Introduction
Squamous cell carcinoma of the lung, also known as squamous cell lung cancer, is a type of non-small cell lung cancer (NSCLC). Among NSCLC, adenocarcinoma is the most common, followed by squamous cell carcinoma of the lung, especially in women. This is attributed to the change in the pattern of cigarette smoking, though there is no definitive evidence. Squamous cell lung tumors often occur in the central part of the lung or the main airway, such as the left or right bronchus. The principal causative agent of cellular transformation is smoking. Approximately 80% of lung cancer cases in men and 90% of patients in women are associated with smoking. SCC is more strongly associated with smoking than any other type of NSCLC. Other risk factors for squamous cell carcinoma of the lung include age, family history, exposure to second-hand smoke, and occupational exposure to minerals, metal particles, or asbestos.
Clinical symptoms of NSCLCs include cough, chest pain, shortness of breath, blood in sputum, wheezing, hoarseness, recurring chest infections (eg, bronchitis and pneumonia), weight loss, loss of appetite, and fatigue. Following the clinical examination, computed tomography (CT) imaging is frequently utilized initially to evaluate SCC, provided the tumor is sufficiently sized to be detected on the CT. Additional diagnostic studies, including imaging modalities, histological analysis, and immunohistochemistry evaluation, are typically performed to characterize tumors further, obtain tissue specimens for diagnostic confirmation, and stage the disease. Squamous cell carcinoma of the lung treatment varies with the stage of cancer and usually consists of a combination of various modalities (eg, surgical resection, chemotherapy, radiotherapy, and immunotherapy).
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Approximately 80% of lung cancer cases in men and 90% of cases in women are associated with smoking.[1][2] SCC is more strongly associated with smoking than any other type of NSCLC. Other risk factors for SCC include age, family history, exposure to second-hand smoke, and exposure to minerals, metal particles, or asbestos.
Epidemiology
In 2023, lung cancer was the third most common cancer in terms of incidence and the most common cause of cancer-related mortality in the US, as per the National Cancer Institute's Surveillance Epidemiology and End Results (NCI SEER) database. Lung cancer incidence was estimated at 238,340 cases, accounting for 12% of the cancer burden in the US. The estimated mortality for lung cancer in 2023 was 127,070 deaths, constituting around 21% of all US cancer-related deaths. Approximately 85% of all lung cancers are NSCLCs. Adenocarcinoma and squamous cell carcinoma are the most common subtypes, accounting for 50% and 30% of NSCLC cases, respectively.[3]
Pathophysiology
SCC of the lungs originates from the transformation of the squamous cells lining the airways. Squamous cells are thin, flat cells that are found lining many organs of the human body. Squamous cell lung tumors often occur in the central part of the lung or the primary airway, such as the left or right bronchus. The primary causative agent of cellular transformation is tobacco smoke, which contains more than 300 harmful agents and 40 potential carcinogens. Transformed squamous cells are characterized by keratinization and intercellular bridges and often exhibit high mutation frequency.[4]
Histopathology
A correct histologic diagnosis is becoming increasingly important because it may predict response and toxicity to therapies.[5] A diagnosis of SCC is confirmed through histologic examination when a minimum of 10% of the tumor bulk of resected samples exhibits transformation features such as keratinization or intracellular bridges. If the differentiated squamous element of the tumor is minimal, a diagnosis of poorly differentiated SCC is made. An immunohistochemistry (IHC) panel and a mucin stain can help identify NSCLC subtypes. SCC also shows a strong expression of squamous biomarkers, including p63 and p40 proteins. In 2015, the World Health Organization revised the classification to recognize the 3 following variants of SCC based on histological examination:[6]
- Keratinizing
- Nonkeratinizing
- Basaloid, when the basaloid component is more than 50% of the tissue with minimal areas of squamous differentiation
History and Physical
Clinical symptoms of NSCLCs include cough, chest pain, shortness of breath, blood in sputum, wheezing, hoarseness, recurring chest infections (eg, bronchitis and pneumonia), weight loss, loss of appetite, and fatigue. However, NSCLC patients often are asymptomatic in the early stages of the disease. Social history may include extensive smoking history or occupational exposure to heavy metals, asbestos, and radon exposure. Metastases may occur in advanced disease, and symptoms may include bone pain, brain metastasis, or spinal cord compression with neurologic symptoms (eg, headache, weakness or numbness of limbs, dizziness, and seizures).
Evaluation
Diagnostic Imaging Studies
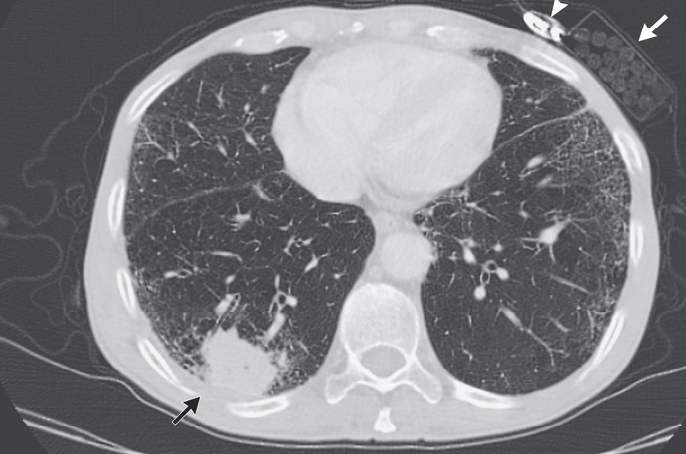
Following the clinical examination, computed tomography (CT) imaging is frequently utilized initially to evaluate SCC, provided the tumor is sufficiently sized to be detected on the CT. The presence of a cavity, filled by either gas or fluid, within a tumor mass is a classic sign of SCC.[1] Further signs may include pulmonary nodules, mass or infiltrate, mediastinal widening, atelectasis, hilar enlargement, or pleural effusion (see Image. Squamous Cell Carcinoma of the Lung).
Suspected lung malignancies require a thorough workup individualized to evaluate the extent of the disease efficiently. For small incidental nodules discovered on the chest CT, the Fleischner Society guidelines for management are typically followed. If a diagnosis of lung cancer is likely, then proceeding with the staging workup, rather than obtaining a tissue specimen first, can be more efficient in some patients, as this may allow metastatic areas to be detected. These areas could be more accessible and easier to biopsy, thus confirming the diagnosis and establishing the advanced stage.
High-quality imaging is essential to the staging process. A Positron emission tomography (PET) scan, typically fused with a CT of the body, is utilized. False positives may occur in situations such as infection, sarcoidosis, rheumatoid nodules, etc. False negatives can occur with carcinoid tumors, ground glass opacities, and or lesions less than 1 cm in size. For lesions less than 1 cm in size, the false negative rate approaches 15%. Although PET/CT staging is generally widespread, they are not indicated in patients with pure ground glass opacities or peripheral cT1a type tumors.[7] MRI brain imaging also becomes essential in evaluating patients for possible brain metastasis. Usually, this is indicated in patients with at least clinical stage II disease and is considered optional for stage IB.
Despite imaging advances, invasive mediastinal nodal evaluation is indicated in a broad cross-section of patients. Even with PET/CT scans, the false positive and false negative rates are 15% to 20% for mediastinal involvement.[7] Invasive mediastinal staging is generally indicated in patients with either discrete enlargement of mediastinal nodes on CT regardless of PET avidity or normal-appearing nodes on CT but is PET avid. Staging can be omitted in patients with established metastatic disease, small peripheral clinical T1a tumors, and bulky mediastinal adenopathy on imaging.[7]
Additional Diagnostic Studies
Multiple techniques can be utilized to sample the mediastinum, which can be divided into surgical, endobronchial, or esophageal, and differ in the number of locations of accessible nodes. Surgical techniques include mediastinoscopy, video-mediastinoscopy, anterior mediastinoscopy, and video-assisted thoracic surgery. Traditional mediastinoscopy can access lymph node stations 1, 2 right and left (R/L), 3, 4 R/L, and 7, and video-assisted mediastinoscopy can also obtain levels 5, 8, and 10 R. The false negative rates for these procedures are approximately 2%, but this is likely dependent on the operator.
Endobronchial techniques involve using an ultrasound probe to identify specific landmarks associated with certain mediastinal nodal levels. Endoscopic bronchial ultrasound with needle aspiration (EBUS-NA) is one of these techniques. It can access nodal levels 2 R/L, 3, 4 R/L, 7, 10, 11, and 12. Esophageal ultrasound with needle aspiration (EUS-NA) can access 4L, 5, 7,8, and 9. The false negative rate may be as high as 20% but highly depends on the operator's and institution's skill and experience. EBUS or EUS is typically the first approach offered to patients requiring mediastinal nodal evaluation.
Baseline pulmonary function testing (PFT) is also essential, especially in patients who may be surgical candidates. (Refer to the Surgical Oncology section for more information on pulmonary function testing in presurgical evaluation). Furthermore, genetic assessment of PD-L1 status and EGFR mutations are also performed as they are targetable, including neoadjuvant and adjuvant therapies. However, EGFR mutations in SCC are rare, estimated at less than 5%.
Treatment / Management
The treatment varies with the stage of cancer. Surgical resection is the first line of therapy for stages I and II. For stage IA, chemotherapy typically has no role. For IB, surgical resection with adjuvant chemotherapy is considered in some cases if the tumor size is greater than 4 cm. Stage II is treated by surgery followed by chemotherapy; usually, lobectomy is preferred, but in poor surgical candidates, sub-lobar resection can be considered. In stages I and II, if postsurgical margins are positive and in poor surgical candidates, radiation therapy is considered.
Most stage III tumors are unresectable. Stage IIIA, which is definitively staged during resection surgery, can be considered followed by adjuvant chemotherapy, but chemotherapy with radiation is the usual choice. More recently, there is increasing evidence favoring the use of perioperative systemic therapy using chemoimmunotherapy combinations. For stage IIIB, combined chemotherapy and radiation are used, followed by maintenance immunotherapy for 1 year. In stage IV, systemic treatment with palliative radiation is used. Multiple randomized, controlled trials and large meta-analyses confirm the efficacy of combination chemotherapy and immunotherapy or single-agent immunotherapy regimens for advanced NSCLC.[2] Also, depending on mutation status, targeted therapy is used more often in non-squamous cell lung cancers. In advanced NSCLC, treatment should be based on the molecular features of the tumor.(B3)
In NSCLC with somatic-driven mutation, specific inhibitor therapy is indicated.[8] For epidermal growth receptor mutation, tyrosine kinase inhibitors (TKI) like erlotinib, gefitinib, and afatinib are used. NSCLC, which lacks this mutation depending on programmed cell death-ligand 1 (PD-L1) expression, immunotherapy alone or combined with chemotherapy is considered. Additionally, chemotherapy for neoadjuvant and adjuvant therapy may be considered, including cisplatin with paclitaxel, gemcitabine, or pemetrexed for non-squamous histologies. In patients who cannot tolerate cisplatin, paclitaxel with carboplatin is considered. Having all patients with tumors >4 cm or positive nodes be evaluated for preoperative therapy is recommended, including immunotherapy using nivolumab or pembrolizumab in addition to chemotherapy. However, if patients are not candidates for immune checkpoint inhibitors due to autoimmune diseases, being on immunosuppressants, or carrying EGFR mutations or ALK rearrangements, then combination chemotherapy alone is recommended.
Targeted Therapy
For advanced cancers, treatment should be tailored according to genetic and molecular testing, including ALK rearrangement, ROS1 fusion, EGFR, BRAF, NTRK 1/2/3, MET exon 14 skipping, RET rearrangement, Her2 mutations, and PD-L1 testing.
- PD-L1 ≥50%: In patients with advanced SCC of the lung with PD-L1 ≥50%, preferred options for first-line treatment include single-agent pembrolizumab, cemiplimab, or atezolizumab, the combination of carboplatin with paclitaxel or albumin-bound paclitaxel and pembrolizumab, or cemiplimab with chemotherapy.
- PD-L1 1% to 49%: For SCC of the lung in this category, preferred first-line treatment options include combination chemotherapy with either pembrolizumab or cemiplimab.
- EGFR exon 19 deletion or exon 21 L858R mutations: Osimertinib is the preferred agent.[9] At progression there, patients can be treated with doublet chemotherapy and immune checkpoint inhibitor therapy. Patients treated with either erlotinib, afatinib, gefitinib, or dacomitinib in the first-line setting can be switched to osimertinib at progression after confirming T790M mutation either on liquid biopsy or tumor sample.[10]
- EGFR S768I, L861Q, or G719X mutations: Preferred agents for these groups of patients include afatinib or osimertinib.
- KRAS G12C: These patients are initially treated based on PD-L1 status and, at progression, are treated with either sotorasib or adagrasib.[11][12]
- ALK rearrangement: When ALK rearrangement is detected before the initiation of any systemic therapy for advanced SCC of the lung, it is preferred to use TKI therapy using agents alectinib, brigatinib, or lorlatinib.[13][14][15]
- ROS1 rearrangement: Patients with advanced ROS1 rearrangement cancers can be treated in a first-line setting with TKI therapies, including entrectinib, crizotinib, or repotrectinib.[16]
- BRAF V600E mutation: In patients with BRAF V600E mutation that is identified before any systemic therapies, it is preferred to BRAF/MEK inhibitor therapy, including either a combination of dabrafenib and trametinib or encorafenib and binimetinib.[17]
- NTRK 1/2/3 fusion: Larotrectinib or entrectinib can be used first-line to treat advanced SCC of the lung harboring NTRK gene fusion.[18][16]
- MET exon 14 skipping mutation: Advanced NSCLC patients with MET exon 14 skip mutation now have the option of first-line targeted therapy with capmatinib or tepotinib.[19][20]
- RET rearrangement: Preferred first-line targeted therapies of either selpercatinib or pralsetinib are currently available for NSCLC harboring RET rearrangements.[21]
- Her2 mutation: For patients with NSCLC in advanced stages harboring her2 mutations, trastuzumab deruxtecan is currently approved for second-line use after progression on first-line systemic chemotherapy to chemoimmunotherapy.[22]
Differential Diagnosis
SCC of the lung must be differentiated from other lung cancers, including small cell lung cancers (SCLC) and other NSCLCs such as adenocarcinoma and large cell carcinoma.
Surgical Oncology
Surgical resection is considered the standard of care for early-stage lung cancers in all medically fit patients.[23] Conventionally, Stage IIIA (T3N1) was considered the limit of surgical resection. Anatomic pulmonary resection, largely consisting of a lobectomy, is considered the gold standard. Video Assisted Thoracic Surgery (VATS) is the preferred noninvasive approach to anatomic lung resections. It has reduced the length of hospital and recovery times for patients compared to an open thoracotomy. Long-term oncologic outcomes appear to be equivalent.[24][25]
More limited resections consisting of segmentectomy or wedge resection for T1N0 NSCLC result in higher local recurrence rates (6% versus 18%) and higher death rates than a lobectomy but with better pulmonary function.[26] Despite this, more limited resections may be necessary in patients with poor pulmonary reserve or small nodules lesser than or equal to 2 cm that are pure AIS, doubling times more than 400 days, or greater than or equal to 50% GG on CT.[23] Indications for a VATS lobectomy include stage I to II disease, tumor sizes less than 6cm, more than 3cm from carina, more than 1cm from fissure line, and no mediastinal lymph node involvement. A pneumonectomy, which consists of total lung removal, may be necessary, but only after less radical interventions have been considered. They are usually indicated when the tumor is centrally located, involving a mainstem bronchus, or crosses major fissure lines. Pneumonectomies can have higher mortality rates and a substantial reduction in pulmonary reserve.[27] The representative N1 and ipsilateral N2 nodal stations should be evaluated regardless of the resection type. A minimum of 3 N2 nodal stations should be sampled.[23] This is critical for pathologic staging and determination of the need for adjuvant chemotherapy. The more extensive T3 and T4 tumors will require en-bloc resection.
Preoperative assessment is critical in identifying suitable candidates for surgery. A patient's history of cardiac disease, prior lung resections, COPD, bronchiectasis, and chronic pulmonary infections (eg, tuberculosis, interstitial lung diseases, pulmonary hypertension) can all affect the patient's ability to tolerate surgery. Determining a patient's pulmonary reserve and predicting their postoperative lung capacity is essential. The consequences of not taking this into account are severe disability and death. A simple and vital first step in preoperative assessment is the chest CT scan, which the patient would have already received. This can be used to count the number of lung segments, determine the extent of surgery required (ie, lobectomy versus pneumonectomy), and predict postoperative lung volumes.
Quantitative pulmonary function testing with spirometry and diffusion capacity is essential to determining a patient's eligibility for surgery. Forced expiratory volume in 1 second (FEV1) and diffusion capacity for carbon monoxide (DLCO) are considered the most important predictors of postoperative complications. The American College of Chest Physicians (ACCP) endorses these metrics and provides a valuable algorithm to determine the need for further testing. If a patient's FEV1 and DLCO is greater than or equal to 80%, no further testing is required; if DLCO or FEV1 falls below 80%, then predicted postoperative lung function testing should be performed.[28] Predicted postoperative values are determined by the preoperative values, the amount of lung to be resected, and its overall contribution to lung function. This is accomplished with either segment counting on CT chest (preferred in lobectomy) or lung scintigraphy, preferred in pneumonectomy.[28] From this information, a predicted postoperative FEV1 or predicted postoperative DLCO can be determined. No further testing is required if the predicted postoperative FEV1 or predicted postoperative DLCO is greater than or equal to 60%.[28] Below 60%, further risk stratification is needed, usually with a cardiopulmonary exercise test or a simple walking test would be indicated. Based on the VOmax, the patients are risk stratified into low, moderate, and high risk. In low-risk patients, mortality is less than 1%, while in high-risk patients, the risk of morbidity and mortality exceeds 10%.
Mortality for lobectomies ranges from 1.4% to 2.6%.[29][30] The most common complications include air leaks, with a reported incidence of 15% to 25%. Atrial fibrillation can occur in up to 40% of patients.[29] Pneumonia has been reported in 2.5% to 6% of all patients.[30] Less common complications include hemorrhage, chylothorax, phrenic nerve injury, recurrent laryngeal nerve injury, bronchopleural fistula, and right middle lobe torsion.[29] Pneumonectomies tend to carry higher rates of mortality and morbidity. Mortality ranges from 5% to 11%, and morbidity can be as high as 60%. The most common complications include bronchopulmonary fistula, cardiac arrhythmias (19%), pneumonia (3.5%), and empyema (4.8%).[31][32]
Radiation Oncology
Radiotherapy alone was a primary mode of treatment for patients with inoperable lungs before the introduction of chemotherapy in the 1980s. Since that time, the delivery and role of radiation therapy in squamous cell lung cancer and non-small cell lung cancer, in general, have evolved considerably. Early-stage lung cancers can be cured with radiotherapy alone, while more advanced disease typically requires the use of concurrent chemoradiation. Radiotherapy can be utilized in definitive, adjuvant, recurrent, and palliative settings with or without chemotherapy. Various techniques and fractionation schedules can be employed depending on the situation and treatment goals.
Definitive Radiotherapy
In general, patients with locally advanced inoperable squamous cell lung cancer and NSCLC are typically offered concurrent platinum-based chemoradiotherapy followed by adjuvant Durvalumab as the standard of care.[23] However, it may also be offered after induction chemotherapy as well.[23] The use of chemotherapy in this setting, in addition to radiotherapy, demonstrated a survival improvement over radiotherapy alone.[33] Further trials demonstrated that the ideal sequencing delivers radiotherapy and chemotherapy concurrently, improving 5-year survival rates by 5%.[34][35] A meta-analysis for concurrent versus sequential showed a 5.7% improvement in overall survival at 3 years.[35]
Preoperative Radiotherapy
Preoperative radiotherapy is typically accompanied by platinum-based chemotherapy. Doses are usually lower than definitive therapy, ranging from 45 to 50 Gy.[23] Indications for this approach are typically for resectable superior sulcus tumors but could be utilized in several circumstances. Randomized evidence comparing definitive chemoradiotherapy to 61Gy alone or preoperative chemoradiotherapy to 45Gy followed by surgical resection in technically resectable Stage IIIA NSCLC demonstrated no difference in overall survival.[27] However, subgroup analysis of the surgical patients found superior 5-year overall survival (18% versus 36%) in patients who received a lobectomy rather than a pneumonectomy. In patients with superior sulcus tumors (T3-4 N0-1 M0), the primary approach considered is preoperative chemoradiotherapy to 45Gy followed by resection if there is no progression on follow-up imaging.[23] This approach had excellent outcomes, with a 5-year overall survival rate of 44% for all patients and 53% for complete resections.[36]
Postoperative Radiotherapy
Postoperative radiotherapy is typically indicated in patients who have residual disease postoperatively. Doses vary from 54 to 60 Gy for an R1 resection or extracapsular extension to 60 to 70 Gy for patients with gross residual disease. The use of postoperative radiotherapy for those with N2 disease has been controversial until recently. The LUNG-ART trial randomized patients with completely resected N2 NSCLC to receive 54 Gy to the primary surgical bed and mediastinal nodes or no radiation. No difference in disease-free survival was found.[37]
Hypofractionated Radiotherapy
Hypofractionated radiotherapy utilizes doses that exceed more than 2 Gy/fraction but are lower than what can be delivered with SBRT and is typically a more protracted course. It can be employed in early-stage disease where the 5 fraction dose constraints for SBRT cannot be met, such as in ultra-central tumors defined as the GTV or PTV abutting or overlapping the proximal bronchial tree, trachea, or esophagus, tumors more than 5 cm, or in palliative cases.[38] Doses typically range from 60 to 70 Gy in 8 to 15 fractions.[23][39][40] Local control rates range from 85% to 92% at 2 years with acceptable toxicity.[40][41][42] Several ongoing trials, such as the SUNSET and LUSTRE trials, are currently examining the role of hypofractionated regimens.[43][44]
Radiotherapy Dosing and Constraints
The ideal dosing for conventionally fractionated radiotherapy was established by the RTOG 7301 trial from the 1970s, which tested doses ranging from 30 to 60 Gy delivered continuously or as a split course.[45] It asserted that the ideal dosing was 60 Gy in 2 Gy per fraction. NCCN guidelines allow a range of 60 to 70 Gy in 2Gy/fraction.[23] Several dose-escalation trials have been attempted with varying degrees of success since that time. Randomized evidence of 60 Gy versus 74 Gy for unresectable stage III NSCLC resulted in inferior overall survival at 5 years (32% versus 23%) and higher rates of pneumonitis and esophagitis.[46] More contemporary trials, such as CRTOG 1601, have attempted selective dose escalation based on positron emission tomography (PET) or computed tomography (CT) adaptive planning, demonstrating an improvement in median survival of 44.6 versus 28 months. Patients were randomized to 60 Gy in 30 fractions or underwent dose escalation with an extra 22 to 32 Gy in 10 fractions based on PET or CT adaptive planning.
Dose constraints have been established for various organs at risk (OARs) that clinicians may encounter in the thorax. The most common include the spinal cord, heart, esophagus, normal lung, and brachial plexus. The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) guides appropriate dose constraints relative to the risk of toxicity. The spinal cord should generally be less than 45 Gy point max dose for a near zero risk of cord myelopathy. The mean esophagus dose should be kept at less than 34 Gy and V50 less than 35%. Normal lung dose constraints have been studied with various metanalysis. Ideally, the lung V20 less than 20%, which had an 18% pneumonitis rate with no deaths.[47] Unfortunately, it may not be possible to attain this, and clinical decisions will need to be made regarding target coverage relative to the risk of acute and late toxicities.
Radiotherapy Complications
Intensity-modulated radiotherapy has modestly reduced the rates of toxicity associated with conventional radiation compared to 3D conformal but without a change in oncologic outcomes.[46] Pneumonitis is a common complication with lung radiotherapy that can result in significant long-term disability and death in some cases. Historically, pneumonitis rates were approximately 30% in the 3D conformal era, but with IMRT, the rate is now around 8%.[46] The V20 is the most frequently used dosimetric parameter to evaluate radiation plans, and the risk of pneumonitis A V20 Gy ranging from 20% to 40% has a 30% to 40% rate of pneumonitis.[48] The addition of chemotherapy agents such as carboplatin/paclitaxel can increase this risk.[48] Esophagitis occurs in roughly 30% to 40% of patients and depends on the tumor's location and the dose to the esophagus. Cardiovascular toxicity ranges from 5% to 8% and is correlated with the V40 parameter. For each additional gray received by the heart, there appears to be a 7.4% increase in the relative risk of major coronary events with no apparent threshold dose.[49]
Stereotactic Body Radiotherapy
Stereotactic body radiotherapy (SBRT) is defined as the use of imaged guided high-dose radiation therapy delivered in 5 fractions or less to ablate the tumor.[50] In the setting of lung SCC, it typically has a role in the treatment of central and peripheral early-stage primary lung tumors (T1-T3N0) and an emerging role in the treatment of oligometastatic disease, defined as less than 3 to 5 sites of metastasis.[51] [52]
While VATS lobectomy remains the standard of care for early-stage lung cancers, SBRT can be considered in patients who are medically inoperable, technically inoperable, or who refuse surgery. SBRT is a noninvasive and well-tolerated technique with 3-year primary tumor control rates exceeding 90% for peripheral and 80% for centrally located tumors.[52][53][54] Prospective trials comparing surgery to SBRT have been attempted but have closed due to poor accrual.[55] However, the results suggest comparable rates of disease control with acceptable toxicity but do suggest comparable relapse-free survival rates (80% surgery versus 86% SABR).[55]
These trials had several caveats, specifically low patient enrollment (58 patients) and very few patients who underwent VATS lobectomy, which would be expected to improve outcomes compared to open resection. Current prospective trials are again investigating resection versus SBRT. The STABL-MATES trial (NCT02468024) is investigating sub lobar resection versus 54 Gy in 3 fractions of SBRT, while the VALOR trial (NCT02984761) is investigating anatomic resection compared to SBRT. Larger retrospective reviews examining SBRT versus surgery for the early-stage disease have found comparable cancer-specific survival despite having inferior overall survival.[56] Other studies have shown similar OS and DFS after adjusting for age and operability. Local control was also comparable (85% versus 87% at 3 years).[57]
A unique approach utilizing neoadjuvant SBRT followed by resection has been investigated in the MISSILE trial, demonstrating 100% 2-year local control and 77% 2-year overall survival with excellent quality of life.[58] The pathologic complete response rate was 60%, and it was lower than expected, which may have been due to the short interval between SBRT and surgery.[58] Other caveats include short follow-up and a small number of enrolled patients.[58]
Stereotactic body radiotherapy dosing and constraints
Local control is highly dependent on the biologically equivalent dose (BED). Tumors receiving BED <100 Gy have a 5-year local control rate of 36.5%, while those with ≥100 Gy are 84.2%.[59] The 5-year overall survival in patients receiving BED less than 100 Gy was 19.7% and 53.9% for those greater than or equal to 100 Gy. Therefore, the dose and fractionation are critical but must be balanced against potential toxicity. Peripheral tumors are defined as being at least 2 cm away from the proximal bronchial tree. They can typically receive higher doses in smaller fractions than centrally located tumors within 2 cm of the bronchial tree.[54][60]
NCCN guidelines provide a comprehensive list of potential SBRT doses and fractionation schedules.[23] Peripheral tumors less than 2 cm in size and more than 1 cm from the chest wall can receive 25 to 34 Gy in a single fraction, and larger tumors can receive 45 to 60 Gy in 3 fractions, provided they are more than 1 cm from the chest wall. Doses of 50 to 55 Gy in 5 fractions are more versatile and can be used with peripheral tumors less than 1 cm from the chest wall and central tumors. More protracted hypofractionated regimens can also be considered for central tumors. SBRT dose constraints for these regimens can be found in the NCCN guidelines for non-small cell lung cancer or the AAPM Task-group 101.[23][61] Major structures of concern in the thorax include the spinal cord, normal lung, heart, great vessels, and ribs. These constraints are meant to help minimize the risk of late adverse toxicities and should be followed closely. If there are difficulties meeting dose constraints, a more fractionated approach with a lower dose per fraction can be considered. Target coverage evaluation is also critical, and certain metrics have been developed to evaluate and compare target coverage. Conformity less than 1.2 to 1.5, gradient R ranging from 2.9 to 5.9 dependent on target volume, and overall coverage V-100% are evaluated.[60][62]
Set-up and planning
Given the high doses and the small number of fractions in SBRT, it is critical to have highly reproducible positioning and targeting accuracy. A CT simulation is typically the first step with the patient placed in the supine position on a stereotactic body frame and a CT slice thickness of ≤5 mm but recommended 1 to 3 mm.[61] The scan range should be from the cricoid superiorly to the L2 vertebrae inferiorly.
The movement of the target caused by the respiratory cycle is an inherent problem with lung SBRT. While there is relatively little movement with tumors in the upper lobes of the lung, the closer the tumor is to the diaphragm, the more tumor motion can be expected. If tumor motion exceeds 5 mm, it is recommended that some motion management techniques be employed.[63] Several techniques to mitigate this issue include 4-dimensional CT (4D-CT), respiratory gating, abdominal compression, and fiducial marker tracking.
A 4-dimensional CT is a simulation scan containing both spatial and temporal information regarding the tumor's location in each respiratory cycle phase. A series of "slow" CT scans are acquired and binned according to the respiratory phase. They are typically divided into 10 phases. These sequences can then be "played," and tumor motion can be appreciated. If the tumor motion exceeds 1 cm, additional devices, such as abdominal compression, may be used to limit tumor motion. Abdominal compression devices can range from a relatively simple belt placed around the patient's abdomen to more sophisticated devices that are attached to the treatment table and apply pressure to the abdomen in predetermined increments.
Respiratory gating is another technique that isolates a particular portion of the respiratory cycle to deliver radiation. Breathing is monitored with either spirometry or surface monitoring.[64] The spirometric techniques typically occlude breathing either by a valve (ie, active breathing control) or voluntarily (ie, deep inspiratory breath hold). These techniques typically require coaching the patient and determining the most comfortable degree of inspiration for optimal breath hold, usually 70% to 80% of inspiration.[64] The surface monitoring technique uses a plastic box with markers that reflect infrared light. The apparatus is placed on the patient and can be tracked in 3 dimensions in real-time. These techniques tend to result in longer treatment times but can reduce the volume of uninvolved lung radiated.
Fiducial marker tracking is another option for targeting. Depending on the tumor's location, it requires implanting a radiopaque marker, typically gold, either with endoscopy or CT guidance. The fiducial markers can be tracked using cone beam CT, kilovoltage x-ray imaging (kV imaging), or an automated tracking system to identify the offset and calculate the required shifts. The disadvantage is that it can add additional time, cost, and treatment complications but allows for smaller expansions, thus sparing additional uninvolved lung tissue. Each marker must remain in the same place relative to the other markers and the tumor. The markers also risk migration, requiring a second implantation. Migration rates range from 1% to 19%, depending on the type of marker implanted.[65]
Once the patient has been simulated, target delineation can begin. The CT from the initial simulation and a fused PET/CT can ensure the target is completely outlined. Contouring in lung windowing is especially helpful. Once the gross tumor volume has been outlined, expansions to the volume account for tumor motion and set-up uncertainty. If the institution has 4D-CT capabilities, the GTV is contoured on every phase of respiration and then combined to form an internal target volume (ITV). Alternatively, the maximal intensity projection (MIP) could also be used to construct an ITV. However, it is vulnerable to error in cases of irregular breathing or close proximity to the diaphragm. A planning target volume expansion (PTV) is typically 5 mm.
Stereotactic body radiotherapy delivery
Treatment delivery of SBRT can be delivered with dedicated stereotactic systems such as Cyberknife or with various linac-based systems utilizing intensity modulation. What is critical in SBRT delivery is the need for onboard image guidance to ensure accurate targeting. This can be accomplished with kV imaging, megavoltage (MV)-based planar imaging, or volumetric with cone beam CT.
Stereotactic body radiotherapy complications
SBRT is generally well tolerated, but the risk of acute or long-term toxicities should be considered when recommending treatment. The risks are determined by the tumor's location (eg, peripheral, central, or ultra-central), dose, and volume of irradiated tissue. Radiation pneumonitis is a significant source of toxicity in lung radiotherapy and can result in lung fibrosis, the need for oxygen therapy, and even mechanical ventilation. The incidence ranges from 0% to 29%. Still, the severity is usually non-life threatening, even in patients with known lung disease, which is likely due to the smaller volumes radiated with SBRT in general.[66]
Centrally located tumors treated tend to have higher toxicity rates than peripheral lesions. They also have a unique set of complications according to their location. Esophageal toxicity ranging from stenosis to tracheoesophageal fistula has been reported with an overall incidence of 12%.[67] Other complications, such as spontaneous pneumothorax, fatal hemoptysis, and vascular injury, are uncommon but have been reported in the literature and have a higher risk in the repeated irradiation setting.
Chest wall pain is usually observed with peripherally located tumors in approximately 30% of cases several months to years posttreatment. Rib fractures can occur up to 2 years posttreatment with an incidence of 5%, provided threshold volume and dose thresholds are met.[66] Skin toxicity is not as common, ranging from 1.2% to 14%.[66] It depends on the body's habitus and the distance between the overlying skin and the tumor. Brachial plexopathy is unique to tumors in the apex of the lungs.[66] The incidence is dose-dependent, but if the max dose to the brachial plexus can be kept at less than 26 Gy, the risk of plexopathy is around 8%.
Repeated Irradiation
Most evidence for reirradiation of lung tumors after primary definitive radiotherapy is confined mainly to retrospective studies. Local relapse for NSCLC can occur within 2 years in about one-third of patients, and the rate of second primary lung tumors is 14% at 10 years.[68] Any patient being evaluated for repeated irradiation should also be assessed for repeated resection. Reirradiation is increasing with time, likely due to more posttreatment surveillance CT scans and improved radiation delivery methods, which allow for more normal tissue sparing. The survival rates in locally recurrent lung cancer are quite poor, with 2-year survival rates of 21%.[69]
Several technical challenges are associated with reirradiation, including the lack of a standard definition of what constitutes reirradiation, disagreement regarding an acceptable time interval between treatments, the ideal method of delivering radiation, and the lack of readily accepted cumulative dose tolerances. Several papers have attempted to address these issues with varying degrees of success. To establish a consensus, an expert survey was taken. Although a consensus failed to emerge, most experts defined reirradiation as any overlap in the previously radiated PTV or organs at risk.[68] The question of how much overlap is considered significant is traditionally considered at the 50% isodose line; however, this is also controversial. The minimum interval of 6 months is generally considered acceptable, although no official consensus exists.[68] Patients are unlikely to benefit from retreatment if they progressed in such a short amount of time or had Grade 3 or higher toxicity from the initial course.
Highly conformal radiation delivery with SBRT, IMRT/VMAT, or proton therapy can be considered.[68] Using conventional fractionation or moderate hypofractionation, 60 Gy in 30 or 55 Gy in 20 fractions are deemed acceptable. SBRT dosing depends on the location of the lesion being treated (ie, central versus peripheral) and ranges from 20 to 60 Gy in 1 to 5 fractions.[68] It is the preferred treatment method for peripheral recurrences and may be considered for central disease. However, SBRT is not regarded as appropriate for retreatment of ultra-central recurrences due to high rates of toxicity and death.[70]
Devising acceptable dose constraints has been particularly challenging due to the lack of data regarding normal tissue recovery and dose or toxicity data in humans. Several groups have attempted to assemble cumulative dose constraints. However, there is no agreed-upon set of constraints or agreement on how to evaluate or report cumulative doses. The methods used to compare and report cumulative doses are the equieffective dose in 2Gy fractions (EQD2) or the biologic effective dose (BED). Both of these methods attempt to ensure a uniform comparison of doses delivered. There are several published putative cumulative dose constraints listed in EQD2.[68]
Complications resulting from retreatment include radiation pneumonitis, esophagitis, chest wall pain, cardiac toxicity, and death. Esophagitis was the most commonly reported toxicity, with a rate of 17%, followed by pneumonitis, with a rate of 12.3%. Rib fractures and spinal cord myelopathy occurred in <1% of cases. The risk of cardiac toxicity is related to the mean heart dose, and the relative risk increases by 7% for every additional 1Gy.[70] Treatment-related deaths were 1.6%.[71]
Pulmonary function testing should be considered, although there are no defined thresholds under which retreatment should not be offered. PFTs may be unnecessary for small peripheral recurrences treated with SBRT.[68] Retreatment should be individualized and discussed in a multidisciplinary tumor board setting.
Palliative Radiotherapy
There are several circumstances where radiotherapy can be used for palliative purposes, including chest wall pain, bronchial obstruction, superior vena cava syndrome, hemoptysis, bone metastasis, and brain metastasis. Chest wall pain can be treated with doses ranging from 20 to 30 Gy in 5 to 10 fractions daily or in patients with particularly poor functional status, 17 Gy in 2 fractions given weekly.[23] Superior vena cava syndrome is not considered an oncologic emergency if the patient is stable. Typical doses range from 30 to 45 Gy in 10 to 15 fractions. Bone metastasis can be treated to 8 to 30 Gy in 1 to 10 fractions. Radiation is also helpful in promoting hemostasis in low-volume hemoptysis with doses of 8 to 30 Gy in 1 to 10 fractions. Various means can treat brain metastasis depending on the number of lesions, size of the brain lesions, and institutional resources. In patients with >10 lesions, whole brain radiotherapy is usually recommended at a dose of 30 Gy in 10 fractions.
Staging
Staging in lung cancer is based on CT scan images according to the Tumor-Node-Metastasis (TNM) staging system. The following is a summary of the eighth Edition of TNM in Lung Cancer, issued by the IASLC (International Association for the Study of Lung Cancer) and replaced by the TNM seventh edition.[72]
Table. Eighth Edition of TNM Staging in Lung Cancer
| T: Primary tumor | |
| Tx | Primary tumors cannot be assessed or tumor-proven by the presence of malignant cells in sputum or bronchial washings but are not visualized by imaging or bronchoscopy. |
| T0 | No evidence of a primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤3 cm in its greatest dimension surrounded by lung or visceral pleura without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus) |
|
Minimally invasive adenocarcinoma |
|
Tumor ≤1 cm in greatest dimension |
|
Tumor >1 cm but ≤2 cm in greatest dimension |
|
Tumor >2 cm but ≤3 cm in the greatest dimension |
| T2 | Tumor >3 cm but ≤5 cm or tumor with any of the following features : |
|
|
|
Tumor >3 cm but ≤4 cm in greatest dimension |
|
Tumor >4 cm but ≤5 cm in greatest dimension |
| T3 | Tumor >5 cm but ≤7 cm in greatest dimension or associated with separate tumor nodule(s) in the same lobe as the primary tumor or directly invades any of the following structures: chest wall (including the parietal pleura and superior sulcus tumors), phrenic nerve, the parietal pericardium |
| T4 | Tumor >7 cm in greatest dimension or associated with separate tumor nodule(s) in a different ipsilateral lobe than that of the primary tumor or invades any of the following structures: diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, and carina. |
| N: Regional lymph node involvement | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in ipsilateral peribronchial and ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Metastasis in ipsilateral mediastinal and subcarinal lymph node(s) |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| M: Distant metastasis | |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
|
Separate tumor nodule(s) in a contralateral lobe; tumor with pleural or pericardial nodule(s) or malignant pleural or pericardial effusion |
|
Single extrathoracic metastasis |
|
Multiple extrathoracic metastases in ≥1 organs |
- a: The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a.
- b: Solitary adenocarcinoma, ≤3 cm with a predominately lepidic pattern and ≤5 mm invasion in any focus.
- c: T2 tumors with these features are classified as T2a if ≤4 cm in greatest dimension or if the size cannot be determined, and T2b if >4 cm but ≤5 cm in greatest dimension.
- d: Most pleural (pericardial) effusions with lung cancer are due to tumors. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is non-bloody and not an exudate. When these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging descriptor.
- e: This includes the involvement of a single distant (nonregional) lymph node.
Table. Summary of TNM Staging for Lung Cancer, Eight Edition. Note: Changes from the seventh edition are in bold.
| Occult carcinoma | TX | N0 | M0 |
| Stage 0 | Tis | N0 | M0 |
| Stage IA1 | T1a(mi) | N0 | M0 |
| T1a | N0 | M0 | |
| Stage IA2 | T1b | N0 | M0 |
| Stage IA3 | T1c | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T2b | N0 | M0 |
| Stage IIB | T1a–c | N1 | M0 |
| T2a | N1 | M0 | |
| T2b | N1 | M0 | |
| T3 | N0 | M0 | |
| Stage IIIA | T1a–c | N2 | M0 |
| T2a –b | N2 | M0 | |
| T3 | N1 | M0 | |
| T4 | N0 | M0 | |
| T4 | N1 | M0 | |
| Stage IIIB | T1a–c | N3 | M0 |
| T2a–b | N3 | M0 | |
| T3 | N2 | M0 | |
| T4 | N2 | M0 | |
| Stage IIIC | T3 | N3 | M0 |
| T4 | N3 | M0 | |
| Stage IVA | Any T | Any N | M1a |
| Any T | Any N | M1b | |
| Stage IVB | Any T | Any N | M1c |
Prognosis
SCC of the lungs can spread to multiple sites, including the brain, spine, bones, adrenal glands, and liver. Due to the lower incidence of biologically relevant molecular targets for SCC and the late detection stage, the prognosis is often poor for these patients. However, it is to be noted that in advanced stages with higher levels of PD-L1 expression, checkpoint inhibitor therapies have provided better and prolonged responses in comparison to nonsquamous histologies.
Complications
Complications from SCC of the lung can include:
- Shortness of breath if the tumor obstructs the major airways or causes fluid to accumulate around the lungs (ie, pleural effusion)
- Bleeding in the airway, resulting in hemoptysis
- Metastasis resulting in pain and neurological complications
Postoperative and Rehabilitation Care
Preoperative exercises and rehabilitation programs have shown significant postoperative outcomes and reduced postoperative pulmonary complications.[73] However, postoperative pulmonary rehabilitation without preoperative exercise showed only a small to moderate effect on postoperative exercise capacity on the short-term follow-up. Still, the long-term impact on functional capacity is unknown.[74]
Consultations
Clinicians that are typically consulted in the management of SCC of the lung include:
- Pulmonology
- Cardiothoracic surgery
- Medical oncology
- Surgical oncology
- Palliative care
- Psychiatry
Deterrence and Patient Education
Education on avoiding or mitigating risk factors such as tobacco products and causes of occupational disease (eg, using personal protective equipment) can reduce SCC development. Education about early cancer screening with low-dose CT scan of the chest is essential for early recognition and treatment to prevent tumor burden. Counseling patients about the stages of cancer and the chance of a complete cure in case of early detection and counseling on palliative care in advanced stages is critical to reducing stress both for patients and loved ones.
Enhancing Healthcare Team Outcomes
Given the complexity of care required to treat this condition, an interprofessional healthcare team is needed, including primary clinicians, pulmonologists, cardiothoracic surgeons, medical oncologists, radiation oncologists, surgical oncologists, and palliative care. Nurses, pharmacists, and respiratory and physical therapists round out the care team. Strong collaboration and communication within the interprofessional team and meticulous record keeping, allowing all care team members access to the most accurate and updated patient information, are vital to improving outcomes.
Clinicians will direct the overall direction of the case, with other specialties working in their particular discipline. Nurses will play a pivotal role in helping with patient assessment, assisting in surgery, providing postsurgical care, administering chemotherapy, and offering patient counsel. An oncology-specialized pharmacist should oversee all chemotherapy regimens and can also check for drug interactions, answer clinician and patient questions about the drugs, and help monitor the patient's progress. All interprofessional team members must be open to communication from the rest of the team and report any concerns to everyone involved in patient care. This interprofessional team approach to case management for squamous cell lung cancer will yield optimal benefits with minimal adverse events.
Media
(Click Image to Enlarge)
References
Chaudhuri MR. Primary pulmonary cavitating carcinomas. Thorax. 1973 May:28(3):354-66 [PubMed PMID: 4353362]
Gridelli C, Ardizzoni A, Douillard JY, Hanna N, Manegold C, Perrone F, Pirker R, Rosell R, Shepherd FA, De Petris L, Di Maio M, de Marinis F. Recent issues in first-line treatment of advanced non-small-cell lung cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Lung cancer (Amsterdam, Netherlands). 2010 Jun:68(3):319-31. doi: 10.1016/j.lungcan.2009.11.018. Epub 2009 Dec 24 [PubMed PMID: 20036027]
Level 3 (low-level) evidencePerez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 May 1:18(9):2443-51. doi: 10.1158/1078-0432.CCR-11-2370. Epub 2012 Mar 8 [PubMed PMID: 22407829]
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012 Sep 27:489(7417):519-25. doi: 10.1038/nature11404. Epub 2012 Sep 9 [PubMed PMID: 22960745]
Dietel M, Bubendorf L, Dingemans AM, Dooms C, Elmberger G, García RC, Kerr KM, Lim E, López-Ríos F, Thunnissen E, Van Schil PE, von Laffert M. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax. 2016 Feb:71(2):177-84. doi: 10.1136/thoraxjnl-2014-206677. Epub 2015 Nov 3 [PubMed PMID: 26530085]
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I, WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015 Sep:10(9):1243-1260. doi: 10.1097/JTO.0000000000000630. Epub [PubMed PMID: 26291008]
Level 3 (low-level) evidenceSilvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May:143(5 Suppl):e211S-e250S. doi: 10.1378/chest.12-2355. Epub [PubMed PMID: 23649440]
Level 1 (high-level) evidenceLindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013 Jul:8(7):823-59. doi: 10.1097/JTO.0b013e318290868f. Epub [PubMed PMID: 23552377]
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine. 2018 Jan 11:378(2):113-125. doi: 10.1056/NEJMoa1713137. Epub 2017 Nov 18 [PubMed PMID: 29151359]
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. The New England journal of medicine. 2017 Feb 16:376(7):629-640. doi: 10.1056/NEJMoa1612674. Epub 2016 Dec 6 [PubMed PMID: 27959700]
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, Kato T, Curioni-Fontecedro A, Sacher A, Spira A, Ramalingam SS, Takahashi T, Besse B, Anderson A, Ang A, Tran Q, Mather O, Henary H, Ngarmchamnanrith G, Friberg G, Velcheti V, Govindan R. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. The New England journal of medicine. 2021 Jun 24:384(25):2371-2381. doi: 10.1056/NEJMoa2103695. Epub 2021 Jun 4 [PubMed PMID: 34096690]
Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, Bazhenova L, Negrao MV, Pennell NA, Zhang J, Anderes K, Der-Torossian H, Kheoh T, Velastegui K, Yan X, Christensen JG, Chao RC, Spira AI. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. The New England journal of medicine. 2022 Jul 14:387(2):120-131. doi: 10.1056/NEJMoa2204619. Epub 2022 Jun 3 [PubMed PMID: 35658005]
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T, ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017 Aug 31:377(9):829-838. doi: 10.1056/NEJMoa1704795. Epub 2017 Jun 6 [PubMed PMID: 28586279]
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, Gridelli C, Delmonte A, Garcia Campelo R, Kim DW, Bearz A, Griesinger F, Morabito A, Felip E, Califano R, Ghosh S, Spira A, Gettinger SN, Tiseo M, Gupta N, Haney J, Kerstein D, Popat S. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2018 Nov 22:379(21):2027-2039. doi: 10.1056/NEJMoa1810171. Epub 2018 Sep 25 [PubMed PMID: 30280657]
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, Solomon BJ, CROWN Trial Investigators. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. The New England journal of medicine. 2020 Nov 19:383(21):2018-2029. doi: 10.1056/NEJMoa2027187. Epub [PubMed PMID: 33207094]
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD, trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. The Lancet. Oncology. 2020 Feb:21(2):271-282. doi: 10.1016/S1470-2045(19)30691-6. Epub 2019 Dec 11 [PubMed PMID: 31838007]
Planchard D, Besse B, Groen HJM, Hashemi SMS, Mazieres J, Kim TM, Quoix E, Souquet PJ, Barlesi F, Baik C, Villaruz LC, Kelly RJ, Zhang S, Tan M, Gasal E, Santarpia L, Johnson BE. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2022 Jan:17(1):103-115. doi: 10.1016/j.jtho.2021.08.011. Epub 2021 Aug 26 [PubMed PMID: 34455067]
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. The New England journal of medicine. 2018 Feb 22:378(8):731-739. doi: 10.1056/NEJMoa1714448. Epub [PubMed PMID: 29466156]
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, Tan DSW, Hida T, de Jonge M, Orlov SV, Smit EF, Souquet PJ, Vansteenkiste J, Hochmair M, Felip E, Nishio M, Thomas M, Ohashi K, Toyozawa R, Overbeck TR, de Marinis F, Kim TM, Laack E, Robeva A, Le Mouhaer S, Waldron-Lynch M, Sankaran B, Balbin OA, Cui X, Giovannini M, Akimov M, Heist RS, GEOMETRY mono-1 Investigators. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. The New England journal of medicine. 2020 Sep 3:383(10):944-957. doi: 10.1056/NEJMoa2002787. Epub [PubMed PMID: 32877583]
Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van Meerbeeck J, Raskin J, Reinmuth N, Conte P, Kowalski D, Cho BC, Patel JD, Horn L, Griesinger F, Han JY, Kim YC, Chang GC, Tsai CL, Yang JC, Chen YM, Smit EF, van der Wekken AJ, Kato T, Juraeva D, Stroh C, Bruns R, Straub J, Johne A, Scheele J, Heymach JV, Le X. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. The New England journal of medicine. 2020 Sep 3:383(10):931-943. doi: 10.1056/NEJMoa2004407. Epub 2020 May 29 [PubMed PMID: 32469185]
Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, Peled N, Weiss J, Kim YJ, Ohe Y, Nishio M, Park K, Patel J, Seto T, Sakamoto T, Rosen E, Shah MH, Barlesi F, Cassier PA, Bazhenova L, De Braud F, Garralda E, Velcheti V, Satouchi M, Ohashi K, Pennell NA, Reckamp KL, Dy GK, Wolf J, Solomon B, Falchook G, Ebata K, Nguyen M, Nair B, Zhu EY, Yang L, Huang X, Olek E, Rothenberg SM, Goto K, Subbiah V. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2020 Aug 27:383(9):813-824. doi: 10.1056/NEJMoa2005653. Epub [PubMed PMID: 32846060]
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E, Pacheco JM, Pérol M, Paz-Ares L, Saxena K, Shiga R, Cheng Y, Acharyya S, Vitazka P, Shahidi J, Planchard D, Jänne PA, DESTINY-Lung01 Trial Investigators. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. The New England journal of medicine. 2022 Jan 20:386(3):241-251. doi: 10.1056/NEJMoa2112431. Epub 2021 Sep 18 [PubMed PMID: 34534430]
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico TA, DeCamp M, Dilling TJ, Dowell J, Gettinger S, Grotz TE, Gubens MA, Hegde A, Lackner RP, Lanuti M, Lin J, Loo BW, Lovly CM, Maldonado F, Massarelli E, Morgensztern D, Ng T, Otterson GA, Pacheco JM, Patel SP, Riely GJ, Riess J, Schild SE, Shapiro TA, Singh AP, Stevenson J, Tam A, Tanvetyanon T, Yanagawa J, Yang SC, Yau E, Gregory K, Hughes M. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2022 May:20(5):497-530. doi: 10.6004/jnccn.2022.0025. Epub [PubMed PMID: 35545176]
Level 1 (high-level) evidenceBerry MF, D'Amico TA, Onaitis MW, Kelsey CR. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. The Annals of thoracic surgery. 2014 Jul:98(1):197-202. doi: 10.1016/j.athoracsur.2014.03.018. Epub 2014 May 10 [PubMed PMID: 24820392]
Level 2 (mid-level) evidenceMurakawa T, Ichinose J, Hino H, Kitano K, Konoeda C, Nakajima J. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World journal of surgery. 2015 May:39(5):1084-91. doi: 10.1007/s00268-014-2918-z. Epub [PubMed PMID: 25561187]
Level 2 (mid-level) evidenceGinsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of thoracic surgery. 1995 Sep:60(3):615-22; discussion 622-3 [PubMed PMID: 7677489]
Level 1 (high-level) evidenceAlbain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet (London, England). 2009 Aug 1:374(9687):379-86. doi: 10.1016/S0140-6736(09)60737-6. Epub 2009 Jul 24 [PubMed PMID: 19632716]
Level 1 (high-level) evidenceBrunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May:143(5 Suppl):e166S-e190S. doi: 10.1378/chest.12-2395. Epub [PubMed PMID: 23649437]
Level 1 (high-level) evidenceZiarnik E, Grogan EL. Postlobectomy Early Complications. Thoracic surgery clinics. 2015 Aug:25(3):355-64. doi: 10.1016/j.thorsurg.2015.04.003. Epub 2015 Jun 12 [PubMed PMID: 26210931]
Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ, Rusch VW, Putnam JB Jr. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. The Journal of thoracic and cardiovascular surgery. 2011 Mar:141(3):662-70. doi: 10.1016/j.jtcvs.2010.11.008. Epub [PubMed PMID: 21335122]
Level 1 (high-level) evidenceJoo JB, DeBord JR, Montgomery CE, Munns JR, Marshall JS, Paulsen JK, Anderson RC, Meyer LE, Estes NC. Perioperative factors as predictors of operative mortality and morbidity in pneumonectomy. The American surgeon. 2001 Apr:67(4):318-21; discussion 321-2 [PubMed PMID: 11307996]
Level 2 (mid-level) evidenceKlemperer J, Ginsberg RJ. Morbidity and mortality after pneumonectomy. Chest surgery clinics of North America. 1999 Aug:9(3):515-25, vii [PubMed PMID: 10459427]
Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. Journal of the National Cancer Institute. 1996 Sep 4:88(17):1210-5 [PubMed PMID: 8780630]
Level 1 (high-level) evidenceCurran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. Journal of the National Cancer Institute. 2011 Oct 5:103(19):1452-60. doi: 10.1093/jnci/djr325. Epub 2011 Sep 8 [PubMed PMID: 21903745]
Level 2 (mid-level) evidenceAupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 May 1:28(13):2181-90. doi: 10.1200/JCO.2009.26.2543. Epub 2010 Mar 29 [PubMed PMID: 20351327]
Level 1 (high-level) evidenceRusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, Johnson DH, Shulman L, Shepherd F, Deschamps C, Livingston RB, Gandara D. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Jan 20:25(3):313-8 [PubMed PMID: 17235046]
Le Pechoux C, Pourel N, Barlesi F, Lerouge D, Antoni D, Lamezec B, Nestle U, Boisselier P, Dansin E, Paumier A, Peignaux K, Thillays F, Zalcman G, Madelaine J, Pichon E, Larrouy A, Lavole A, Argo-Leignel D, Derollez M, Faivre-Finn C, Hatton MQ, Riesterer O, Bouvier-Morel E, Dunant A, Edwards JG, Thomas PA, Mercier O, Bardet A. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. The Lancet. Oncology. 2022 Jan:23(1):104-114. doi: 10.1016/S1470-2045(21)00606-9. Epub 2021 Dec 15 [PubMed PMID: 34919827]
Level 1 (high-level) evidenceChaudhuri AA, Tang C, Binkley MS, Jin M, Wynne JF, von Eyben R, Hara WY, Trakul N, Loo BW Jr, Diehn M. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung cancer (Amsterdam, Netherlands). 2015 Jul:89(1):50-6. doi: 10.1016/j.lungcan.2015.04.014. Epub 2015 May 4 [PubMed PMID: 25997421]
Patibandla A, Featherstone C, Maclaren V, Lumsden G, Harrow S, Jones R, Chalmers AJ, McLoone P, Hicks J. Hypofractionated Accelerated Concurrent Chemoradiotherapy in Inoperable Stage III Non-small Cell Lung Cancer: SOCCAR. A Large Single-Centre Experience. Clinical oncology (Royal College of Radiologists (Great Britain)). 2020 Oct:32(10):e211. doi: 10.1016/j.clon.2020.06.006. Epub 2020 Jul 11 [PubMed PMID: 32665103]
Lodeweges JE, van Rossum PSN, Bartels MMTJ, van Lindert ASR, Pomp J, Peters M, Verhoeff JJC. Ultra-central lung tumors: safety and efficacy of protracted stereotactic body radiotherapy. Acta oncologica (Stockholm, Sweden). 2021 Aug:60(8):1061-1068. doi: 10.1080/0284186X.2021.1942545. Epub 2021 Jun 30 [PubMed PMID: 34191670]
Bogart JA, Hodgson L, Seagren SL, Blackstock AW, Wang X, Lenox R, Turrisi AT 3rd, Reilly J, Gajra A, Vokes EE, Green MR. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Jan 10:28(2):202-6. doi: 10.1200/JCO.2009.25.0753. Epub 2009 Nov 23 [PubMed PMID: 19933904]
Zhao Y, Khawandanh E, Thomas S, Zhang S, Dunne EM, Liu M, Schellenberg D. Outcomes of stereotactic body radiotherapy 60 Gy in 8 fractions when prioritizing organs at risk for central and ultracentral lung tumors. Radiation oncology (London, England). 2020 Feb 27:15(1):61. doi: 10.1186/s13014-020-01491-w. Epub 2020 Feb 27 [PubMed PMID: 32106868]
Giuliani M, Mathew AS, Bahig H, Bratman SV, Filion E, Glick D, Louie AV, Raman S, Swaminath A, Warner A, Yau V, Palma D. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clinical lung cancer. 2018 Jul:19(4):e529-e532. doi: 10.1016/j.cllc.2018.04.001. Epub 2018 Apr 18 [PubMed PMID: 29759332]
Swaminath A, Wierzbicki M, Parpia S, Wright JR, Tsakiridis TK, Okawara GS, Kundapur V, Bujold A, Ahmed N, Hirmiz K, Kurien E, Filion E, Gabos Z, Faria S, Louie AV, Owen T, Wai E, Ramchandar K, Chan EK, Julian J, Cline K, Whelan TJ. Canadian Phase III Randomized Trial of Stereotactic Body Radiotherapy Versus Conventionally Hypofractionated Radiotherapy for Stage I, Medically Inoperable Non-Small-Cell Lung Cancer - Rationale and Protocol Design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE Trial. Clinical lung cancer. 2017 Mar:18(2):250-254. doi: 10.1016/j.cllc.2016.08.002. Epub 2016 Oct 3 [PubMed PMID: 27876603]
Level 1 (high-level) evidencePerez CA, Stanley K, Rubin P, Kramer S, Brady L, Perez-Tamayo R, Brown GS, Concannon J, Rotman M, Seydel HG. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980 Jun 1:45(11):2744-53 [PubMed PMID: 6991092]
Level 1 (high-level) evidenceBradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, Bogart JA, Forster KM, Magliocco AM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Koprowski CD, Olson MR, Meng J, Paulus R, Curran WJ Jr, Choy H. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Mar 1:38(7):706-714. doi: 10.1200/JCO.19.01162. Epub 2019 Dec 16 [PubMed PMID: 31841363]
Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2013 Feb 1:85(2):444-50. doi: 10.1016/j.ijrobp.2012.04.043. Epub 2012 Jun 9 [PubMed PMID: 22682812]
Level 1 (high-level) evidenceAdebahr S, Hechtner M, Schräder N, Schimek-Jasch T, Kaier K, Duncker-Rohr V, Gkika E, Momm F, Gaertner J, Becker G, Grosu AL, Nestle U. Early Impact of Pulmonary Fractionated Stereotactic Body Radiotherapy on Quality of Life:Benefit for Patients With Low Initial Scores (STRIPE Trial). Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2019 Mar:14(3):408-419. doi: 10.1016/j.jtho.2018.10.170. Epub 2018 Dec 3 [PubMed PMID: 30521969]
Level 2 (mid-level) evidenceDarby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England journal of medicine. 2013 Mar 14:368(11):987-98. doi: 10.1056/NEJMoa1209825. Epub [PubMed PMID: 23484825]
Level 2 (mid-level) evidencePotters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman R, Welsh JS, Rosenthal SA, American Society for Therapeutic Radiology and Oncology, American College of Radiology. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. International journal of radiation oncology, biology, physics. 2010 Feb 1:76(2):326-32. doi: 10.1016/j.ijrobp.2009.09.042. Epub [PubMed PMID: 20117285]
Level 1 (high-level) evidencePalma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Schlijper R, Bauman GS, Laba J, Qu XM, Warner A, Senan S. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Sep 1:38(25):2830-2838. doi: 10.1200/JCO.20.00818. Epub 2020 Jun 2 [PubMed PMID: 32484754]
Level 1 (high-level) evidenceTimmerman RD, Hu C, Michalski JM, Bradley JC, Galvin J, Johnstone DW, Choy H. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA oncology. 2018 Sep 1:4(9):1287-1288. doi: 10.1001/jamaoncol.2018.1258. Epub [PubMed PMID: 29852036]
Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG, Schiff PB, Chang G, Loo BW Jr, Bradley JD, Choy H. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA oncology. 2018 Sep 1:4(9):1263-1266. doi: 10.1001/jamaoncol.2018.1251. Epub [PubMed PMID: 29852037]
Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, McGarry RC, Iyengar P, Pantarotto JR, Urbanic JJ, Sun AY, Daly ME, Grills IS, Sperduto P, Normolle DP, Bradley JD, Choy H. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019 May 20:37(15):1316-1325. doi: 10.1200/JCO.18.00622. Epub 2019 Apr 3 [PubMed PMID: 30943123]
Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, van den Borne BE, Munsell MF, Hurkmans C, Berry DA, van Werkhoven E, Kresl JJ, Dingemans AM, Dawood O, Haasbeek CJ, Carpenter LS, De Jaeger K, Komaki R, Slotman BJ, Smit EF, Roth JA. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. The Lancet. Oncology. 2015 Jun:16(6):630-7. doi: 10.1016/S1470-2045(15)70168-3. Epub 2015 May 13 [PubMed PMID: 25981812]
Level 1 (high-level) evidenceChen H, Laba JM, Boldt RG, Goodman CD, Palma DA, Senan S, Louie AV. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. International journal of radiation oncology, biology, physics. 2018 May 1:101(1):186-194. doi: 10.1016/j.ijrobp.2018.01.064. Epub 2018 Feb 3 [PubMed PMID: 29619964]
Level 1 (high-level) evidenceZheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, Chang A, Lv F, Orringer M, Spring Kong FM. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. International journal of radiation oncology, biology, physics. 2014 Nov 1:90(3):603-11. doi: 10.1016/j.ijrobp.2014.05.055. Epub 2014 Jul 19 [PubMed PMID: 25052562]
Level 2 (mid-level) evidencePalma DA, Nguyen TK, Louie AV, Malthaner R, Fortin D, Rodrigues GB, Yaremko B, Laba J, Kwan K, Gaede S, Lee T, Ward A, Warner A, Inculet R. Measuring the Integration of Stereotactic Ablative Radiotherapy Plus Surgery for Early-Stage Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA oncology. 2019 May 1:5(5):681-688. doi: 10.1001/jamaoncol.2018.6993. Epub [PubMed PMID: 30789648]
Level 1 (high-level) evidenceOnishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Hirokawa Y, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004 Oct 1:101(7):1623-31 [PubMed PMID: 15378503]
Level 2 (mid-level) evidenceVidetic GM, Paulus R, Singh AK, Chang JY, Parker W, Olivier KR, Timmerman RD, Komaki RR, Urbanic JJ, Stephans KL, Yom SS, Robinson CG, Belani CP, Iyengar P, Ajlouni MI, Gopaul DD, Gomez Suescun JB, McGarry RC, Choy H, Bradley JD. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. International journal of radiation oncology, biology, physics. 2019 Apr 1:103(5):1077-1084. doi: 10.1016/j.ijrobp.2018.11.051. Epub 2018 Dec 1 [PubMed PMID: 30513377]
Level 1 (high-level) evidenceBenedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Medical physics. 2010 Aug:37(8):4078-101 [PubMed PMID: 20879569]
Yaparpalvi R, Garg MK, Shen J, Bodner WR, Mynampati DK, Gafar A, Kuo HC, Basavatia AK, Ohri N, Hong LX, Kalnicki S, Tome WA. Evaluating which plan quality metrics are appropriate for use in lung SBRT. The British journal of radiology. 2018 Feb:91(1083):20170393. doi: 10.1259/bjr.20170393. Epub 2018 Jan 10 [PubMed PMID: 29227151]
Level 2 (mid-level) evidenceKissick MW, Mackie TR. Task Group 76 Report on 'The management of respiratory motion in radiation oncology' [Med. Phys. 33, 3874-3900 (2006)]. Medical physics. 2009 Dec:36(12):5721-2 [PubMed PMID: 20095285]
Level 3 (low-level) evidenceGiraud P, Morvan E, Claude L, Mornex F, Le Pechoux C, Bachaud JM, Boisselier P, Beckendorf V, Morelle M, Carrère MO, STIC Study Centers. Respiratory gating techniques for optimization of lung cancer radiotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011 Dec:6(12):2058-68. doi: 10.1097/JTO.0b013e3182307ec2. Epub [PubMed PMID: 22052228]
Casutt A, Kinj R, Ozsahin EM, von Garnier C, Lovis A. Fiducial markers for stereotactic lung radiation therapy: review of the transthoracic, endovascular and endobronchial approaches. European respiratory review : an official journal of the European Respiratory Society. 2022 Mar 31:31(163):. doi: 10.1183/16000617.0149-2021. Epub 2022 Jan 12 [PubMed PMID: 35022258]
Kang KH, Okoye CC, Patel RB, Siva S, Biswas T, Ellis RJ, Yao M, Machtay M, Lo SS. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers. 2015 Jun 15:7(2):981-1004. doi: 10.3390/cancers7020820. Epub 2015 Jun 15 [PubMed PMID: 26083933]
Wu AJ, Williams E, Modh A, Foster A, Yorke E, Rimner A, Jackson A. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2014 Aug:112(2):267-71. doi: 10.1016/j.radonc.2014.07.001. Epub 2014 Jul 23 [PubMed PMID: 25064471]
Level 2 (mid-level) evidenceRulach R, Ball D, Chua KLM, Dahele M, De Ruysscher D, Franks K, Gomez D, Guckenberger M, Hanna GG, Louie AV, Moghanaki D, Palma DA, Peedell C, Salem A, Siva S, Videtic GMM, Chalmers AJ, Harrow S. An International Expert Survey on the Indications and Practice of Radical Thoracic Reirradiation for Non-Small Cell Lung Cancer. Advances in radiation oncology. 2021 Mar-Apr:6(2):100653. doi: 10.1016/j.adro.2021.100653. Epub 2021 Jan 20 [PubMed PMID: 33851065]
Level 3 (low-level) evidenceWu KL, Jiang GL, Qian H, Wang LJ, Yang HJ, Fu XL, Zhao S. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. International journal of radiation oncology, biology, physics. 2003 Dec 1:57(5):1345-50 [PubMed PMID: 14630272]
Level 2 (mid-level) evidenceTekatli H, Haasbeek N, Dahele M, De Haan P, Verbakel W, Bongers E, Hashemi S, Nossent E, Spoelstra F, de Langen AJ, Slotman B, Senan S. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016 Jul:11(7):1081-9. doi: 10.1016/j.jtho.2016.03.008. Epub 2016 Mar 21 [PubMed PMID: 27013408]
Drodge CS, Ghosh S, Fairchild A. Thoracic reirradiation for lung cancer: a literature review and practical guide. Annals of palliative medicine. 2014 Apr:3(2):75-91. doi: 10.3978/j.issn.2224-5820.2014.03.04. Epub [PubMed PMID: 25841506]
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016 Jan:11(1):39-51. doi: 10.1016/j.jtho.2015.09.009. Epub [PubMed PMID: 26762738]
Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interactive cardiovascular and thoracic surgery. 2016 Sep:23(3):486-97. doi: 10.1093/icvts/ivw152. Epub 2016 May 25 [PubMed PMID: 27226400]
Level 1 (high-level) evidenceSommer MS, Staerkind MEB, Christensen J, Vibe-Petersen J, Larsen KR, Holst Pedersen J, Langberg H. Effect of postsurgical rehabilitation programmes in patients operated for lung cancer: A systematic review and meta-analysis. Journal of rehabilitation medicine. 2018 Feb 28:50(3):236-245. doi: 10.2340/16501977-2292. Epub [PubMed PMID: 29392334]
Level 1 (high-level) evidence