymes oxidize and, therefore, inactivate the monoamines. They do this by using oxygen to remove the amine group. These are split into MAO-A and MAO-B based on substrates. MAO-A is mostly responsible for breaking down serotonin, melatonin, norepinephrine, and epinephrine. Both forms break down dopamine, tyramine, and tryptamine equally. MAO-B also breaks down phenethylamine and benzylamine.
- Catechol-O-Methyltransferase (COMT): Generally, COMT is responsible for degrading catecholamines, including dopamine, epinephrine, and norepinephrine, as well as most substances with a catechol structure.
It is important to note that both of the above enzymes are very frequent targets of therapeutic medications. Eliminating these enzymes allows the neurotransmitter to remain in the synapse for longer, which can be beneficial in eliminating the symptoms of many disease processes.
- Diffusion: In the simplest termination form, the neurotransmitter can simply diffuse out of the synaptic cleft, away from the receptors, and into nearby blood vessels. This decreases the concentration of the neurotransmitter in the synapse, gradually reducing the effect the neurotransmitter has on the post-synaptic neuron.[5][6]
Clinical Significance
The synapse is the fundamental functional unit of neuronal communication. Because of this, diseases that target the synapse can present with severe clinical consequences. A few examples are listed below:
- Myasthenia Gravis is an auto-immune disease process that causes muscle weakness that usually presents in a descending fashion. It can cause ptosis, diminished facial expression, respiratory depression, and other signs/symptoms of weakness. In general, it is worse after activity and better with rest. The pathogenesis of myasthenia gravis involves diminished communication between the neuron and the muscle at the neuromuscular junction (NMJ). This is because antibodies either block or destroy the acetylcholine receptors at the NMJ, preventing the ACh from binding and depolarizing the muscle, inhibiting contraction. These antibodies block step 3 (receptor activation) of the synaptic communication pathway.
- Lambert-Eaton Syndrome is also an auto-immune condition producing dysfunction at the neuromuscular junction; however, it involves the pre-synaptic neuron. Instead of antibodies directed against the ACh receptors as in myasthenia gravis, the antibodies here are directed against the calcium channels on the pre-synaptic neuron. This prevents calcium influx from occurring, which prevents the fusion of vesicles with the pre-synaptic membrane and the release of the neurotransmitters into the synapse. These antibodies prevent step 2 (neurotransmitter release) of the synaptic communication pathway.
- In both disease processes, the causative agent is a toxin produced by a bacteria that acts as a protease that cleaves the SNARE proteins. This prevents the release of neurotransmitters at the junction by inhibiting vesicular fusion.
- Botulism: The botulinum toxin, produced by Clostridium botulinum, prevents the release of acetylcholine, a stimulatory neurotransmitter. This inhibits stimulatory effects, which prevents muscle contraction and causes flaccid paralysis.
- Tetanus: The tetanus toxin, produced by Clostridium tetani, prevents the release of GABA and glycine, both inhibitory neurotransmitters. Specifically, their release is inhibited in the Renshaw cells in the spinal cord. This produces symptoms resembling an upper motor neuron lesion: spastic paralysis, lockjaw, and opisthotonus.
Media
(Click Image to Enlarge)
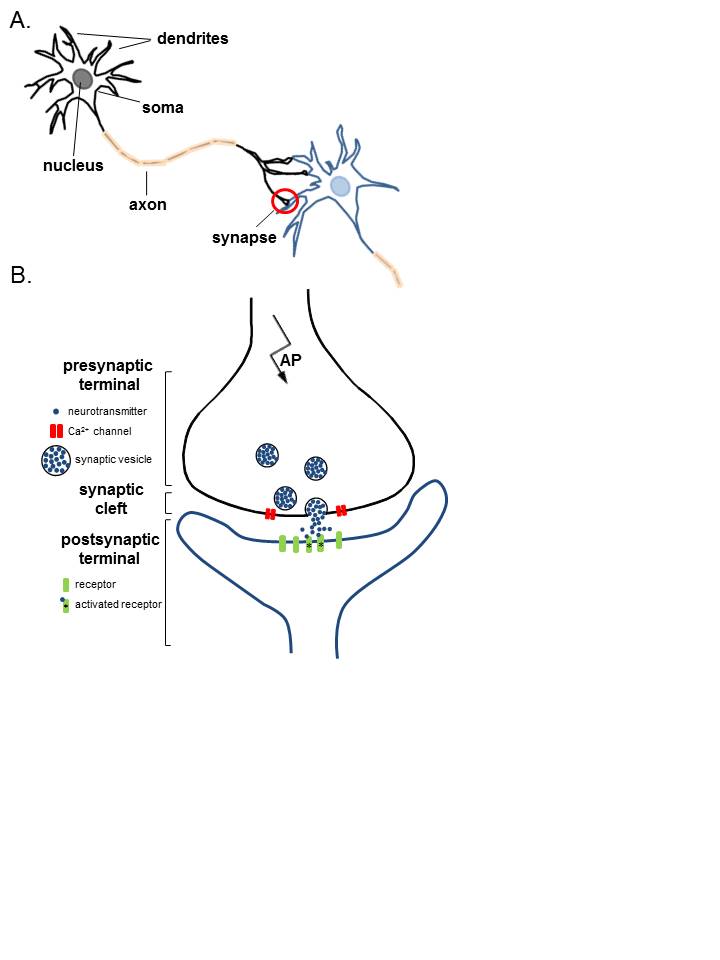
Anatomy of Neurons. A. Two connected neurons. Neurons have a soma that contains a nucleus, an axon, and a dendritic tree. A single synapse (red circle) is formed at the point where an axon's neuron (black) connects to another neuron's dendrite, soma, or axon (blue). B. A magnified view of a single synapse. On the arrival of an action potential at the presynaptic terminal, calcium triggers the release of neurotransmitters from the synaptic vesicles into the synaptic cleft. Neurotransmitters diffuse across the synaptic cleft to activate postsynaptic receptors.
Contributed by K Aubrey, MD
References
Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, Oladiran OA, Gale A, Lamont DJ, Simpson H, Simmen MW, Soeller C, Wishart TM, Gillingwater TH. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell reports. 2017 Nov 28:21(9):2348-2356. doi: 10.1016/j.celrep.2017.11.008. Epub [PubMed PMID: 29186674]
Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. The Journal of comparative neurology. 1988 Aug 8:274(2):168-77 [PubMed PMID: 3209740]
Level 3 (low-level) evidenceHarris AL. Electrical coupling and its channels. The Journal of general physiology. 2018 Dec 3:150(12):1606-1639. doi: 10.1085/jgp.201812203. Epub 2018 Nov 2 [PubMed PMID: 30389716]
Südhof TC. Towards an Understanding of Synapse Formation. Neuron. 2018 Oct 24:100(2):276-293. doi: 10.1016/j.neuron.2018.09.040. Epub [PubMed PMID: 30359597]
Level 3 (low-level) evidenceSüdhof TC. The presynaptic active zone. Neuron. 2012 Jul 12:75(1):11-25. doi: 10.1016/j.neuron.2012.06.012. Epub [PubMed PMID: 22794257]
Level 3 (low-level) evidenceLisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nature reviews. Neuroscience. 2007 Aug:8(8):597-609 [PubMed PMID: 17637801]
Level 3 (low-level) evidence