Introduction
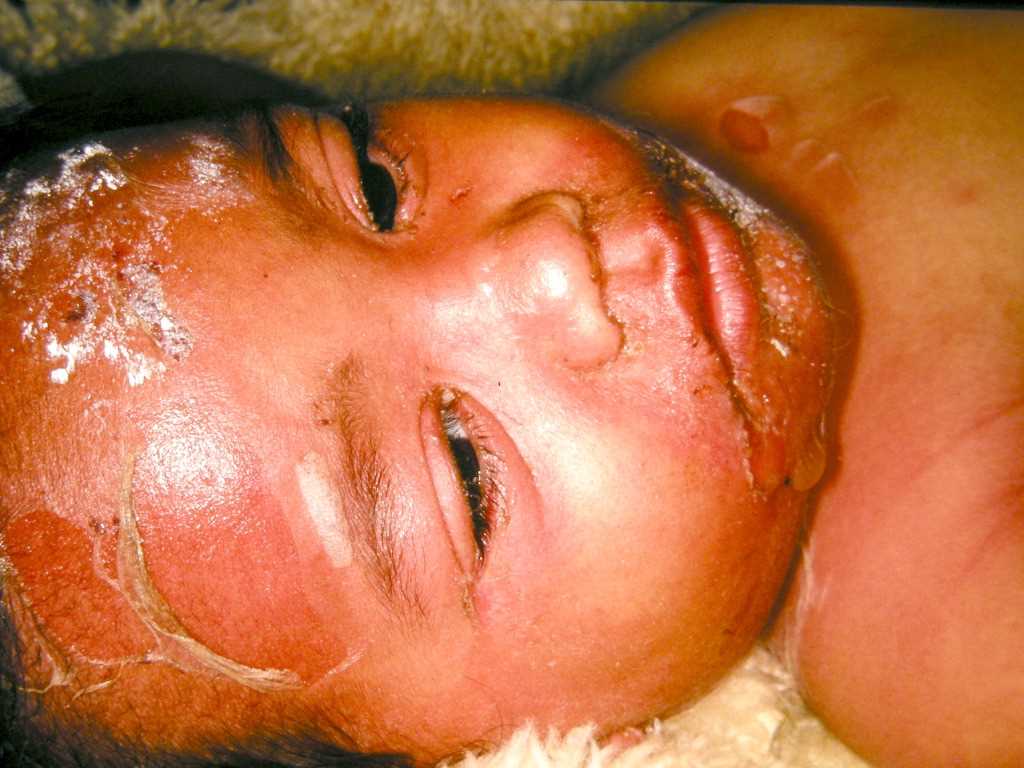
Staphylococcal scalded skin syndrome (SSSS), also known as Ritter disease or pemphigus neonatorum, is a dangerous cutaneous infection caused by certain exotoxin-producing strains of Staphylococcus species (see Image. Staphylococcal Scalded Skin Syndrome).[1] The condition typically presents with superficial skin blistering and denudation and affects children more often than adults. SSSS in adult patients is often associated with renal dysfunction or immunocompromise.[2][3]
Skin desquamation is typically caused by exotoxins from a distant location rather than a local infection spreading via the blood.[4][5] In the pediatric population, SSSS can present as early as 48 hours after birth and becomes less frequent in children older than 6 years.[6] SSSS is a clinical diagnosis, but the diagnostic evaluation focuses on identifying bacteremia, distinguishing SSSS from mimicking conditions like bullous impetigo, and selecting appropriate management. Therapy includes antibiotics, specifically penicillinase-resistant penicillins, supportive care, and monitoring for possible sequelae, including hypothermia, hemodynamic instability, and relapse.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Staphylococcus aureus, a gram-positive coccus, causes most cases of SSSS. Many individuals are colonized, particularly in areas such as the umbilicus, groin, buttocks, and the face, especially the nares.[7][8] Methicillin-sensitive S aureus (MSSA) is more commonly identified than methicillin-resistant S aureus (MRSA).[9] Exfoliative or epidermolytic exotoxins A and B are serine proteases released by S aureus at the primary infection site. These toxins are produced by less than 5% of Staphylococcus species, although all species produce some form of toxin.[10][11][12]
Phage group II types 3A, 3B, 3C, 55, and 71 and the strain ST121 are the most likely S aureus species implicated in producing exotoxins A and B.[13][14][15] The accumulated toxins directly target cadherin desmoglein-1 at distant sites after hematogenous dissemination, causing loss of cellular adhesion among keratinocytes in the stratum granulosum. Notably, desmoglein-3, which predominates in the lower epidermis, is not affected by the exotoxins A and B produced by S aureus.[16][17][18] However, testing for the specific phage type is often not helpful or available.
The primary source of infection is usually distant from areas of skin denudation. However, no specific source can be identified in many cases, and the organism can come from sites that include the upper respiratory tract, ears, conjunctivae, or an abscess, fistula, or joint infection.[19]
Epidemiology
The incidence of SSSS is low. A study in the Czech Republic reported an incidence of approximately 25 cases per 100,000 children younger than 1 year.[20] Most cases involve children younger than 5 years, with most cases occurring before 3 years. However, neonates are relatively protected from SSSS due to the predominance of desmoglein-3 in the epidermis, which is not targeted by staphylococcal exotoxins.[21][22] The disease is much less common in adults because of neutralizing antibodies and mature kidney function, which allow for efficient clearance of exotoxins from the bloodstream.[23] Thus, SSSS in adults typically affects individuals with immunosuppression, including people with HIV/AIDS and advanced malignancies or those with severe kidney impairment.[24]
SSSS may occur as an outbreak in daycares or nurseries. The frequent occurrence of prodromal symptoms resembling those of upper respiratory viral infections, such as fever and irritability, has led some authors to suggest a role for viral infection as a promoter of the proliferation of exotoxin-producing strains of S aureus. Viral coinfection may explain the autumnal predominance of the condition and the prodromal upper respiratory infection symptoms observed in patients with SSSS.
Pathophysiology
Some toxin-producing strains of Staphylococcus cause SSSS. These toxins target the desmoglein-1 complex in the zona granulosa of the epidermis, resulting in skin exfoliation. The manifestations can range from a mild localized form to exfoliation of the entire body. Individuals who have developed antibodies against the bacteria and its products are more likely to have the localized form. The resultant skin loss predisposes patients to hypothermia, large fluid volume losses, and additional or worsening infections.
Histopathology
SSSS is primarily a clinical diagnosis, rarely necessitating additional evaluation. However, a biopsy can support the diagnosis when clarity is lacking. Histopathological evaluation of a skin biopsy from a patient with SSSS typically reveals superficial intraepidermal cleavage beneath the stratum corneum.[25] Cleavage at the stratum granulosum may show sparse neutrophils or a mixed inflammatory infiltrate, complicating the diagnosis in adult patients with coexisting conditions, such as malignancy, where toxic erythema of chemotherapy may present similarly.[26]
History and Physical
SSSS initially presents with irritability, fever, and malaise, followed by the rapid development of a tender, erythematous desquamative rash on the face and flexures, such as the groin, axillae, and neck, within 24 to 48 hours. Subsequently, large, fragile, and potentially purulent blisters form bullae, exhibiting a positive Nikolsky sign. Progressive desquamation and healing without scarring occur over the next 2 weeks.[27] Adults exhibit findings similar to those seen in children.
Evaluation
SSSS is primarily a clinical diagnosis. However, further evaluation may be beneficial in certain circumstances. As with other systemic illnesses, clinicians may perform routine blood tests, including a complete blood count, urinalysis, comprehensive metabolic profile, and blood cultures. However, blood cultures and blister fluid cultures are usually negative, except in adults, who often present with sepsis from bacteremia. Adults with severe disease may require additional evaluation to identify a source of infection, such as infected fistulae in patients with renal failure, bacteremia, or septic arthritis. Testing for Staphylococcus species through nasopharyngeal and periorificial swabs may help confirm the diagnosis. Blood cultures typically provide limited information since the toxins, not the organisms, spread hematogenously.
Treatment / Management
Antibiotics effective against MSSA, such as cefazolin, nafcillin, and oxacillin, should be administered promptly. In children, nafcillin or oxacillin should be initiated at 100 to 150 mg/kg daily, divided every 6 hours. Cefazolin should be administered at 50 to 100 mg/kg daily, divided every 8 hours. Oral alternatives like cephalexin or dicloxacillin at 500 mg every 6 hours or trimethoprim/sulfamethoxazole at 160/800 mg every 12 hours may be used for mild infections.
Vancomycin should be administered if MRSA is suspected, particularly in patients with recent healthcare exposure, including those residing in a skilled nursing facility, recently hospitalized, or living in areas with a high MRSA prevalence.[28] Clindamycin should be avoided due to resistance. Topical antimicrobial therapies are generally ineffective, though they may be used for decolonization at the primary site of infection.[29] Treatment should continue for 10 days unless clinical judgment dictates a longer course.(B2)
Additional antibiotics with Pseudomonas coverage should be initiated if concern for a secondary bacterial skin infection arises. Intravenous fluids become necessary for patients showing signs of dehydration or sepsis. Some children may require intravenous immunoglobulin or fresh frozen plasma to address volume loss. Admission to an intensive care unit may be necessary, as saline-soaked gauze and wound care may be required.[30](B2)
Emollients and nonadherent dressings should be applied to denuded areas to promote healing and reduce heat loss. Supportive care, including management of dehydration, temperature regulation, and nutrition, proves essential. Patients with significant skin involvement are prone to hypothermia and fluid deficits due to epidermal loss. The application of silver sulfadiazine should be avoided, given the potential for increased systemic absorption and resultant toxicity.[31] Acetaminophen and opioids may be needed for pain control, but nonsteroidal anti-inflammatory drugs, such as ibuprofen, should be avoided due to the risk of kidney impairment.(B2)
Differential Diagnosis
The differential diagnosis for SSSS includes other blistering infections, such as:
- Bullous impetigo is typically seen in newborns and also results from cleavage of desmoglein-1. However, this condition features a large dermal inflammatory infiltrate and demonstrates a negative Nikolsky sign.[32]
- Stevens-Johnson syndrome and toxic epidermal necrolysis present with dusky areas that show necrotic keratinocytes. As opposed to clinical patterns associated with SSSS, Stevens-Johnson syndrome and toxic epidermal necrolysis are commonly linked to medications and affect older children and adults.[33]
- Acute generalized exanthematous pustulosis occurs more frequently in women. This disorder is characterized by nonfollicular pustules on flexural sites, exhibiting subcorneal pustules with eosinophilic and neutrophilic inflammation on biopsy.[34][35]
- Toxic shock syndrome may be caused by S aureus, by a mechanism involving the toxic shock syndrome toxin-1, or by Streptococcus pyogenes. This condition presents with necrolysis of keratinocytes, fever, hypotension, and involvement of multiple organ systems.[36][37]
- Kawasaki disease affects a similar age group and is diagnosed based on criteria that include a fever lasting 5 days and at least 4 minor criteria, one of which is acral desquamation.[38][39]
- Scarlet fever, caused by S pyogenes, typically affects older children. This condition presents with flu-like symptoms followed by a characteristic sandpaper rash.[40]
A thorough clinical investigation and judicious diagnostic testing can differentiate SSSS from similar conditions, guide therapeutic strategies, and improve outcomes.
Prognosis
Pediatric patients generally do well with minimal to no scarring. Healing typically occurs within 2 weeks. The mortality rate in children is less than 5%. In contrast, adults with SSSS have a much higher mortality risk, as high as 60%.
Complications
Potential complications of SSSS include dehydration, secondary infections, electrolyte imbalance, sepsis, acute kidney injury, and scarring.[41]
Consultations
Consultation with a dermatologist should be considered if the diagnosis is uncertain. Burn centers and burn unit or critical care physicians should be consulted in severe cases.
Deterrence and Patient Education
Preventing SSSS in children and adults involves practicing good hygiene, including regular handwashing and proper wound care. Parents and caregivers should keep the skin clean and monitor for any signs of infection, especially in children with cutaneous disorders such as eczema. Additionally, limiting exposure to infected individuals and maintaining good sanitation can help minimize the risk of transmission. Strict adherence to infection control protocols is critical to prevent outbreaks in healthcare settings.
Enhancing Healthcare Team Outcomes
SSSS cases should be approached as a team, particularly with unclear cases. Expert consultation with dermatologists can help eliminate alternative diagnoses and guide treatment. Colonized caregivers and individuals with patient contact require treatment with chlorhexidine. Nasal carriers should be treated with topical mupirocin. The mortality is relatively low in children when appropriate treatment is initiated, but it is much higher in adults, even with appropriate antibiotics and supportive therapy.[42][43] In severe cases, referral to a burn center with wound care experts should be strongly considered to reduce fluid losses and prevent scarring.
Effective management of SSSS requires timely identification and coordinated efforts across an interprofessional healthcare team. Initial recognition typically begins with primary care providers (PCPs) and nurses, who should be trained to spot characteristic symptoms like skin redness, blistering, and peeling, especially in pediatric or immunocompromised patients. If SSSS is suspected, the PCP should quickly refer the patient to appropriate specialists, such as dermatologists and infectious disease experts, for further diagnostic support, including skin biopsies or cultures to confirm the presence of Staphylococcus aureus. Lab technicians also play a crucial role in analyzing samples, while infectious disease specialists may help guide the choice of antibiotics. Immediate referral to emergency departments ensures rapid stabilization and treatment for severe cases.
In cases requiring hospitalization, collaboration among the inpatient nursing team, pharmacists, and nutritionists is essential to manage symptoms, administer antibiotics, and address hydration and nutritional needs due to potential fluid loss from damaged skin. All team members should maintain thorough documentation in electronic health records throughout the process to support clear communication and effective care coordination. Once the patient stabilizes, discharge coordinators help arrange follow-up care and provide guidance on wound care and infection prevention. This team-based approach, supported by structured referral pathways and regular interdisciplinary communication, ensures comprehensive care for patients with SSSS, optimizing recovery and reducing the risk of complications.
Media
(Click Image to Enlarge)
References
Brazel M, Desai A, Are A, Motaparthi K. Staphylococcal Scalded Skin Syndrome and Bullous Impetigo. Medicina (Kaunas, Lithuania). 2021 Oct 24:57(11):. doi: 10.3390/medicina57111157. Epub 2021 Oct 24 [PubMed PMID: 34833375]
Daniel SS, Peterman C, Awasthi S. Updates on postinfectious skin rashes in pediatric dermatology. Current opinion in pediatrics. 2024 Aug 1:36(4):431-435. doi: 10.1097/MOP.0000000000001376. Epub 2024 Jun 5 [PubMed PMID: 38957128]
Level 3 (low-level) evidenceGeeta Sai G, Devi S, Jameela Wahab A. Unusual Presentation of Staphylococcal Scalded Skin Syndrome in an Elderly Patient With Acute Kidney Injury: A Case Report. Cureus. 2024 Mar:16(3):e56853. doi: 10.7759/cureus.56853. Epub 2024 Mar 24 [PubMed PMID: 38659552]
Level 3 (low-level) evidenceRůzicková V, Pantůcek R, Petrás P, Doskar J, Sedlácek I, Rosypal S. Molecular typing of exfoliative toxin-producing Staphylococcus aureus strains involved in epidermolytic infections. International journal of medical microbiology : IJMM. 2003 Feb:292(7-8):541-5 [PubMed PMID: 12635937]
Nguyen QD, Vu MN, Hebert AA. Recognizing and Managing Staphylococcal Scalded Skin Syndrome in the Emergency Department. Pediatric emergency care. 2022 Mar 1:38(3):133-135. doi: 10.1097/PEC.0000000000002564. Epub [PubMed PMID: 34744158]
Liy-Wong C, Pope E, Weinstein M, Lara-Corrales I. Staphylococcal scalded skin syndrome: An epidemiological and clinical review of 84 cases. Pediatric dermatology. 2021 Jan:38(1):149-153. doi: 10.1111/pde.14470. Epub 2020 Dec 1 [PubMed PMID: 33283348]
Level 2 (mid-level) evidenceWu Y, Wu H, Wu M, Wei W, Wei Y, Li T, Cao C, Yao Z. The Clinical Characteristics and Antimicrobial Resistance of Staphylococcus aureus Isolated from Patients with Staphylococcal Scalded Skin Syndrome (SSSS) in Southwestern China. Antibiotics (Basel, Switzerland). 2024 May 31:13(6):. doi: 10.3390/antibiotics13060516. Epub 2024 May 31 [PubMed PMID: 38927182]
Bwanga F, Mukashyaka C, Kateete DP, Tumuhamye J, Okeng A, Aboce E, Namugga O, Kwizera R, Sommerfelt H, Nankabirwa V. Vaginal colonization with virulent and methicillin resistant Staphylococcus aureus among Ugandan women in Labour. BMC microbiology. 2024 Aug 19:24(1):307. doi: 10.1186/s12866-024-03460-9. Epub 2024 Aug 19 [PubMed PMID: 39155368]
Vernali S, Blasiak RC, Morrell DS. Demographic characteristics, clinical features, and optimal management of hospitalized patients with staphylococcal scalded skin syndrome. Pediatric dermatology. 2021 Jul:38(4):825-830. doi: 10.1111/pde.14629. Epub 2021 May 18 [PubMed PMID: 34008230]
Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014 Nov:28(11):1418-23. doi: 10.1111/jdv.12541. Epub 2014 May 20 [PubMed PMID: 24841497]
Jordan KS. Staphylococcal Scalded Skin Syndrome: A Pediatric Dermatological Emergency. Advanced emergency nursing journal. 2019 Apr/Jun:41(2):129-134. doi: 10.1097/TME.0000000000000235. Epub [PubMed PMID: 31033660]
Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World journal of pediatrics : WJP. 2018 Apr:14(2):116-120. doi: 10.1007/s12519-018-0150-x. Epub 2018 Mar 5 [PubMed PMID: 29508362]
Dancer SJ, Noble WC. Nasal, axillary, and perineal carriage of Staphylococcus aureus among women: identification of strains producing epidermolytic toxin. Journal of clinical pathology. 1991 Aug:44(8):681-4 [PubMed PMID: 1890203]
Azarian T, Cella E, Baines SL, Shumaker MJ, Samel C, Jubair M, Pegues DA, David MZ. Genomic Epidemiology and Global Population Structure of Exfoliative Toxin A-Producing Staphylococcus aureus Strains Associated With Staphylococcal Scalded Skin Syndrome. Frontiers in microbiology. 2021:12():663831. doi: 10.3389/fmicb.2021.663831. Epub 2021 Aug 18 [PubMed PMID: 34489877]
Mohammed AA, Al-Gadi MA. Neonatal Staphylococcal scalded skin syndrome complicating ileal atresia. Saudi medical journal. 2003 May:24(5):538-41 [PubMed PMID: 12847634]
Sato S, Ragab RF, Guo X, Elfadadny A, Kozono T, Nishikawa A, Nishifuji K, Tonozuka T. Crystal structure of exfoliative toxin D from Staphylococcus aureus. Biochemical and biophysical research communications. 2024 Nov 12:733():150689. doi: 10.1016/j.bbrc.2024.150689. Epub 2024 Sep 11 [PubMed PMID: 39276694]
Rolle CE, Chen J, Pastar I, Cardenas TC, Perez R, Hower S, Ferracci F, Snyder R, Tomic-Canic M, Plano LR. Keratinocytes produce IL-6 in response to desmoglein 1 cleavage by Staphylococcus aureus exfoliative toxin A. Immunologic research. 2013 Dec:57(1-3):258-67. doi: 10.1007/s12026-013-8467-y. Epub [PubMed PMID: 24287883]
Iyori K, Futagawa-Saito K, Hisatsune J, Yamamoto M, Sekiguchi M, Ide K, Son WG, Olivry T, Sugai M, Fukuyasu T, Iwasaki T, Nishifuji K. Staphylococcus pseudintermedius exfoliative toxin EXI selectively digests canine desmoglein 1 and causes subcorneal clefts in canine epidermis. Veterinary dermatology. 2011 Aug:22(4):319-26. doi: 10.1111/j.1365-3164.2011.00952.x. Epub 2011 Mar 17 [PubMed PMID: 21410798]
Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clinical microbiology reviews. 1999 Apr:12(2):224-42 [PubMed PMID: 10194458]
Lipový B, Brychta P, Chaloupková Z, Suchánek I. Staphylococcal scalded skin syndrome in the Czech Republic: an epidemiological study. Burns : journal of the International Society for Burn Injuries. 2012 Mar:38(2):296-300. doi: 10.1016/j.burns.2011.08.005. Epub 2011 Oct 28 [PubMed PMID: 22035884]
Level 2 (mid-level) evidenceAmagai M, Stanley JR. Desmoglein as a target in skin disease and beyond. The Journal of investigative dermatology. 2012 Mar:132(3 Pt 2):776-84. doi: 10.1038/jid.2011.390. Epub 2011 Dec 22 [PubMed PMID: 22189787]
Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nature medicine. 2000 Nov:6(11):1275-7 [PubMed PMID: 11062541]
Mishra AK, Yadav P, Mishra A. A Systemic Review on Staphylococcal Scalded Skin Syndrome (SSSS): A Rare and Critical Disease of Neonates. The open microbiology journal. 2016:10():150-9. doi: 10.2174/1874285801610010150. Epub 2016 Aug 31 [PubMed PMID: 27651848]
Lamand V, Dauwalder O, Tristan A, Casalegno JS, Meugnier H, Bes M, Dumitrescu O, Croze M, Vandenesch F, Etienne J, Lina G. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012 Dec:18(12):E514-21. doi: 10.1111/1469-0691.12053. Epub 2012 Oct 19 [PubMed PMID: 23078129]
Level 2 (mid-level) evidenceHanakawa Y, Schechter NM, Lin C, Garza L, Li H, Yamaguchi T, Fudaba Y, Nishifuji K, Sugai M, Amagai M, Stanley JR. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. The Journal of clinical investigation. 2002 Jul:110(1):53-60 [PubMed PMID: 12093888]
Lee JJ, Tsibris HC, Mostaghimi A, Lian CG. Staphylococcal Scalded Skin Syndrome in an Adult on Chemotherapy. Dermatopathology (Basel, Switzerland). 2014 Aug-Dec:1(2):75-80. doi: 10.1159/000368599. Epub 2014 Oct 31 [PubMed PMID: 27047925]
Kanathur S, Sarvajnyamurthy S, Somaiah SA. Characteristic facies: an index of the disease. Indian journal of dermatology, venereology and leprology. 2013 May-Jun:79(3):439-43. doi: 10.4103/0378-6323.110801. Epub [PubMed PMID: 23619458]
Bamberger DM, Boyd SE. Management of Staphylococcus aureus infections. American family physician. 2005 Dec 15:72(12):2474-81 [PubMed PMID: 16370403]
Wang Z, Feig JL, Mannschreck DB, Cohen BA. Antibiotic sensitivity and clinical outcomes in staphylococcal scalded skin syndrome. Pediatric dermatology. 2020 Jan:37(1):222-223. doi: 10.1111/pde.14014. Epub 2019 Oct 18 [PubMed PMID: 31626359]
Level 2 (mid-level) evidenceBraunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatric dermatology. 2014 May-Jun:31(3):305-8. doi: 10.1111/pde.12195. Epub 2013 Aug 23 [PubMed PMID: 24033633]
Level 2 (mid-level) evidenceBaartmans MG, Dokter J, den Hollander JC, Kroon AA, Oranje AP. Use of skin substitute dressings in the treatment of staphylococcal scalded skin syndrome in neonates and young infants. Neonatology. 2011:100(1):9-13. doi: 10.1159/000317997. Epub 2010 Dec 9 [PubMed PMID: 21150225]
Level 2 (mid-level) evidenceHartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. American family physician. 2014 Aug 15:90(4):229-35 [PubMed PMID: 25250996]
Shah H, Parisi R, Mukherjee E, Phillips EJ, Dodiuk-Gad RP. Update on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Diagnosis and Management. American journal of clinical dermatology. 2024 Nov:25(6):891-908. doi: 10.1007/s40257-024-00889-6. Epub 2024 Sep 15 [PubMed PMID: 39278968]
Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. Journal of the American Academy of Dermatology. 2015 Nov:73(5):843-8. doi: 10.1016/j.jaad.2015.07.017. Epub 2015 Sep 6 [PubMed PMID: 26354880]
Feldmeyer L, Heidemeyer K, Yawalkar N. Acute Generalized Exanthematous Pustulosis: Pathogenesis, Genetic Background, Clinical Variants and Therapy. International journal of molecular sciences. 2016 Jul 27:17(8):. doi: 10.3390/ijms17081214. Epub 2016 Jul 27 [PubMed PMID: 27472323]
Burnham JP, Kollef MH. Understanding toxic shock syndrome. Intensive care medicine. 2015 Sep:41(9):1707-10. doi: 10.1007/s00134-015-3861-7. Epub 2015 May 14 [PubMed PMID: 25971393]
Level 3 (low-level) evidenceVuzevski VD, van Joost T, Wagenvoort JH, Dey JJ. Cutaneous pathology in toxic shock syndrome. International journal of dermatology. 1989 Mar:28(2):94-7 [PubMed PMID: 2661452]
Gupta A, Singh S. Kawasaki disease for dermatologists. Indian dermatology online journal. 2016 Nov-Dec:7(6):461-470 [PubMed PMID: 27990380]
Agarwal S, Agrawal DK. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert review of clinical immunology. 2017 Mar:13(3):247-258. doi: 10.1080/1744666X.2017.1232165. Epub 2016 Sep 13 [PubMed PMID: 27590181]
Ferretti JJ, Stevens DL, Fischetti VA, Wessels MR. Streptococcus pyogenes Pharyngitis and Scarlet Fever. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. 2022 Oct 8:(): [PubMed PMID: 36479768]
Haggerty J, Grimaldo F. A Desquamating Skin Rash in a Pediatric Patient. Clinical practice and cases in emergency medicine. 2019 May:3(2):112-114. doi: 10.5811/cpcem.2019.1.41162. Epub 2019 Feb 26 [PubMed PMID: 31061964]
Level 3 (low-level) evidenceParanthaman K, Bentley A, Milne LM, Kearns A, Loader S, Thomas A, Thompson F, Logan M, Newitt S, Puleston R. Nosocomial outbreak of staphyloccocal scalded skin syndrome in neonates in England, December 2012 to March 2013. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014 Aug 21:19(33):. pii: 20880. Epub 2014 Aug 21 [PubMed PMID: 25166346]
Mazori DR, Leonard A, Alexander JB, Glick SA. The spectrum of staphylococcal scalded skin syndrome: a case series in children. Clinical and experimental dermatology. 2020 Apr:45(3):333-336. doi: 10.1111/ced.14116. Epub 2019 Nov 22 [PubMed PMID: 31587342]
Level 2 (mid-level) evidence