Introduction
Spina bifida is a congenital anomaly arising from incomplete neural tube development. See Image. Infant With Spina Bifida. "Spina bifida" is a nonspecific term referring to any degree of neural tube closure. This condition can be subdivided into spina bifida occulta and spina bifida aperta. Spina bifida occulta, or closed spinal dysraphism, is the mildest form of neural tube defect that involves a hidden vertebral defect and minimal neural involvement.
Spina bifida aperta, or open spinal dysraphism, refers to a defect in which neural tissues communicate with the external environment, such as meningocele and myelomeningocele (see Image. Types of Spina Bifida). These conditions result in a varied spectrum of neurological deficits. Spina bifida is commonly associated with several other developmental abnormalities, making a multidisciplinary medical plan paramount to patient survival and outcomes.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Spinal dysraphisms are due to incomplete closure of the posterior spinal elements and typically occur between 17 and 30 days of fetal development. The process of neuralization occurs in 2 phases: primary and secondary neuralization. Primary neuralization refers to the closure of the neural tube, forming the brain and spinal cord. Secondary neuralization involves the formation of the caudal structures of the neural tube, which form the sacral and coccygeal portions. These caudal structures develop around day 26 of gestation, and failure to close results in varying degrees of spinal dysraphisms.[1]
Defects in neural tube development are thought to be multifactorial, including environmental and genetic influences. The most common environmental cause is folate deficiency, with most cases deemed “folic acid-sensitive.”[2] Programs for dietary folate fortification have been implemented across the globe, and these have decreased the prevalence of anencephaly and spina bifida by 28%.
Other environmental risk factors include maternal obesity, maternal diabetes, and teratogens such as valproic acid. Valproic acid has the highest association with the development of neural tube defects (NTDs), carrying about a 10-fold increase in risk. Some genetic factors have also been correlated with poor neuralization, including several chromosomal syndromes and genetic polymorphism. Research has implicated polymorphism of the gene encoding the MTHFR enzyme, which is involved in folate metabolism, as a likely genetic risk factor. While NTDs are typically isolated, some are associated with chromosomal syndromes, most often trisomy 13 and 18.[1][3]
Epidemiology
The prevalence and incidence of NTDs have varied since they were first recognized, but overall, they have decreased with several interventional programs and increased fetal screening. In the United States, the overall prevalence of spina bifida between 1999 and 2007 was 3.17/10,000 live births. Study results show that about 1300 healthy babies born each year would have had NTDs if folic acid fortification was not introduced into common prenatal practice.
Hispanic women seem to have a high rate of pregnancies affected by NTD. Worldwide, Hispanic individuals have a prevalence of spinal bifida in 3.8/10,000 live births as compared to non-Hispanic Black Americans, who have a rate of 2.73/10,000, and non-Hispanic White individuals with a prevalence of 3.09/10,000.[3][4] There is also an increased risk for recurrence associated with family history, geographic location, and defect severity. There is an increased risk of recurrence of about 3% to 8% after 1 affected pregnancy or maternal history of the defect, and the risk worsens with an increasing number of affected children.[5]
History and Physical
Patients with NTDs should be evaluated during prenatal screening, and at-risk women should be counseled before conception to improve outcomes. However, in certain areas, especially underserved communities and those of low socioeconomic backgrounds, mothers may not have access to proper screening and may present at birth or in infancy. Standard prenatal screening includes serum alpha-fetal protein levels at 16 to 18 weeks to evaluate for NTD. Follow-up can be obtained with ultrasound, which has a sensitivity of 88% to 89%.[5] Given the wide variety of neuralization, patients can present with various symptoms and conditions.
Physically, visualization of the lower portions of the spine can determine whether the patient has an open or closed spinal dysraphism. Spina bifida occulta shows no obvious deformity; however, a hairy skin patch or dimple can sometimes be seen, indicating an underlying lesion. Meningocele involves a defect of the posterior elements of the spine with extrusion of meninges and cerebrospinal fluid (CSF) without the involvement of the neural elements. Myelomeningocele involves extrusion of meninges, CSF, and functional neural elements such as nerve or spinal cord contents.
Myelomeningocele typically affords more functional limitations in the future as nervous tissue is involved in the defect. Patients may present with symptoms of spasticity, pain, motor deficits, neurogenic bowel and/or bladder, cognitive deficits, seizures, and even endocrine disorders such as precocious puberty.[1] Neurological dysfunction is common with open dysraphisms such as meningocele and myelomeningocele, as neural tissue can extrude outside the defect and be affected. Patients often also present with latex allergies, with a prevalence of 10% and 73% in those with NTDs.[6] Once these signs or symptoms are encountered, a detailed gestational, birth, and family history should be obtained to understand the likely contributors and help guide family counseling.
Evaluation
As mentioned above, women should undergo routine screening to identify NTD early and help with therapeutic intervention and counseling. Initial screening is done with serum alpha fetal protein, but amniocentesis can be pursued for confirmation in cases of high suspicion. However, given the risk of amniocentesis and the accuracy of ultrasound, the latter has become the gold standard for diagnosis in utero and can even detect abnormalities in the first trimester.[7]
Several ultrasound signs have been identified as reliably diagnostic, as many patients also have intracranial abnormalities.[8] The small biparietal diameter has been associated with NTD. However, the most cited signs include the lemon and banana signs. The lemon sign describes the overlapping of the frontal bones due to a posterior shift of intracranial contents. The banana sign is that of a curved cerebellum due to its downward displacement, often leading to Arnold-Chiari II malformation at birth.[5]
Ventriculomegaly, even without hydrocephalus, can be seen on ultrasound as well. Ultrasound of the spine can help visualize the affected vertebral levels and give prognostic information regarding the bowel, bladder, and gait.[9] The functional level after birth correlates with prenatal ultrasound-identified level of injury in 60% of patients.[10] Magnetic resonance imaging can also be used after birth for prognostication within 1 to 2 vertebral levels in 89% of cases.[5]
Treatment / Management
The mainstay of treatment of NTDs such as spina bifida is prevention. Women of childbearing age should supplement their diet with folate to prevent NTDs. The United States began to mandate the fortification of grains with folic acid to help diminish cases.[3] Women who are trying to become pregnant should take 0.4 mg of folic acid daily before conception. In comparison, women who have a history of NTD or a previously affected pregnancy should take 4 mg of folic acid supplementation.[5] Most patients with spina bifida occulta will not require surgical correction of the defect. However, surgical intervention is warranted in cases of open spinal dyspraphism.
In some patients, an intra-uterine repair can be pursued to close the defect earlier to improve outcomes. The Management of Myelomeningocele Study (MOMS) was a randomized clinical trial that evaluated prenatal vs standard postnatal intervention to repair myelomeningocele. The study's results found that prenatal repair decreased the incidence of hydrocephalus and hindbrain herniation, reduced the need for ventriculoperitoneal shunt placement in the future, and provided improved leg function and ambulation in children 12 to 30 months of age.[1][11] However, there are risks associated with intrauterine repair, including increased risk of prematurity and maternal complications.[12] The MOMs 2 trial reevaluated patients at school age and found that the functional benefits of prenatal repair extended into school age. These children tended to be more adept in self-care tasks, were more likely to be community ambulators, and had higher mobility skills than the postnatal repair group.[13](A1)
Several implications and sequela must be monitored and managed in the neonatal stage.[8] Early closure within 72 hours is preferred to prevent significant neurologic decline and minimize the risk of infection or injury to any exposed neural tissue. Patients will often require a ventriculoperitoneal shunt for hydrocephalus after the defect is closed. Arnold-Chiari malformations are common and may require repair if they become symptomatic.
Long-term management involves an interdisciplinary approach, as several organ systems can be affected. Neurogenic bowel and bladder are common manifestations of the condition. Neurogenic bladder typically involves detrusor-sphincter dyssynergia, which can lead to renal failure if poorly managed. Patients should have biannual renal ultrasounds for surveillance and may require intermittent catheterization for long-term management. Patients also present with neurogenic bowel caused by impaired sensation and poor sphincter control. For adequate control, patients may need to learn a consistent bowel program, which includes stool softeners, motility agents, and digital stimulation.
Children with NTDs can present with a wide range of motor involvement, including weakness, flaccidity, spasticity, and contractures. Tendon lengthening procedures can be considered for patients with severe contractures that interfere with ambulation, hygiene, or positioning. Foot deformities, including equinovarus, calcaneus, and rocker bottom deformities, are common in NTDs. Splinting, passive stretching, and serial casting can help with significant spasticity and contractures.
Differential Diagnosis
The diagnosis of spina bifida is typically accurate based on the physical presentation of the disease. However, it is important to consider several differentials in cases where the physical findings or radiographic diagnosis is not clear:
- Cord compression
- Diastematolyelia
- Isolated Chiari malformations
- Mass lesions
- Tethered cord
Prognosis
Few recent longitudinal studies on spina bifida and long-term outcomes exist, making it challenging to prognosticate for patients, given the significant improvements in spina bifida management. Most studies also combine data from patients with both open and closed dysraphisms. Overall, the prognosis depends mainly on the presence of hydrocephalus, defect level, and Chiari malformation severity.
Results from a recent study showed that survivability up to 1 year of age was about 71% overall. Of those with and without hydrocephalus, 56% and 88% survived beyond age 1, respectively. The rates of survival drop slightly as patients with hydrocephalus age, to about 50% by age 20.[14] Most deaths beyond age 5 are attributed to seizures, pulmonary emboli, hydrocephalus, and acute renal failure/sepsis.[5] However, there is significant morbidity associated with NTDs if patients are not adequately managed.
Urinary tract infections are the most common complication faced by those with NTDs secondary to neurogenic bladder, affecting about 48% of patients, with 6% ultimately developing renal failure.[5] The severity of Chiari malformation is a significant risk factor for mortality in infants with NTDs and can have several effects, such as sleep apnea, vocal cord paralysis, and bradycardia. Patients often are concerned about functional outcomes, specifically the prognosis regarding ambulation. In the MOMS trial, only 7% of participants were wheelchair dependent for ambulation. The remaining percentage could ambulate with assistive devices, and about 29% were independent.[15]
Complications
Patients can present with several complications, a few of which have been discussed above. The most common complication is acute renal failure/urosepsis, which is secondary to ureteral reflux caused by a neurogenic bladder. Introducing aggressive treatment and surveillance of urologic dysfunction is essential. Many patients will require intermittent catheterization for urinary management. Scoliosis is also a common complication, with about 33% of patients affected, followed by chronic pain in 29% and epilepsy in 12%.[5]
Patients may also present with tethered cords or hydromyelia, which can present with a sudden neurologic decline, spasticity, or pain. Increased fracture risk is also associated with NTD, likely secondary to osteopenia, contractures, decreased sensation, and immobilization. There is also a unique association with a latex allergy, as about 10% and 73% of patients with spina bifida present with latex allergies.[6]
Postoperative and Rehabilitation Care
Early rehabilitative intervention is key to improving functional outcomes and quality of life. Early mobilization and stretching can help patients maintain a range of motion for future applications with gait, hygiene, and activities of daily living (ADLs). Most patients have a combination of upper and lower motor neuron symptoms and have varying motor control, requiring individualized therapy plans. Patients will also typically require orthotics for joint stabilization, prevention of deformity, gait, and overall improved function. They will also require assistive devices such as crutches, canes, walkers, or wheelchairs for support. Some may need assistive devices for ADLs.
Patients should participate in physical and occupational therapy over the long term. Some may require speech therapy for dysphagia, dysarthria, vocal cord paralysis, and cognitive training. Neuropsychology would also be important in the early stages of adolescence to assist with adjustment to complications as they arrive. However, research results show that despite the risk of significant complications, only 5% of patients develop depression.[5]
Classifying a patient's functional capacity based on the level of injury and presentation can help medical professionals, allied health staff, families, and patients understand expected outcomes and therapy goals. Several scales used in rehabilitation practice generally depict a patient's functional mobility. A study by Dias et al. has proposed a classification system specific to myelomeningocele that combines the Functional Mobility Scale (FMS) and clinical and ambulatory characteristics of myelomeningocele to encompass functional levels, expected mobility outcomes and expected associated bracing.[16]
The FMS rates a patient's mobility from 1 to 6 (1 needing the most support, 6 needing the least) in their environment over 5 m (eg, home), 50 m (eg, school), and 500 m (eg, community). This scale is designed for pediatric cerebral palsy populations but has been applied to other childhood-onset conditions with neuromuscular effects.[17] The clinical and ambulatory characteristics of patients with spina bifida are described by the Swaroop and Dias Spina Bifida Classification scale. This scale associates the level of vertebral defect and likely associated affected muscles with ambulatory capacity.[18] Together, Dias et al present the Myelomeningocele Functional Classification (MMFC), which combines the level of injury, muscle activation pattern, ambulatory capacity, and functional mobility scale to demonstrate the expected level of ambulation and what assistive devices may be required to achieve said goals.
Consultations
Important consultations for spina bifida include:
- Neurosurgery
- Endocrinology
- Orthopedics
- Urology
- Rehabilitation/therapy
- Neurology
- Maternal-fetal medicine
Deterrence and Patient Education
Women should be counseled during child-bearing age to take folic acid supplementation to prevent NTDs. As discussed, women planning pregnancy should take 0.4 mg daily, and those with a family history or previous child with NTD should take 4 mg daily. In the early stages of the condition, caretakers require the most education to empower them to advocate effectively for the patient during childhood. Throughout growth, patients should be given increasing autonomy based on their cognitive development; patients and their caretakers should feel like they are part of the medical team.
Families must be trained during their child’s therapy to effectively care for their children as they age. Important considerations include mobilization of the patient, appropriate use of orthotics and assistive devices, and proper body mechanics. They must also be educated on the signs and symptoms of serious conditions that may develop, including urinary tract infections, hydrocephalus, or seizures.
Once children are old enough to understand their condition and needs, they should be included in the decision-making process, providing them with education on their self-care. Patients with spina bifida occulta may not have many comorbidities, but those with open dysraphisms may require closer monitoring. Patients may need to be taught to self-catheterize or provide their bowel program.[19] Many patients will express concern regarding ambulation as they age. As mentioned above, most patients will obtain functional ambulation, with only 7% requiring wheelchair-level ambulation. Psychosocial aspects of the patient’s health must also be addressed to improve patient participation in healthcare and improve outcomes. Results from a randomized controlled trial revealed cognitive behavioral therapy in a high-intensity rehabilitation program provided improved outcomes in self-care, cognition, mood, and independence.[20]
Enhancing Healthcare Team Outcomes
A multidisciplinary approach, medically and from a psychosocial standpoint, represents the best outcomes for patients with spina bifida. A team approach involving several specialists and therapy staff can holistically address the spectrum of disease. In an article by Dicianno, the “specialty medical home” is proposed as the ideal management method of spina bifida cases as a variation of the often-referenced “medical home model.” In the medical home model, the primary care physician serves as a central hub with all compiled medical information and acts as the referring clinician, managing the team dynamic between specialists. However, it has been suggested that the specialty physician act as the medical home in certain conditions, as most managed comorbidities stem from the underlying condition. Dicianno recommends this specialty medical home model for spina bifida to encourage teamwork driven by a subspecialist well-versed in spina bifida and its sequelae.[21]
This team-based medical care should follow the patient through adulthood, but many patients find barriers to a seamless transfer of care into adulthood. Involving parents, caretakers, the transitioning youths, and pediatric and adult providers in the transition is important. Early preparation is key to a smooth transition and should be considered early in the process. Transition to adult care can be difficult for patients, caretakers, and clinicians, as patients usually spend several years of their lives with a specific physician team. The transition timing should consider not only the patient’s age but also their cognitive level to ensure the patient is ready to transition out of pediatric care. This transition requires significant cooperation between the pediatric clinician, the new specialty clinician, and all other possible consultants on the team.[19] This team-based approach involves clinicians, physical therapists, occupational therapists, speech therapists, and neuropsychologists to help navigate the disease and its sequelae. Results from a randomized controlled trial by Kahn et al showed that high-intensity, multidisciplinary rehabilitation with incorporated cognitive behavioral therapy had significantly improved outcomes in cognitive function, mood, independence, and bowel/bladder dysfunction.[20]
Media
(Click Image to Enlarge)
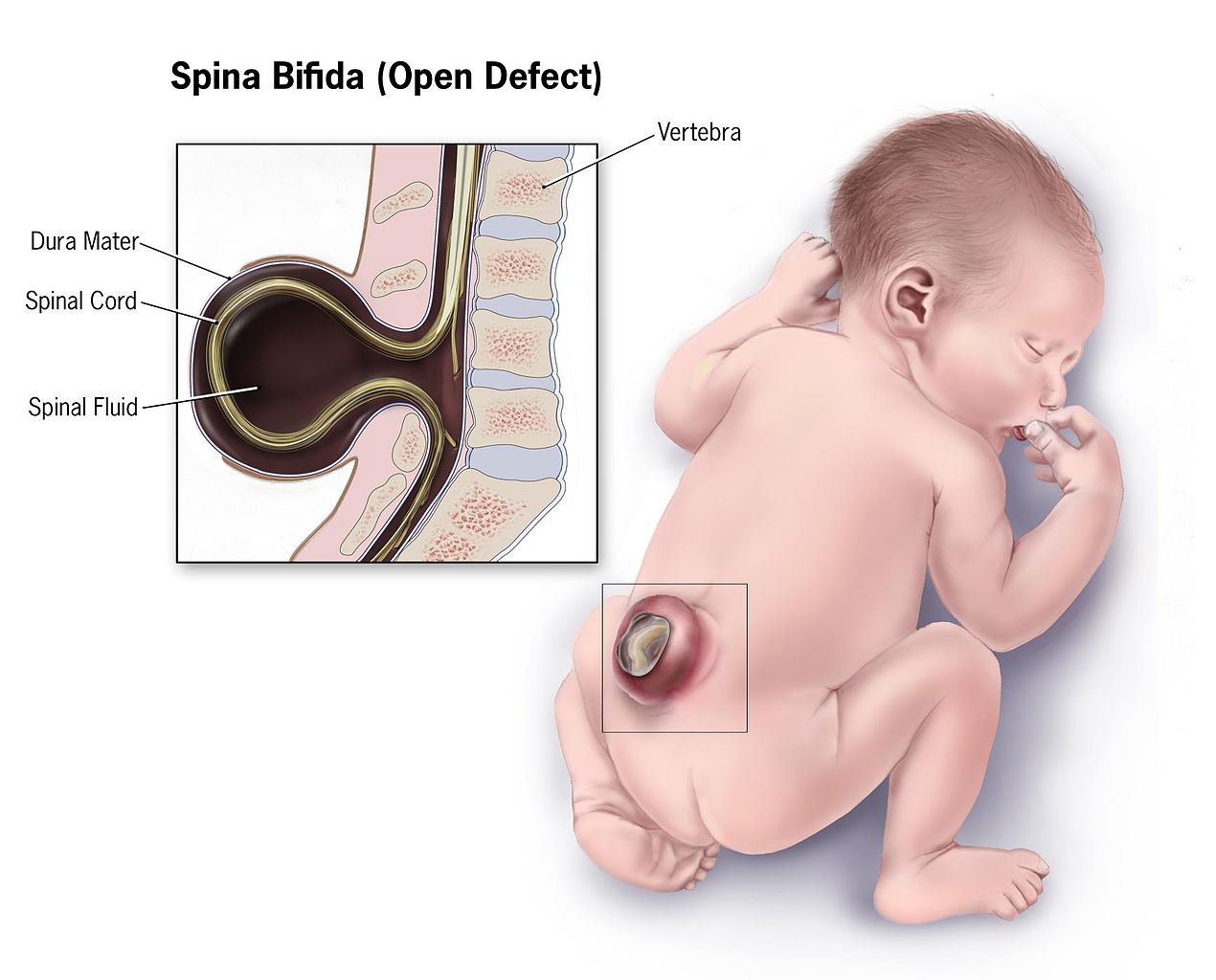
Infant With Spina Bifida. This illustration depicts the characteristics of an infant with spina bifida.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

Types of Spina Bifida. Meningocele is a type of neural tube defect (NTD) that occurs when the meninges, the protective membranes around the spinal cord, protrude through a spinal defect, forming a sac filled with cerebrospinal fluid (CSF). Meningoceles are typically covered by skin and usually present without neurological symptoms.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
References
Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nature reviews. Disease primers. 2015 Apr 30:1():15007. doi: 10.1038/nrdp.2015.7. Epub 2015 Apr 30 [PubMed PMID: 27189655]
Aydin S, Jenkins A, Detchou D, Barrie U. Folate fortification for spina bifida: preventing neural tube defects. Neurosurgical review. 2024 Oct 4:47(1):724. doi: 10.1007/s10143-024-02959-z. Epub 2024 Oct 4 [PubMed PMID: 39365348]
Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS, Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995-2011. MMWR. Morbidity and mortality weekly report. 2015 Jan 16:64(1):1-5 [PubMed PMID: 25590678]
Canfield MA, Mai CT, Wang Y, O'Halloran A, Marengo LK, Olney RS, Borger CL, Rutkowski R, Fornoff J, Irwin N, Copeland G, Flood TJ, Meyer RE, Rickard R, Alverson CJ, Sweatlock J, Kirby RS, National Birth Defects Prevention Network. The association between race/ethnicity and major birth defects in the United States, 1999-2007. American journal of public health. 2014 Sep:104(9):e14-23. doi: 10.2105/AJPH.2014.302098. Epub 2014 Jul 17 [PubMed PMID: 25033129]
Trudell AS, Odibo AO. Diagnosis of spina bifida on ultrasound: always termination? Best practice & research. Clinical obstetrics & gynaecology. 2014 Apr:28(3):367-77. doi: 10.1016/j.bpobgyn.2013.10.006. Epub 2013 Dec 3 [PubMed PMID: 24373566]
Martínez-Lage JF, Moltó MA, Pagán JA. [Latex allergy in patients with spina bifida: prevention and treatment]. Neurocirugia (Asturias, Spain). 2001:12(1):36-42 [PubMed PMID: 11706433]
Zhu Z, Li H. First-trimester diagnosis of open spina bifida using three-dimensional ultrasound. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2023 Jun:161(3):1095-1097. doi: 10.1002/ijgo.14708. Epub 2023 Feb 15 [PubMed PMID: 36728582]
Paschereit F, Schindelmann KH, Hummel M, Schneider J, Stoltenburg-Didinger G, Kaindl AM. Cerebral Abnormalities in Spina Bifida: A Neuropathological Study. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2022 Mar-Apr:25(2):107-123. doi: 10.1177/10935266211040500. Epub 2021 Oct 6 [PubMed PMID: 34614376]
Biggio JR Jr, Owen J, Wenstrom KD, Oakes WJ. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? American journal of obstetrics and gynecology. 2001 Nov:185(5):1016-20 [PubMed PMID: 11717624]
Appasamy M, Roberts D, Pilling D, Buxton N. Antenatal ultrasound and magnetic resonance imaging in localizing the level of lesion in spina bifida and correlation with postnatal outcome. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006 May:27(5):530-6 [PubMed PMID: 16619377]
Level 2 (mid-level) evidenceFarmer DL, Thom EA, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Gupta N, Adzick NS, Management of Myelomeningocele Study Investigators. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. American journal of obstetrics and gynecology. 2018 Feb:218(2):256.e1-256.e13. doi: 10.1016/j.ajog.2017.12.001. Epub 2017 Dec 12 [PubMed PMID: 29246577]
Levin-Decanini T, Houtrow A, Katz A. The Evolution of Spina Bifida Treatment Through a Biomedical Ethics Lens. HEC forum : an interdisciplinary journal on hospitals' ethical and legal issues. 2017 Sep:29(3):197-211. doi: 10.1007/s10730-017-9327-2. Epub [PubMed PMID: 28555303]
Houtrow AJ, MacPherson C, Jackson-Coty J, Rivera M, Flynn L, Burrows PK, Adzick NS, Fletcher J, Gupta N, Howell LJ, Brock JW 3rd, Lee H, Walker WO, Thom EA. Prenatal Repair and Physical Functioning Among Children With Myelomeningocele: A Secondary Analysis of a Randomized Clinical Trial. JAMA pediatrics. 2021 Apr 1:175(4):e205674. doi: 10.1001/jamapediatrics.2020.5674. Epub 2021 Apr 5 [PubMed PMID: 33555337]
Level 1 (high-level) evidenceTennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet (London, England). 2010 Feb 20:375(9715):649-56. doi: 10.1016/S0140-6736(09)61922-X. Epub 2010 Jan 19 [PubMed PMID: 20092884]
Houtrow AJ, Thom EA, Fletcher JM, Burrows PK, Adzick NS, Thomas NH, Brock JW 3rd, Cooper T, Lee H, Bilaniuk L, Glenn OA, Pruthi S, MacPherson C, Farmer DL, Johnson MP, Howell LJ, Gupta N, Walker WO. Prenatal Repair of Myelomeningocele and School-age Functional Outcomes. Pediatrics. 2020 Feb:145(2):. doi: 10.1542/peds.2019-1544. Epub [PubMed PMID: 31980545]
Dias LS, Swaroop VT, de Angeli LRA, Larson JE, Rojas AM, Karakostas T. Myelomeningocele: a new functional classification. Journal of children's orthopaedics. 2021 Feb 1:15(1):1-5. doi: 10.1302/1863-2548.15.200248. Epub [PubMed PMID: 33643452]
Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS). Journal of pediatric orthopedics. 2004 Sep-Oct:24(5):514-20 [PubMed PMID: 15308901]
Swaroop VT, Dias L. Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. Journal of children's orthopaedics. 2009 Dec:3(6):441-9. doi: 10.1007/s11832-009-0214-5. Epub 2009 Oct 25 [PubMed PMID: 19856195]
Binks JA, Barden WS, Burke TA, Young NL. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Archives of physical medicine and rehabilitation. 2007 Aug:88(8):1064-73 [PubMed PMID: 17678671]
Khan F, Amatya B, Ng L, Galea M. Rehabilitation outcomes in persons with spina bifida: A randomised controlled trial. Journal of rehabilitation medicine. 2015 Sep:47(8):734-40. doi: 10.2340/16501977-1999. Epub [PubMed PMID: 26181910]
Level 1 (high-level) evidenceDicianno BE. 21st century challenges to the provision of health care to adults with spina bifida: a rehabilitation approach. Archives of physical medicine and rehabilitation. 2014 Sep:95(9):1601-2. doi: 10.1016/j.apmr.2014.01.011. Epub 2014 Jan 29 [PubMed PMID: 24486240]
Abdelmageed S, Votoupal M, Lam SK, Garcia RM. Epidemiology and morbidity of spina bifida in Hispanic Americans: a systematic review. BMJ public health. 2024 Jun:2(1):e000746. doi: 10.1136/bmjph-2023-000746. Epub 2024 Jun 22 [PubMed PMID: 40018258]
Level 1 (high-level) evidenceKrause M, Leibnitz F, Knüpfer MM, Merkenschlager A, Griessenauer CJ, Gburek-Augustat J. The potential impact of intraoperative neurophysiological monitoring on neurological function outcomes after postnatal spina bifida repair. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2025 Feb 24:41(1):119. doi: 10.1007/s00381-025-06778-5. Epub 2025 Feb 24 [PubMed PMID: 39992435]
Zargarzadeh N, Sambatur E, Abiad M, Rojhani E, Javinani A, Northam W, Chmait RH, Krispin E, Aagaard K, Shamshirsaz A. Gestational Age at Birth Varies by Surgical Technique in Prenatal Open Spina Bifida Repair: A Systematic Review and Meta-Analysis. American journal of obstetrics and gynecology. 2025 Feb 19:():. pii: S0002-9378(25)00094-8. doi: 10.1016/j.ajog.2025.02.014. Epub 2025 Feb 19 [PubMed PMID: 39983885]
Level 1 (high-level) evidence