Introduction
Cardiac arrest is defined as a sudden cessation of cardiac activity resulting in hemodynamic collapse. Sudden cardiac death (SCD) is defined as death presumed to be of a cardiac cause that occurs within 1 hour of the onset of cardiac symptoms or 24 hours of last being seen healthy and alive. Autopsies may reveal a cardiac etiology, though not all SCD cases have an identifiable cause.[1] SCD may be the first presentation of cardiovascular diseases and accounts for half of cardiovascular deaths.[2] SCD has a strong association with age. Men are at a higher risk of SCD as compared to age-matched women. SCD has a low incidence in infancy, but the condition's annual incidence reaches as high as 200 per 100,000 person-years in the 8th decade of life.[3]
Coronary artery disease (CAD) is responsible for more than 75% of SCD cases in the developed world. The incidence of CAD has increased over the last few decades. However, a significant decline in cardiovascular mortality is also evident.[4] Early CAD treatment is the most effective SCD preventive method. Studies show that cardiac arrest and SCD may be the first presentation of CAD in genetically predisposed individuals. Myocardial infarction or ischemia is the typical diagnosis in these patients.[5] Early CAD identification and management of atherosclerotic cardiovascular disease (ASCVD) risk factors are the best strategies for minimizing the risk of cardiac arrest and SCD. Early cardiopulmonary resuscitation (CPR) is paramount in preventing SCD in patients with witnessed cardiac arrest.[6]
In younger individuals with inherited arrhythmias, identifying and appropriately treating the underlying condition can effectively prevent SCD. Implantable cardioverter-defibrillator (ICD) use is the only way to prevent SCD in most inherited cardiac arrhythmias.
Heart Anatomy
The human heart is a muscular organ in the thoracic cavity, slightly left of the center. The heart circulates blood, supplying oxygen and nutrients to tissues and organs. Structurally, the heart consists of 4 chambers: 2 atria and 2 ventricles. The right atrium receives deoxygenated blood returning from the body via the superior and inferior vena cavae, while the left atrium receives oxygenated blood from the lungs through the pulmonary veins. Blood flows from the atria into the ventricles through the atrioventricular valves—the tricuspid valve on the right side and mitral valve on the left. Ventricular contraction pumps blood out of the heart through the semilunar valves—the pulmonary valve on the right ventricle and aortic valve on the left ventricle—into the pulmonary artery and aorta, respectively.
The coronary arteries supply oxygen-rich blood to the heart muscle (myocardium). The left coronary artery arises from the left side of the aorta and branches into 2 main arteries. The left anterior descending artery supplies the anterior surfaces of the left ventricle and interventricular septum. The left circumflex artery supplies the left atrium and posterolateral side of the left ventricle. The right coronary artery, originating from the aorta's right side, supplies the right atrium and ventricle and part of the left ventricle's posterior wall. The coronary arteries ensure the heart receives an adequate blood supply to meet its high metabolic demands.
The cardiac conduction system comprises specialized cells that generate and transmit electrical impulses regulating the heart's rhythm and coordinating its contractions. The sinoatrial node, located in the right atrium near the entrance of the superior vena cava, serves as the heart's natural pacemaker. The electrical impulses then travel through the atria, causing them to contract and forcing blood into the ventricles. The impulses reach the atrioventricular (AV) node, situated at the junction of the atria and ventricles. The impulses are momentarily delayed in the AV node, allowing the ventricles to fill completely before contracting. From the AV node, the impulses travel through specialized conduction pathways—the bundle of His and Purkinje fibers—stimulating the ventricles to contract and pump blood to the lungs and the rest of the body.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The common risk factors for SCA and SCD, as identified in population-based studies, include risk factors for ASCVD, left ventricular hypertrophy, and cardiac conduction abnormalities. Smoking directly predicts the risk of SCD. The Framingham study revealed that people who smoked had a 2.5-fold higher annual SCD incidence than those who never smoked.[7] Population-based studies showed that the risk of SCA and SCD is higher in people with structural heart diseases and ASCVD. SCD risk is relatively low in populations with lower incidences of ASCVD and structural heart diseases.[8]
The etiology varies with age, but CAD is responsible for most cases of SCD overall.[9] In young individuals, inherited cardiac arrhythmias, inherited cardiomyopathies, myocarditis, and coronary artery anomalies are the common SCD causes.[10][11] Up to half of SCD cases in the 4th decade of life arise from acute coronary syndrome (ACS).[12] Rare SCD causes include drug toxicity, coronary artery spasm, and cardiac trauma.
The literature shows that 40% to 80% of the survivors of cardiac arrest are found to have greater than 70% cross-sectional luminal stenosis in at least 1 major coronary artery. These findings vary with the age and gender of the studied population. Although less than 50% of the patients with resuscitated ventricular fibrillation have evidence of myocardial injury, autopsy studies report occlusive coronary artery thrombosis in up to 64% of the patients with SCD. Other common autopsy findings include plaque instability, fissuring, or hemorrhage, and nonobstructive coronary artery thrombosis.[13]
CAD is responsible for most SCD cases in older patients. Other SCD causes include nonischemic cardiomyopathy and valvular heart disease. Inherited arrhythmias are relatively rare in older populations.[14] SCD's cardiac causes are classified into the following groups:
Coronary Artery–Related Causes
- Myocardial infarction or ischemia
- Anomalous coronary artery origin
- Coronary spasm
- Vasculitides
- Myocardial bridging
Primary Arrhythmogenic Diseases
- Long QT syndrome (LQTS)
- Short QT syndrome (SQTS)
- Brugada syndrome
- Early repolarization syndrome
- Catecholaminergic polymorphic ventricular tachycardia (CPVT)
- Idiopathic ventricular fibrillation
- Congenital heart blocks
- Wolf-Parkinson-White syndrome
Cardiomyopathies
- Hypertrophic cardiomyopathy (HCM)
- Arrhythmogenic right ventricular cardiomyopathy (ARVC)
- Myocarditis
- Idiopathic dilated cardiomyopathy
- Noncompaction cardiomyopathy
- Infiltrative cardiomyopathy
- Restrictive cardiomyopathy
- Alcohol-related cardiomyopathy
- Peripartum cardiomyopathy
- Tokatsubo cardiomyopathy
Heart Failure
- Heart failure with reduced ejection fraction
- Heart failure with preserved ejection fraction
Valvular Heart Diseases
- Aortic stenosis
- Mitral valve prolapse
Congenital Heart Diseases
- Tetralogy of Fallot
- Transposition of great arteries
- Fontan circulation
- Ebstein anomaly
- Eisenminger syndrome
- Single ventricular physiology
- Coarctation of aorta
- Double-outlet right ventricle
- Interrupted aortic arch
- Tricuspid atresia
- Pulmonary atresia
- Total anomalous pulmonary venous connection
Miscellaneous
Epidemiology
Approximately 0.1% of the United States population experience a medical services-assessed, out-of-hospital cardiac arrest (OHCA) annually. European studies have a similar incidence, ranging from 0.04% to 0.1% of the population.[17] SCD is estimated to account for 10% to 20% of deaths in Europe, and 300,000 people are brought to the emergency department every year with OHCAs.[18] The median age in the US is between 66 and 68. Male individuals are more likely to experience SCD.[19]
Certain ethnic groups have a higher incidence of SCD than others. The literature reports a higher SCD risk in black than white people, with the difference being more pronounced among women. The reasons behind this ethnic difference include differences in income, education, and traditional risk factors for ASCVD.[20] SCD incidence rises proportionately with age, with the highest risk reported in the 8th decade of life. Overall, men have a higher SCD risk than women.[21]
Inherited cardiac arrhythmias, cardiomyopathies, and coronary artery anomalies are the major SCD causes in younger people. In contrast, CAD is the most common cause of SCD in older patients.[22][23] SCD is the leading cause of nontraumatic cause of death among young athletes. In the general population, sports-related, sudden death from any cause has an incidence of 0.5 to 2.1 per 100,000 yearly. Sports-related, sudden deaths are more frequent in elite than other student-athletes, with an incidence of 1:8,253 per year per the National Collegiate Athletic Association (NCAA). NCAA Division I male basketball players have a 1:5200 incidence of sudden death.[24][25]
SCD's circadian variation is well documented and attributed to circadian variations in the secretion of adrenaline and other hormones. SCD incidence reportedly peaks between 6 am and 12 pm. A small peak occurs during the late afternoon from ventricular fibrillation-induced OHCA. β-adrenergic blocking drugs reduce the early morning SCD peak. The reported incidence of SCD is highest on Mondays.[26]
Many individuals have SCA as their first medical encounter before SCD. Identifying people in the general population at high SCA risk facilitates primary prevention strategies and reduces the risk of SCD and SCA-associated morbidities.[27] Although limited data on the incidence of SCD exists, especially in low-middle-income countries, the global SCD incidence is reported to be as high as 100 cases per 100,000 person-year. Around 2 million SCD cases are reported each year worldwide.[28][29] This high incidence and the associated mortality and morbidity make SCA and SCD major global public health problems.
Pathophysiology
SCD mostly results from an electrical accident in the form of ventricular arrhythmias or asystole. Some patients have an anatomic and functional substrate for developing life-threatening ventricular arrhythmias or asystole. However, many have transient events in the form of myocardial ischemia or infarction, metabolic abnormalities, and drug toxicities leading to ventricular tachyarrhythmias or asystole. This reflects an interplay between the arrhythmogenic substrates and transient events perturbing myocardial hemostasis and predisposing patients to life-threatening arrhythmias, SCA, and SCD.[30]
SCD risk is highest during the first few months after a myocardial infarction due to fatal tachyarrhythmias, reinfarction, or myocardial rupture.[16] Ventricular fibrillation and tachycardia were initially identified as the most common causes of OHCA. However, recent studies show that asystole and pulseless electrical activity (PEA) are the most frequent initial rhythms in OHCAs. Approximately 50% of patients initially have asystole, and 19% to 23% have PEA as the first identifiable rhythm.[31] Blood flow to the brain slows to essentially zero immediately following OHCA, leading to death.
History and Physical
History
People with SCA present to the hospital unconscious, with no cardiac tone and respirations. Immediate resuscitation is warranted in patients in cardiorespiratory arrest, regardless of cause. Airway, breathing, circulation, disability, and exposure must be evaluated during a quick primary survey and addressed without delay. A more detailed investigation may be pursued once the patient is stable and hooked to cardiac and vital signs monitors.
On history, patients may experience palpitations, dizziness, or near-syncope before SCA. Almost half of people who had SCA report no symptoms before losing consciousness.[32] Other signs and symptoms that may be reported by a person who had SCA include the following:
- Chest pain, discomfort, tightness, pressure related to exertion
- Excessive exertional and unexplained dyspnea, fatigue, or palpitations associated with exercise
- Prior recognition of a heart murmur
- Elevated systemic blood pressure
- Sensorineural deafness, which may indicate LQTS
Relevant information that may be elicited during history-taking includes a prior history of cardiogenic or arrhythmia-related syncope or SCA and a family history of SCD or inherited cardiac arrhythmias.[33] Other information that a person who had SCA may disclose in their past medical and family history includes the following:
- Prior restriction from sports participation
- Prior physician-ordered cardiac testing
- Premature death (before age 40) in more than 1 relative attributed to heart disease
- Disability from heart disease in a close relative younger than 50
- Hypertrophic or dilated cardiomyopathy, LQTS or other ion channelopathies, Marfan syndrome, clinically significant arrhythmia, or specific knowledge of certain cardiac conditions in family members
The relevant history provides clues to certain high-risk patients. In individuals with CAD leading to cardiomyopathy and severe left ventricular systolic dysfunction, a history of syncope with a documented ventricular arrhythmia episode, New York Heart Association (NYHA) class III or IV classification, ventricular arrhythmia immediately after myocardial infarction, and previous myocardial infarctions predict high future SCA and SCD risks.[34] A family history of SCD increases the risk of SCD in those with inherited cardiac channelopathies and primary cardiomyopathies.[35]
The American Heart Association recommends cardiovascular screening for high school and collegiate athletes, with physical examination and tools evaluating personal and family history.[36]
Physical Examination
Physical examination findings in people with SCA include unresponsiveness and pulselessness. Blood pressure and cardiac tone are absent. Patients may display agonal respirations or apnea. The skin is usually cyanotic and cold to the touch. Pupils are dilated and nonreactive to light. Muscles become flaccid, with absent bladder and bowel control. Cardiac monitoring may show arrhythmias, commonly asystole, PEA, or ventricular fibrillation.
In contrast, patients with myocardial ischemia not in cardiorespiratory arrest have a range of physical findings. Diaphoresis and pallor are often present. Blood pressure may be elevated or normal, but hypotension may be a sign of cardiac decompensation. Abnormal heart sounds may be appreciated on auscultation. An S3 gallop, S4 sound, and murmurs may be signs of heart failure, which may also manifest with jugular venous distension, bilateral respiratory crackles, an abdominal fluid wave, and bipedal edema. Cardiac monitoring reveals wide rate and rhythm variations. Tachycardia, normocardia, or bradycardia may be noted. Rhythm may be normal with ST and T wave changes or outright abnormal.
Individuals with ACS often have normal neurologic function. However, ACS and stroke may cooccur, with patients exhibiting cardiac abnormalities, eg, new-onset atrial fibrillation, alongside neurologic deficits, eg, new-onset dysphonia or slurred speech. The onset of these signs must be ascertained before deciding to include neuroimaging in the cardiovascular workup. Importantly, people who survive SCA may also manifest neurologic symptoms from brain hypoxia during the arrested state.
Specific physical examination findings may point toward other potential causes of cardiac arrest. A mitral valve prolapse's mid-to-late systolic murmur, HCM's ejection systolic murmur, cyanosis, Tetralogy of Fallot right ventricular outflow murmur, and sarcoidosis' skin signs may help identify the underlying cardiac cause.
Heart murmurs should be evaluated in both supine and standing positions during the cardiovascular examination. Femoral pulses must be evaluated and compared to exclude aortic coarctation. Brachial artery blood pressure may be taken in the sitting position, preferably on both arms.[37]
Evaluation
SCA may be the first manifestation of CAD and other cardiac conditions. Full cardiac assessment is required for individuals who survive this event. Diagnostic tests should include the following:
- Electrocardiogram (ECG) to evaluate for myocardial ischemia or infarction and inherited channelopathies
- Echocardiogram to assess for preexisting heart failure, cardiomyopathies, valvular heart diseases, and congenital cardiac abnormalities
- Coronary angiography for diagnosing ASCVD, coronary artery anomalies, and coronary artery spasms
- Exercise tests in selected patients to evaluate exercise-induced ventricular arrhythmias and ischemia
- Electrophysiology testing in select groups of people to search for cardiac conduction diseases and risk stratification
- Cardiac magnetic resonance imaging (MRI) to establish the diagnosis of cardiomyopathies and assess the risk of SCD
- Genetic testing if the patient has arrhythmogenic right ventricular cardiomyopathy (ARVC), Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT), or LQTS
- A cardiac biopsy may be considered if no other cause is found [38]
The 12-lead ECG helps in diagnosing myocardial infarction or ischemia and inherited channelopathies. Echocardiograms are the best imaging modality for evaluating heart failure, cardiomyopathy, valvular heart disease, and congenital heart disease. Coronary angiography further evaluates CAD, congenital coronary anomalies, and coronary spasms. Exercise testing is helpful for the diagnosis of ischemic heart disease, LQTS, and CPVT. Electrophysiology studies can detect suspected arrhythmia. Procainamide can provoke Brugada syndrome regardless of initial ECG findings. Cardiac MRI is recommended for tissue characterization to diagnose ARVC, sarcoidosis, and myocarditis and estimate the extent of fibrosis with late gadolinium enhancement.[39]
Genetic testing may be recommended in young patients without evidence of structural heart disease but with unexplained syncope or cardiac arrest to identify the inherited channelopathy or arrhythmogenic disorder. Genetic testing may also provide insights into the condition's prognosis and guide family members' cascade screening if a disease-causing genetic mutation is identified in the proband.[40][41]
Recent genome sequencing advances and a better understanding of inherited cardiac disorders have made genetic testing more effective in diagnosing different cardiomyopathies. Genetic testing may be used to screen asymptomatic relatives who may be carriers. Testing guides the appropriate preventive measures for those with a positive genetic mutation in the form of lifestyle modifications, medical therapy, and ongoing monitoring. Contemporary guidelines recommend genetic counseling for patients and their family members when a genetic mutation associated with an inherited arrhythmogenic cardiac disorder is identified.[42]
Treatment / Management
Initial Management
SCA's initial management is a critical and time-sensitive process aimed at restoring cardiac function and improving the chances of survival. Immediate intervention is crucial to increase the likelihood of a positive outcome. Basic (BLS) and Advanced Cardiac Life Support (ACLS) protocols must be performed by trained healthcare professionals.
Resuscitation for cardiac arrest
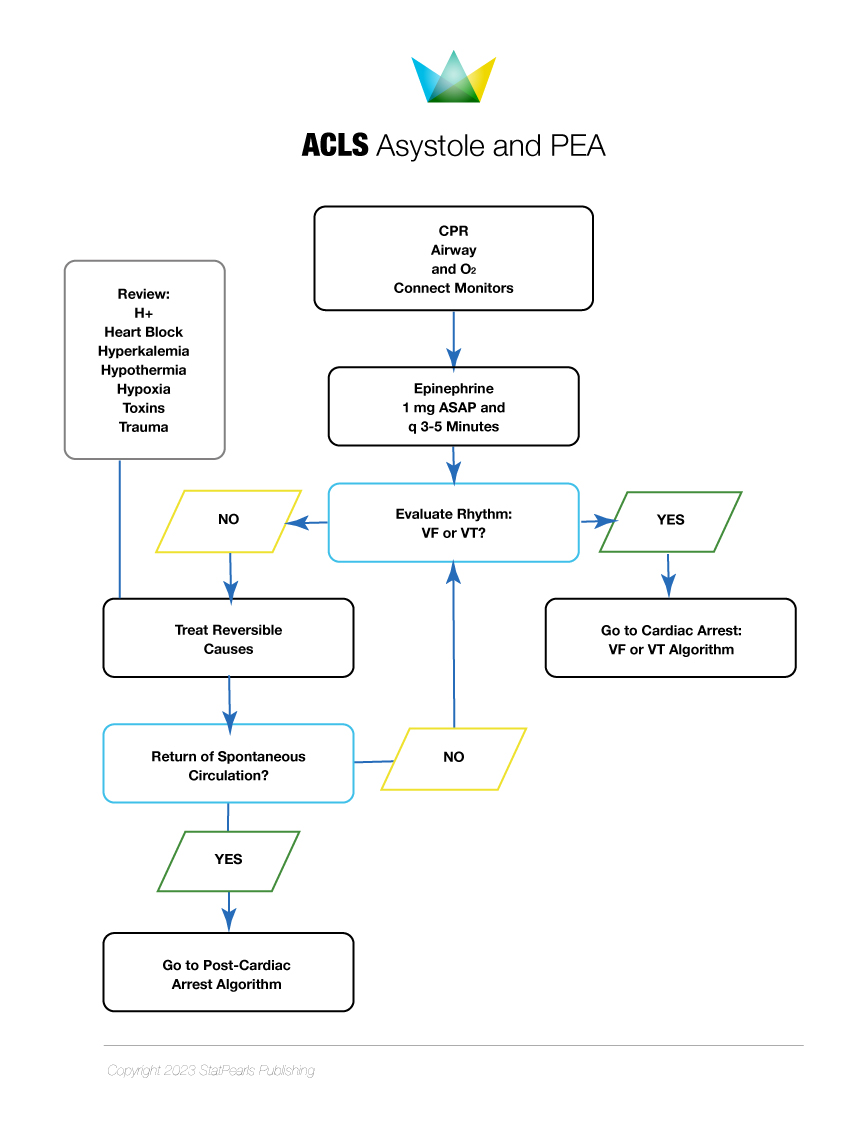
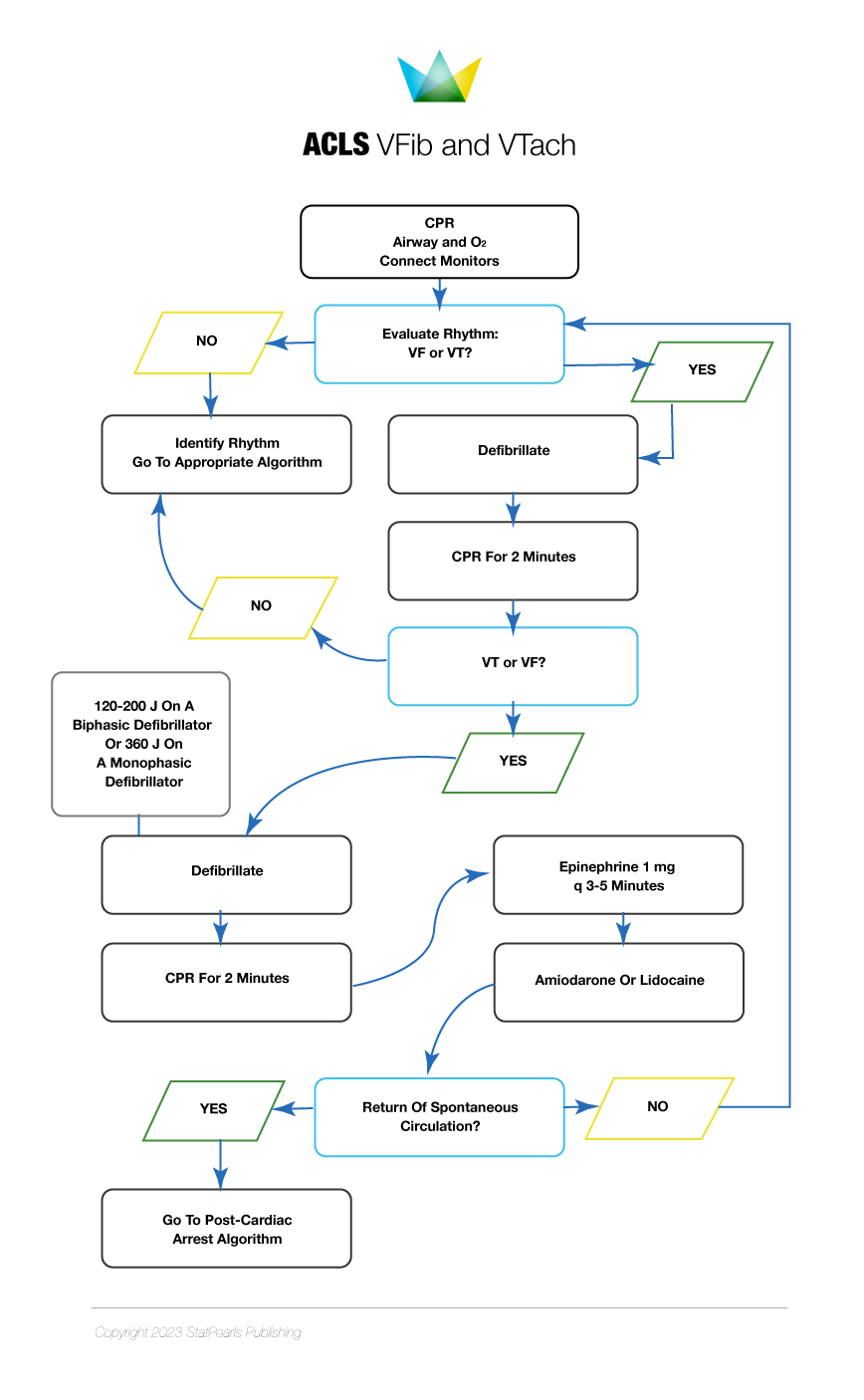
The initial management steps for SCA resuscitation include rapid recognition of cardiac arrest, early and effective CPR, defibrillation, postcardiac arrest care, and treatment of underlying causes. CPR with effective chest compressions and appropriate defibrillator use is paramount for treating SCD. CPR should be performed according to published BLS and ACLS algorithms (see Images. ACLS Algorithm for Asystole and PEA and ACLS Algorithm for VFib and VTach).[43] Early CPR and defibrillation and rapid arrival of emergency medical services (EMS) during cardiac arrest are the major determinants of successful resuscitation.
Strategies that may improve the chances of survival include early CPR initiation for witnessed cardiac arrest, bystander CPR, and rapid defibrillation.[44] Survival after in-hospital cardiac arrest (IHCA) is as high as 90%, especially in intensive care units where the response to ventricular fibrillation is quick. However, survival after OHCA decreases rapidly after the first few minutes from the onset of cardiac arrest. Less than 25% survive at 5 minutes, and close to zero survive 10 minutes after OHCA.[45] (A1)
Intravenous or intraosseous epinephrine every 3 to 5 minutes is an important ACLS component, improving the odds of successful resuscitation. Epinephrine's favorable effects are produced by the α-adrenergic mediated vasoconstriction, which improves coronary and cerebral perfusion pressure during CPR.[46] Amiodarone administration after at least 3 defibrillation attempts in refractory ventricular fibrillation cases increases the rate of successful resuscitation as compared to lidocaine and placebo. However, amiodarone does not improve survival to discharge compared to placebo.[47]
Procainamide administration in OHCA with a shockable rhythm does not have favorable outcomes. The drug has been associated with a higher number of shocks, longer resuscitation times, and a lower survival rate.[48] Cardiac arrest with PEA and asystole have relatively lower chances of survival than ventricular fibrillation. CPR with effective chest compression, epinephrine, and treatment of reversible etiologies is the only way of survival.[49](A1)
Postcardiac arrest care
Patients are evaluated for possible SCA causes after successful resuscitation and return of spontaneous circulation (ROSC). The initial evaluation includes an assessment of airway, hemodynamics, and neurologic status, followed by a 12-lead ECG, baseline laboratory investigations, chest radiograph, ECG, and brain imaging. Cardiac arrest results in multiorgan dysfunction. Death can also occur from shock or dysfunction of organs other than the heart. Postcardiac arrest care is complex. Thus, an interprofessional team with expertise in cardiac arrest care and dedicated postcardiac arrest treatment protocols are critical to improving survival and neurological outcomes after ROSC.[50]
ST-elevation myocardial infarction (STEMI)—diagnosed based on clinical presentation, ECG, and cardiac enzyme levels—should be managed with immediate coronary angiography followed by revascularization (see Image. ST-Elevated Myocardial Infarction on ECG). Percutaneous revascularization can be achieved safely when a postcardiac arrest angiogram reveals significant CAD. Observational studies report improved survival after successful revascularization in patients with SCA.[51] However, in the absence of STEMI, immediate coronary angiography and revascularization do not provide added benefit.[52]
Hypotension may worsen brain injury and outcomes after cardiac arrest due to decreased tissue perfusion. Maintaining systolic blood pressure above 90 mm Hg and mean arterial pressure above 65 mm Hg with proper fluid resuscitation and vasopressor therapy is recommended.[53] Avoiding hypoxia and hypoglycemia is vital in the post-cardiac arrest period, particularly in comatose patients, as these conditions can worsen tissue damage and impact long-term survival.(B2)
Targeted temperature management (32 °C to 35 °C) is recommended for at least 24 hours in patients not following commands after ROSC. Multiple studies document survival and positive neurologic outcomes when targeted temperature management is used after IHCA and OHCA, even in patients with an initially nonshockable rhythm. Fever in the postcardiac arrest period is associated with poor neurological outcomes and thus must be prevented or treated immediately.[54][55](B2)
Hypoxic brain injury is the major contributor to morbidity and mortality in survivors of OHCA and IHCA. A detailed neurological assessment and appropriate prognostication are required to avoid premature withdrawal of life-saving measures in patients who may otherwise achieve neurological recovery. Avoiding unnecessary treatment when a poor outcome is inevitable is helpful.[56] Neuroprognostication is recommended in every comatose patient after 5 days of targeted temperature management. A multimodal approach, including clinical examination, electroencephalogram, and brain imaging, is recommended for neuroprognostication, as a single test may yield false positive or false negative results.[57][58](A1)
Long-Term Management
The long-term management of patients who survive SCA focuses on reducing the risk of recurrence, improving overall prognosis, and addressing potential underlying causes or contributing factors. Patients who survive SCA face unique challenges, including the risk of recurrent arrhythmias, underlying cardiovascular disease, and psychological consequences. Therefore, a comprehensive and interprofessional approach to long-term management is crucial to optimize outcomes and enhance quality of life.
SCD prevention is critical after survival from SCA. Patients have a high risk of SCA recurrence or SCD, especially in the presence of underlying structural heart diseases and primary cardiac arrhythmias. Medical treatment has a limited role in preventing SCD. No antiarrhythmic drugs except for β-adrenergic blocking agents have documented evidence for preventing SCD.[59](A1)
However, the use of antiarrhythmic medications and heart failure therapy is essential in some patients to control arrhythmias and improve symptoms. β-blockers improve survival and reduce SCD risk in patients with left ventricular systolic dysfunction or previous acute myocardial infarction.[60][61] β-blockers, especially propranolol and nadolol, are also recommended as the first-line therapy for preventing the recurrence of arrhythmias and SCA in patients with cardiac channelopathies, eg, LQTS and catecholaminergic polymorphic ventricular tachycardia.[62](A1)
The recommended cardioprotective agents in patients with cardiomyopathy and severely reduced left ventricular systolic function include β-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, angiotensin receptor-neprilysin inhibitors, and sodium-glucose cotransporter-2 inhibitors. These drugs have demonstrated a significant reduction in SCD rate in studies. Contemporary guidelines recommend these drugs for preventing SCD in all patients with heart failure with reduced ejection fraction, irrespective of the underlying cause of left ventricular systolic dysfunction.
Randomized trials demonstrate that ICDs effectively prevent SCD and improve survival in patients who survive SCA due to fatal arrhythmia, as compared to antiarrhythmic drugs.[63] ICD placement is advised to prevent SCD in patients who survive SCA due to ventricular tachycardia or fibrillation without an identified reversible cause. This intervention is supported by class IA recommendations in all contemporary guidelines.[64](A1)
Rehabilitation program
A significant number of patients who survive SCA experience neuropsychiatric disorders, including anxiety, depression, and posttraumatic stress after the event. Easy fatigability is another common symptom in these patients, which may occur due to physical and cognitive impairment.[65] Family members of patients who survive SCA also report significant stress and may benefit from therapy.[66]
Clinical practice guidelines recommend a structured postcardiac arrest rehabilitation program. Patients who survive SCA and their caregivers should undergo periodic, comprehensive assessments for anxiety, depression, and posttraumatic stress disorder (PTSD). Psychosocial support should also be provided to patients and their families. Individuals who survive SCA and their caregivers should receive a detailed discharge plan, including instructions regarding their daily routine.
Differential Diagnosis
The differential diagnoses of SCA and SCD include all causes of syncope and bradyarrhythmias or tachyarrhythmias, including the following:
- Acute myocardial infarction
- Aortic stenosis
- Arrhythmogenic right ventricular cardiomyopathy
- Atrioventricular block
- Brugada syndrome
- Catecholaminergic polymorphic ventricular tachycardia
- Dilated cardiomyopathy
- Ebstein anomaly
- HCM
- Idiopathic ventricular fibrillation
- LQTS
- Mitral stenosis
- Mitral valve prolapse
- Pulmonary embolism
- SQTS
- Teratology of Fallot
- Wolff-Parkinson-White syndrome
Accurately diagnosing SCA allows healthcare providers to identify underlying cardiac conditions, which may require specific management strategies to reduce the risk of recurrence. A thorough medical evaluation and diagnostic examination can help differentiate SCA and SCD from other conditions.
Prognosis
The overall survival of cardiac arrest depends on multiple factors. The global OHCA survival rate is very low. Only 22% of the patients with OHCA reach the emergency department, and only 8.8% survive until hospital discharge. The 1-year survival rate of OHCA is less than 8% in developed countries.[67] SCA survival rate may be improved with early resuscitation, bystander CPR, and defibrillator access in public places.[68][69] Periodic education of public officials and community members about the role of bystander CPR and early defibrillation is critical to improving OHCA survival rates.
Complications
SCA's complications include anoxic brain injury and multiorgan dysfunction. Mental health disorders are also reported in patients who survive SCA and their families. The literature suggests that a significant number of patients who survive cardiac arrest develop anxiety, depression, and PTSD. The incidence of depression is reported to be as high as 45%, while 6% to 15% of patients develop anxiety. PTSD is diagnosed in up to 27% of the survivors of cardiac arrest.[70]
Deterrence and Patient Education
Primary Prevention
SCA has a high mortality. Only a few patients survive OHCA despite current healthcare advances.[71] The first clinical event is almost always fatal, especially in patients who present with ventricular fibrillation. SCA's poor prognosis makes primary prevention critical, as it reduces SCD risk by identification and treatment of high-risk populations.
Primary ASCVD prevention is an effective way to prevent SCD due to myocardial infarction and ischemic cardiomyopathy.[72] Strategies for preventing myocardial infarction-related SCD include early recalculation, optimal heart failure treatment, and implantation of cardiac resynchronization therapy, especially in patients with cardiomyopathy and evidence of left ventricular dyssynchrony. Reduced ejection fraction is reported as an independent SCD predictor. Thus, an ICD is indicated for primary SCD prevention in patients with ischemic cardiomyopathy with an ejection fraction of 35% or less and heart failure symptoms on guideline-directed medical therapy.
The guidelines also recommend ICD placement for primary SCD prevention in people with nonischemic cardiomyopathy, an ejection fraction of 35% or less, and heart failure symptoms on guideline-directed medical therapy. However, ICDs should not be implanted for primary SCD prevention in those with a limited life expectancy due to noncardiac comorbidities.
Most SCD occurs in the presence of normal left ventricular systolic function, and 10% of those patients are found to have arrhythmogenic cardiac conditions. Commonly identifiable arrhythmogenic cardiac conditions include HCM, arrhythmogenic right ventricular cardiomyopathy, and inherited channelopathies. Certain features predict the future risk of ventricular arrhythmias and SCD in patients with inherited arrhythmia syndromes. These features include a family history of premature SCD in first-degree relatives, a history of recurrent syncope, and evidence of nonsustained arrhythmia.[73] In patients with arrhythmogenic cardiac conditions, the contemporary guidelines recommend ICD use for primary SCD prevention in the presence of the abovementioned features.[74]
Patients with inherited channelopathies and arrhythmogenic cardiomyopathies need to adhere to the therapy advised by their cardiologist. Patients with arrhythmia syndromes, symptomatic ventricular arrhythmias, and a family history of SCD require ICDs. Genetic testing of family members identifies asymptomatic individuals carrying a pathogenic mutation, which helps reduce the incidence. Consanguineous marriages, especially in families with inherited cardiac conditions, are reported to increase the incidence of inherited arrhythmias. Thus, genetic counseling is vital in preventing the incidence of SCD in the general population.
Secondary Prevention
Secondary SCA and SCD prevention necessitate patient adherence to long-term management strategies, as previously explained. These strategies focus on addressing underlying structural heart diseases and primary cardiac arrhythmias.
β-blockers have shown efficacy in reducing SCD risk and improving survival, especially in patients with left ventricular systolic dysfunction or previous acute myocardial infarction. Additionally, β-blockers are recommended as first-line therapy for preventing arrhythmia recurrence in patients with cardiac channelopathies. Cardioprotective agents such as β-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, angiotensin receptor-neprilysin inhibitors, and sodium-glucose cotransporter-2 inhibitors have demonstrated significant reductions in SCD rates in patients with cardiomyopathy and severely reduced left ventricular systolic function. ICD placement is the most effective therapy for preventing SCA recurrence due to ventricular tachyarrhythmias.
Mental health issues can contribute to physiological stress responses and increase the risk of arrhythmias or other cardiac events. Providing support and interventions to address mental health concerns can play a significant role in preventing SCA recurrence.
Pearls and Other Issues
SCD refers to an unexpected and abrupt cardiac function cessation. This condition is typically caused by a sudden, life-threatening arrhythmia, disrupting the heart's ability to pump blood effectively. Rarely, the cause of SCD is unknown. Certain risk factors, such as ASCVD, a history of prior heart attack, heart failure, or family history of SCD, increase the likelihood of experiencing a sudden cardiac event. The most significant SCD risk factor is SCA survival. Prompt SCA recognition and resuscitation following BLS and ACLS guidelines are crucial for improving outcomes. Additionally, risk stratification, targeted preventive measures, and ICD use can help reduce the risk of recurrent SCA in high-risk individuals.
Enhancing Healthcare Team Outcomes
SCD and aborted SCA are major public health problems and significant burdens on the healthcare system. Vulnerable patients present with SCD as the first manifestation of the underlying cardiac conditions. CPR and proper SCA management are pivotal in prognostication. In OHCA cases, studies suggest that early SCA identification followed by bystander CPR, timely defibrillation, and early EMS arrival improve patient survival.[75]
While bystanders and EMS personnel are the first SCA responders, an interprofessional team comprising of a cardiologist, electrophysiologist, neurologist, intensive care expert, and geneticist is required to manage SCA and its complications and prevent recurrence in patients who survive the condition. Staff nurses and respiratory therapists are also vital interprofessional team members, assisting in resuscitation and postcardiac arrest care. The rehabilitation team help patients regain functional independence. Mental health professionals can help patients who survive SCA and their families address their psychosocial needs.
The American Heart Association and American College of Cardiology, in collaboration with the Heart Rhythm Society, have developed evidence-based guidelines for managing ventricular arrhythmia and preventing SCD. These guidelines, reviewed by expert committees, are grounded in an extensive literature review to inform treatment approaches for ventricular arrhythmias and inherited cardiac conditions. In cases where literature is lacking or evidence is inadequate, specialist opinion may guide therapeutic decisions.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
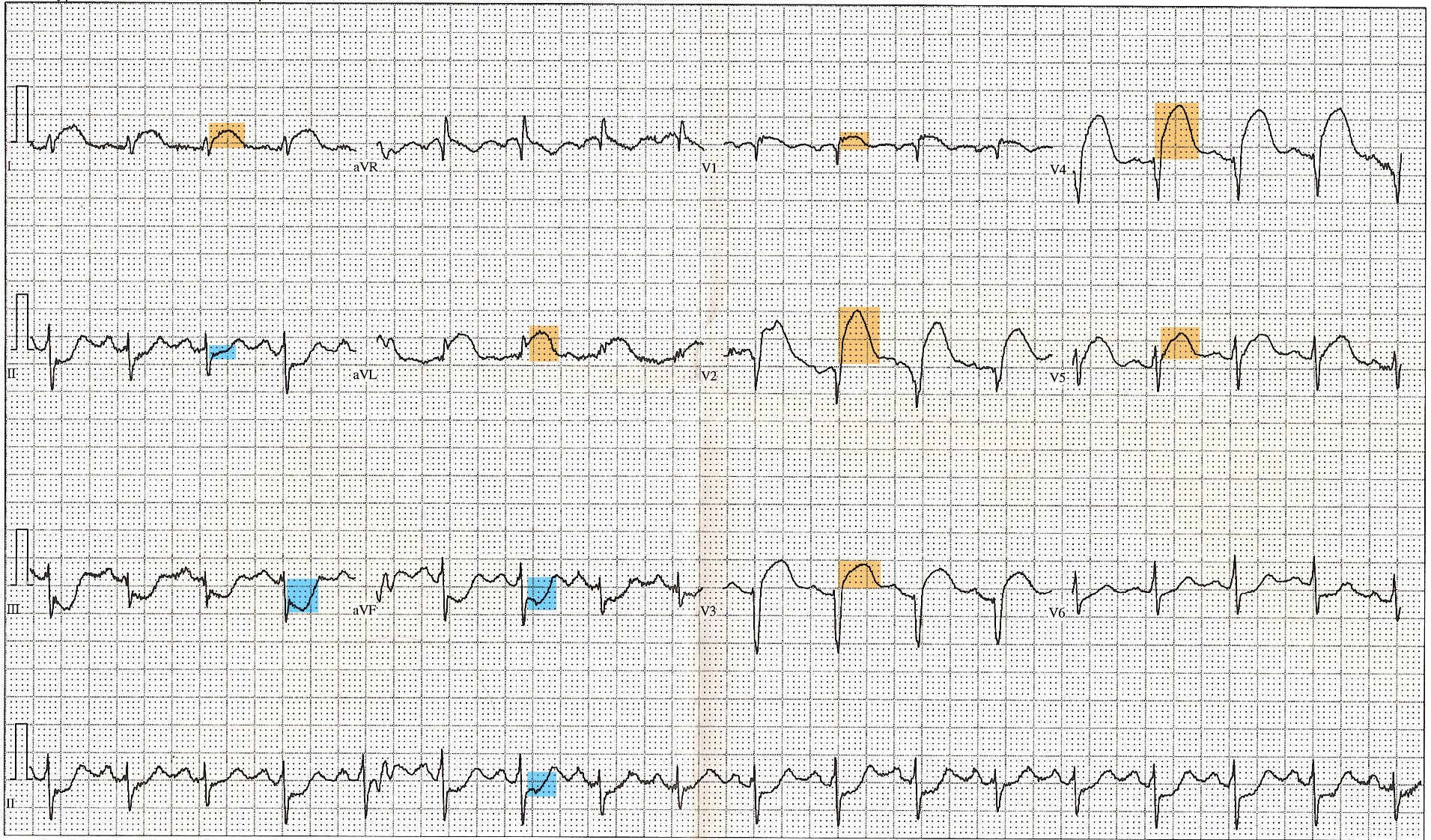
ST-Elevated Myocardial Infarction on Electrocardiogram. This 12-lead electrocardiogram shows ST elevation in the anterior (orange) and inferior (blue) leads. Tachycardia and anterior fascicular block are also noted. A diagnosis of ST-elevated myocardial infarction can be made, along with clinical evaluation and cardiac marker elevation.
Displaced, Public Domain, via Wikimedia Commons
References
ESC Scientific Document Group. [2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death]. Giornale italiano di cardiologia (2006). 2023:24(3 Suppl 1):e1-e132. doi: 10.1714/3986.39669. Epub [PubMed PMID: 36880552]
Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012 Feb 28:125(8):1043-52. doi: 10.1161/CIRCULATIONAHA.111.023846. Epub [PubMed PMID: 22371442]
Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Public health burden of sudden cardiac death in the United States. Circulation. Arrhythmia and electrophysiology. 2014 Apr:7(2):212-7. doi: 10.1161/CIRCEP.113.001034. Epub 2014 Mar 7 [PubMed PMID: 24610738]
Level 2 (mid-level) evidenceDudas K, Lappas G, Stewart S, Rosengren A. Trends in out-of-hospital deaths due to coronary heart disease in Sweden (1991 to 2006). Circulation. 2011 Jan 4:123(1):46-52. doi: 10.1161/CIRCULATIONAHA.110.964999. Epub 2010 Dec 20 [PubMed PMID: 21173352]
Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006 Oct 3:114(14):1462-7 [PubMed PMID: 17000909]
Level 2 (mid-level) evidenceHasselqvist-Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, Ringh M, Jonsson M, Axelsson C, Lindqvist J, Karlsson T, Svensson L. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. The New England journal of medicine. 2015 Jun 11:372(24):2307-15. doi: 10.1056/NEJMoa1405796. Epub [PubMed PMID: 26061835]
Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. The New England journal of medicine. 1997 May 1:336(18):1276-82 [PubMed PMID: 9113930]
Myerburg RJ, Goldberger JJ. Sudden Cardiac Arrest Risk Assessment: Population Science and the Individual Risk Mandate. JAMA cardiology. 2017 Jun 1:2(6):689-694. doi: 10.1001/jamacardio.2017.0266. Epub [PubMed PMID: 28329250]
Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004 Aug 3:110(5):522-7 [PubMed PMID: 15262842]
Level 2 (mid-level) evidenceWinkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunsø S, Tfelt-Hansen J. Nationwide study of sudden cardiac death in persons aged 1-35 years. European heart journal. 2011 Apr:32(8):983-90. doi: 10.1093/eurheartj/ehq428. Epub 2010 Dec 2 [PubMed PMID: 21131293]
Level 2 (mid-level) evidenceEckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, Avedissian L, Pearse LA, Potter RN, Tremaine L, Gentlesk PJ, Huffer L, Reich SS, Stevenson WG, Department of Defense Cardiovascular Death Registry Group. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. Journal of the American College of Cardiology. 2011 Sep 13:58(12):1254-61. doi: 10.1016/j.jacc.2011.01.049. Epub [PubMed PMID: 21903060]
Level 2 (mid-level) evidenceWaldmann V, Karam N, Bougouin W, Sharifzadehgan A, Dumas F, Narayanan K, Spaulding C, Cariou A, Jouven X, Marijon E, Paris-SDEC Investigators. Burden of Coronary Artery Disease as a Cause of Sudden Cardiac Arrest in the Young. Journal of the American College of Cardiology. 2019 Apr 30:73(16):2118-2120. doi: 10.1016/j.jacc.2019.01.064. Epub [PubMed PMID: 31023437]
Roberts WC, Kragel AH, Gertz SD, Roberts CS. Coronary arteries in unstable angina pectoris, acute myocardial infarction, and sudden coronary death. American heart journal. 1994 Jun:127(6):1588-93 [PubMed PMID: 8197987]
Risgaard B, Winkel BG, Jabbari R, Behr ER, Ingemann-Hansen O, Thomsen JL, Ottesen GL, Gislason GH, Bundgaard H, Haunsø S, Holst AG, Tfelt-Hansen J. Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in Denmark. Circulation. Arrhythmia and electrophysiology. 2014 Apr:7(2):205-11. doi: 10.1161/CIRCEP.113.001421. Epub 2014 Mar 6 [PubMed PMID: 24604905]
Level 2 (mid-level) evidenceHayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circulation research. 2015 Jun 5:116(12):1887-906. doi: 10.1161/CIRCRESAHA.116.304521. Epub [PubMed PMID: 26044246]
Katritsis DG, Gersh BJ, Camm AJ. A Clinical Perspective on Sudden Cardiac Death. Arrhythmia & electrophysiology review. 2016:5(3):177-182. doi: 10.15420/aer.2016:11:2. Epub [PubMed PMID: 28116082]
Level 3 (low-level) evidenceTiwari L, Lockey A, Böttiger BW, Rott N, Hoover AV, Chakra Rao S, Garg R, Edara LR, ILCOR WRAH collaborators. More than 302 million people reached and over 2,200,000 trained in cardiopulmonary resuscitation worldwide: The 2021 ILCOR World Restart a Heart initiative. Resuscitation plus. 2023 Jun:14():100375. doi: 10.1016/j.resplu.2023.100375. Epub 2023 Mar 22 [PubMed PMID: 37007185]
Lynge TH, Risgaard B, Banner J, Nielsen JL, Jespersen T, Stampe NK, Albert CM, Winkel BG, Tfelt-Hansen J. Nationwide burden of sudden cardiac death: A study of 54,028 deaths in Denmark. Heart rhythm. 2021 Oct:18(10):1657-1665. doi: 10.1016/j.hrthm.2021.05.005. Epub 2021 May 7 [PubMed PMID: 33965606]
Ågesen FN, Lynge TH, Blanche P, Banner J, Prescott E, Jabbari R, Tfelt-Hansen J. Temporal trends and sex differences in sudden cardiac death in the Copenhagen City Heart Study. Heart (British Cardiac Society). 2021 Aug:107(16):1303-1309. doi: 10.1136/heartjnl-2020-318881. Epub 2021 May 21 [PubMed PMID: 34021040]
. Correction to: Racial Differences in Sudden Cardiac Death: Atherosclerosis Risk in Communities Study (ARIC). Circulation. 2019 Apr 2:139(14):e837. doi: 10.1161/CIR.0000000000000672. Epub [PubMed PMID: 30933613]
Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart, lung & circulation. 2019 Jan:28(1):6-14. doi: 10.1016/j.hlc.2018.08.026. Epub 2018 Sep 24 [PubMed PMID: 30482683]
Level 3 (low-level) evidenceBagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L, Davis AM, Thompson T, Connell V, Wallace J, Naylor C, Crawford J, Love DR, Hallam L, White J, Lawrence C, Lynch M, Morgan N, James P, du Sart D, Puranik R, Langlois N, Vohra J, Winship I, Atherton J, McGaughran J, Skinner JR, Semsarian C. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. The New England journal of medicine. 2016 Jun 23:374(25):2441-52. doi: 10.1056/NEJMoa1510687. Epub [PubMed PMID: 27332903]
Waldmann V, Karam N, Rischard J, Bougouin W, Sharifzadehgan A, Dumas F, Narayanan K, Sideris G, Voicu S, Gandjbakhch E, Jost D, Lamhaut L, Ludes B, Plu I, Beganton F, Wahbi K, Varenne O, Megarbane B, Algalarrondo V, Extramiana F, Lellouche N, Celermajer DS, Spaulding C, Lafont A, Cariou A, Jouven X, Marijon E, On Behalf Paris-SDEC investigators. Low rates of immediate coronary angiography among young adults resuscitated from sudden cardiac arrest. Resuscitation. 2020 Feb 1:147():34-42. doi: 10.1016/j.resuscitation.2019.12.005. Epub 2019 Dec 16 [PubMed PMID: 31857140]
Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL, Ackerman MJ, Drezner JA. Incidence, Cause, and Comparative Frequency of Sudden Cardiac Death in National Collegiate Athletic Association Athletes: A Decade in Review. Circulation. 2015 Jul 7:132(1):10-9. doi: 10.1161/CIRCULATIONAHA.115.015431. Epub 2015 May 14 [PubMed PMID: 25977310]
Level 2 (mid-level) evidenceCurtis AB, Tijssen J. Sex-Related Differences in Sports-Related Sudden Cardiac Death Should Be Reflected in Guideline Screening Recommendations. Journal of the American College of Cardiology. 2023 Mar 21:81(11):1032-1034. doi: 10.1016/j.jacc.2023.01.014. Epub [PubMed PMID: 36922088]
Arntz HR, Willich SN, Schreiber C, Brüggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. European heart journal. 2000 Feb:21(4):315-20 [PubMed PMID: 10653679]
Level 3 (low-level) evidenceNarayan SM, Wang PJ, Daubert JP. New Concepts in Sudden Cardiac Arrest to Address an Intractable Epidemic: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2019 Jan 8:73(1):70-88. doi: 10.1016/j.jacc.2018.09.083. Epub [PubMed PMID: 30621954]
Martens E, Sinner MF, Siebermair J, Raufhake C, Beckmann BM, Veith S, Düvel D, Steinbeck G, Kääb S. Incidence of sudden cardiac death in Germany: results from an emergency medical service registry in Lower Saxony. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014 Dec:16(12):1752-8. doi: 10.1093/europace/euu153. Epub 2014 Jul 24 [PubMed PMID: 25061228]
Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS, Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, Mai JZ, Chu LN, Zhang S. Incidence of sudden cardiac death in China: analysis of 4 regional populations. Journal of the American College of Cardiology. 2009 Sep 15:54(12):1110-8. doi: 10.1016/j.jacc.2009.06.016. Epub [PubMed PMID: 19744622]
de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. Journal of the American College of Cardiology. 1997 Nov 15:30(6):1500-5 [PubMed PMID: 9362408]
Oving I, de Graaf C, Karlsson L, Jonsson M, Kramer-Johansen J, Berglund E, Hulleman M, Beesems SG, Koster RW, Olasveengen TM, Ringh M, Claessen A, Lippert F, Hollenberg J, Folke F, Tan HL, Blom MT. Occurrence of shockable rhythm in out-of-hospital cardiac arrest over time: A report from the COSTA group. Resuscitation. 2020 Jun:151():67-74. doi: 10.1016/j.resuscitation.2020.03.014. Epub 2020 Apr 8 [PubMed PMID: 32278017]
Glinge C, Jabbari R, Risgaard B, Lynge TH, Engstrøm T, Albert CM, Haunsø S, Winkel BG, Tfelt-Hansen J. Symptoms Before Sudden Arrhythmic Death Syndrome: A Nationwide Study Among the Young in Denmark. Journal of cardiovascular electrophysiology. 2015 Jul:26(7):761-7. doi: 10.1111/jce.12674. Epub 2015 May 12 [PubMed PMID: 25807988]
Level 2 (mid-level) evidenceRanthe MF, Winkel BG, Andersen EW, Risgaard B, Wohlfahrt J, Bundgaard H, Haunsø S, Melbye M, Tfelt-Hansen J, Boyd HA. Risk of cardiovascular disease in family members of young sudden cardiac death victims. European heart journal. 2013 Feb:34(7):503-11. doi: 10.1093/eurheartj/ehs350. Epub 2012 Nov 13 [PubMed PMID: 23150455]
Brugada P, Talajic M, Smeets J, Mulleneers R, Wellens HJ. The value of the clinical history to assess prognosis of patients with ventricular tachycardia or ventricular fibrillation after myocardial infarction. European heart journal. 1989 Aug:10(8):747-52 [PubMed PMID: 2792116]
Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998 Jan 20:97(2):155-60 [PubMed PMID: 9445167]
Level 2 (mid-level) evidenceCasa DJ, Almquist J, Anderson SA, Baker L, Bergeron MF, Biagioli B, Boden B, Brenner JS, Carroll M, Colgate B, Cooper L, Courson R, Csillan D, Demartini JK, Drezner JA, Erickson T, Ferrara MS, Fleck SJ, Franks R, Guskiewicz KM, Holcomb WR, Huggins RA, Lopez RM, Mayer T, McHenry P, Mihalik JP, O'Connor FG, Pagnotta KD, Pryor RR, Reynolds J, Stearns RL, Valentine V. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. Journal of athletic training. 2013 Jul-Aug:48(4):546-53. doi: 10.4085/1062-6050-48.4.12. Epub 2013 Jun 6 [PubMed PMID: 23742253]
Sarto P, Zorzi A, Merlo L, Vessella T, Pegoraro C, Giorgiano F, Graziano F, Basso C, Drezner JA, Corrado D. Value of screening for the risk of sudden cardiac death in young competitive athletes. European heart journal. 2023 Mar 21:44(12):1084-1092. doi: 10.1093/eurheartj/ehad017. Epub [PubMed PMID: 36760222]
Somani R, Krahn AD, Healey JS, Chauhan VS, Birnie DH, Champagne J, Sanatani S, Angaran P, Gow RM, Chakrabarti S, Gerull B, Yee R, Skanes AC, Gula LJ, Leong-Sit P, Klein GJ, Gollob MH, Talajic M, Gardner M, Simpson CS. Procainamide infusion in the evaluation of unexplained cardiac arrest: from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER). Heart rhythm. 2014 Jun:11(6):1047-54. doi: 10.1016/j.hrthm.2014.03.022. Epub 2014 Mar 18 [PubMed PMID: 24657429]
Mandoli GE, D'Ascenzi F, Vinco G, Benfari G, Ricci F, Focardi M, Cavigli L, Pastore MC, Sisti N, De Vivo O, Santoro C, Mondillo S, Cameli M. Novel Approaches in Cardiac Imaging for Non-invasive Assessment of Left Heart Myocardial Fibrosis. Frontiers in cardiovascular medicine. 2021:8():614235. doi: 10.3389/fcvm.2021.614235. Epub 2021 Apr 15 [PubMed PMID: 33937354]
Costa J, Lopes CM, Barsheshet A, Moss AJ, Migdalovich D, Ouellet G, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Combined assessment of sex- and mutation-specific information for risk stratification in type 1 long QT syndrome. Heart rhythm. 2012 Jun:9(6):892-8. doi: 10.1016/j.hrthm.2012.01.020. Epub 2012 Jan 28 [PubMed PMID: 22293141]
Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA, Heart Failure Society of America. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. Journal of cardiac failure. 2009 Mar:15(2):83-97. doi: 10.1016/j.cardfail.2009.01.006. Epub [PubMed PMID: 19254666]
Level 1 (high-level) evidence. Correction. Heart rhythm. 2018 Nov:15(11):e276-e277. doi: 10.1016/j.hrthm.2018.09.025. Epub 2018 Sep 27 [PubMed PMID: 30267692]
Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O'Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM, Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020 Oct 20:142(16_suppl_2):S366-S468. doi: 10.1161/CIR.0000000000000916. Epub 2020 Oct 21 [PubMed PMID: 33081529]
Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circulation. Cardiovascular quality and outcomes. 2010 Jan:3(1):63-81. doi: 10.1161/CIRCOUTCOMES.109.889576. Epub 2009 Nov 10 [PubMed PMID: 20123673]
Level 1 (high-level) evidenceWeisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002 Dec 18:288(23):3035-8 [PubMed PMID: 12479769]
Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O'Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015 Nov 3:132(18 Suppl 2):S444-64. doi: 10.1161/CIR.0000000000000261. Epub [PubMed PMID: 26472995]
Kudenchuk PJ, Daya M, Dorian P, Resuscitation Outcomes Consortium Investigators. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. The New England journal of medicine. 2016 Aug 25:375(8):802-3. doi: 10.1056/NEJMc1608041. Epub [PubMed PMID: 27557314]
Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M, Roberts RS. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg . Canadian Implantable Defibrillator Study. European heart journal. 2000 Dec:21(24):2071-8 [PubMed PMID: 11102258]
Level 1 (high-level) evidenceEuropean Heart Rhythm Association, Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology, American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Journal of the American College of Cardiology. 2006 Sep 5:48(5):e247-346 [PubMed PMID: 16949478]
Level 1 (high-level) evidencePanchal AR, Berg KM, Cabañas JG, Kurz MC, Link MS, Del Rios M, Hirsch KG, Chan PS, Hazinski MF, Morley PT, Donnino MW, Kudenchuk PJ. 2019 American Heart Association Focused Update on Systems of Care: Dispatcher-Assisted Cardiopulmonary Resuscitation and Cardiac Arrest Centers: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019 Dec 10:140(24):e895-e903. doi: 10.1161/CIR.0000000000000733. Epub 2019 Nov 14 [PubMed PMID: 31722563]
Kern KB, Lotun K, Patel N, Mooney MR, Hollenbeck RD, McPherson JA, McMullan PW, Unger B, Hsu CH, Seder DB, INTCAR-Cardiology Registry. Outcomes of Comatose Cardiac Arrest Survivors With and Without ST-Segment Elevation Myocardial Infarction: Importance of Coronary Angiography. JACC. Cardiovascular interventions. 2015 Jul:8(8):1031-1040. doi: 10.1016/j.jcin.2015.02.021. Epub 2015 Jun 24 [PubMed PMID: 26117462]
Stær-Jensen H, Nakstad ER, Fossum E, Mangschau A, Eritsland J, Drægni T, Jacobsen D, Sunde K, Andersen GØ. Post-Resuscitation ECG for Selection of Patients for Immediate Coronary Angiography in Out-of-Hospital Cardiac Arrest. Circulation. Cardiovascular interventions. 2015 Oct:8(10):. pii: e002784. doi: 10.1161/CIRCINTERVENTIONS.115.002784. Epub [PubMed PMID: 26453688]
Young MN, Hollenbeck RD, Pollock JS, Giuseffi JL, Wang L, Harrell FE, McPherson JA. Higher achieved mean arterial pressure during therapeutic hypothermia is not associated with neurologically intact survival following cardiac arrest. Resuscitation. 2015 Mar:88():158-64. doi: 10.1016/j.resuscitation.2014.12.008. Epub 2014 Dec 22 [PubMed PMID: 25541429]
Level 2 (mid-level) evidenceSuffoletto B, Peberdy MA, van der Hoek T, Callaway C. Body temperature changes are associated with outcomes following in-hospital cardiac arrest and return of spontaneous circulation. Resuscitation. 2009 Dec:80(12):1365-70. doi: 10.1016/j.resuscitation.2009.08.020. Epub 2009 Oct 4 [PubMed PMID: 19804929]
Gebhardt K, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC, Post Cardiac Arrest Service. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation. 2013 Aug:84(8):1062-7. doi: 10.1016/j.resuscitation.2013.03.038. Epub 2013 Apr 22 [PubMed PMID: 23619740]
Level 2 (mid-level) evidenceGeocadin RG,Callaway CW,Fink EL,Golan E,Greer DM,Ko NU,Lang E,Licht DJ,Marino BS,McNair ND,Peberdy MA,Perman SM,Sims DB,Soar J,Sandroni C, Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation. 2019 Aug 27; [PubMed PMID: 31291775]
Matthews EA, Magid-Bernstein J, Sobczak E, Velazquez A, Falo CM, Park S, Claassen J, Agarwal S. Prognostic Value of the Neurological Examination in Cardiac Arrest Patients After Therapeutic Hypothermia. The Neurohospitalist. 2018 Apr:8(2):66-73. doi: 10.1177/1941874417733217. Epub 2017 Oct 29 [PubMed PMID: 29623156]
Dragancea I, Horn J, Kuiper M, Friberg H, Ullén S, Wetterslev J, Cranshaw J, Hassager C, Nielsen N, Cronberg T, TTM Trial Investigators. Neurological prognostication after cardiac arrest and targeted temperature management 33°C versus 36°C: Results from a randomised controlled clinical trial. Resuscitation. 2015 Aug:93():164-70. doi: 10.1016/j.resuscitation.2015.04.013. Epub 2015 Apr 25 [PubMed PMID: 25921544]
Level 1 (high-level) evidenceEllison KE, Hafley GE, Hickey K, Kellen J, Coromilas J, Stein KM, Lee KL, Buxton AE, Multicenter UnSustained Tachycardia Trial Investigators. Effect of beta-blocking therapy on outcome in the Multicenter UnSustained Tachycardia Trial (MUSTT). Circulation. 2002 Nov 19:106(21):2694-9 [PubMed PMID: 12438295]
Level 1 (high-level) evidencePacker M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. The New England journal of medicine. 1996 May 23:334(21):1349-55 [PubMed PMID: 8614419]
Level 1 (high-level) evidence. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982 Mar 26:247(12):1707-14 [PubMed PMID: 7038157]
Level 1 (high-level) evidenceManolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H, Manolis AS. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends in cardiovascular medicine. 2021 Jul:31(5):290-302. doi: 10.1016/j.tcm.2020.04.011. Epub 2020 May 17 [PubMed PMID: 32434043]
Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The New England journal of medicine. 1997 Nov 27:337(22):1576-83 [PubMed PMID: 9411221]
Level 1 (high-level) evidenceAl-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart rhythm. 2018 Oct:15(10):e73-e189. doi: 10.1016/j.hrthm.2017.10.036. Epub 2017 Oct 30 [PubMed PMID: 29097319]
Level 1 (high-level) evidencePresciutti A, Verma J, Pavol M, Anbarasan D, Falo C, Brodie D, Rabbani LE, Roh DJ, Park S, Claassen J, Agarwal S. Posttraumatic stress and depressive symptoms characterize cardiac arrest survivors' perceived recovery at hospital discharge. General hospital psychiatry. 2018 Jul-Aug:53():108-113. doi: 10.1016/j.genhosppsych.2018.02.006. Epub 2018 May 10 [PubMed PMID: 29776732]
Larsson IM, Wallin E, Rubertsson S, Kristoferzon ML. Relatives' experiences during the next of kin's hospital stay after surviving cardiac arrest and therapeutic hypothermia. European journal of cardiovascular nursing. 2013 Aug:12(4):353-9. doi: 10.1177/1474515112459618. Epub 2012 Sep 14 [PubMed PMID: 22984190]
Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, Zong Q, Chen S, Lv C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Critical care (London, England). 2020 Feb 22:24(1):61. doi: 10.1186/s13054-020-2773-2. Epub 2020 Feb 22 [PubMed PMID: 32087741]
Level 1 (high-level) evidenceNakashima T, Noguchi T, Tahara Y, Nishimura K, Yasuda S, Onozuka D, Iwami T, Yonemoto N, Nagao K, Nonogi H, Ikeda T, Sato N, Tsutsui H, Japanese Circulation Society with Resuscitation Science Study Group. Public-access defibrillation and neurological outcomes in patients with out-of-hospital cardiac arrest in Japan: a population-based cohort study. Lancet (London, England). 2019 Dec 21:394(10216):2255-2262. doi: 10.1016/S0140-6736(19)32488-2. Epub 2019 Dec 17 [PubMed PMID: 31862250]
Pollack RA, Brown SP, Rea T, Aufderheide T, Barbic D, Buick JE, Christenson J, Idris AH, Jasti J, Kampp M, Kudenchuk P, May S, Muhr M, Nichol G, Ornato JP, Sopko G, Vaillancourt C, Morrison L, Weisfeldt M, ROC Investigators. Impact of Bystander Automated External Defibrillator Use on Survival and Functional Outcomes in Shockable Observed Public Cardiac Arrests. Circulation. 2018 May 15:137(20):2104-2113. doi: 10.1161/CIRCULATIONAHA.117.030700. Epub 2018 Feb 26 [PubMed PMID: 29483086]
Naber D, Bullinger M. Psychiatric sequelae of cardiac arrest. Dialogues in clinical neuroscience. 2018 Mar:20(1):73-77 [PubMed PMID: 29946214]
Wong MK, Morrison LJ, Qiu F, Austin PC, Cheskes S, Dorian P, Scales DC, Tu JV, Verbeek PR, Wijeysundera HC, Ko DT. Trends in short- and long-term survival among out-of-hospital cardiac arrest patients alive at hospital arrival. Circulation. 2014 Nov 18:130(21):1883-90. doi: 10.1161/CIRCULATIONAHA.114.010633. Epub [PubMed PMID: 25399397]
Level 2 (mid-level) evidenceKirchhof P, Breithardt G, Eckardt L. Primary prevention of sudden cardiac death. Heart (British Cardiac Society). 2006 Dec:92(12):1873-8 [PubMed PMID: 17105896]
Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, Eckardt L, Friede T, Haugaa KH, Hocini M, Lambiase PD, Marijon E, Merino JL, Peichl P, Priori SG, Reichlin T, Schulz-Menger J, Sticherling C, Tzeis S, Verstrael A, Volterrani M, ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European heart journal. 2022 Oct 21:43(40):3997-4126. doi: 10.1093/eurheartj/ehac262. Epub [PubMed PMID: 36017572]
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2020 Dec 22:76(25):3022-3055. doi: 10.1016/j.jacc.2020.08.044. Epub 2020 Nov 20 [PubMed PMID: 33229115]
Level 1 (high-level) evidenceDorian P, Lin S. Improving Resuscitation Rates After Out-of-Hospital Cardiac Arrest. Circulation. 2019 Mar 5:139(10):1272-1274. doi: 10.1161/CIRCULATIONAHA.118.038822. Epub [PubMed PMID: 30865547]