Anatomy, Abdomen and Pelvis, Sphincter of Oddi (Hepatopancreatic Sphincter)
Anatomy, Abdomen and Pelvis, Sphincter of Oddi (Hepatopancreatic Sphincter)
Introduction
First described by Ruggero Oddi in 1887, the sphincter of Oddi (SO) is a smooth muscle sphincter located in the second portion of the duodenum.[1] It regulates the flow of hepatic and pancreatic substances into the small intestines. Although its function seems simple, it represents a prime example of how hormones and other signals can regulate smooth muscle. The sphincter of Oddi is important in hepatobiliary surgery as it is commonly traversed in endoscopic retrograde cholangiopancreatography (ERCP). This sphincter may also become dysfunctional, requiring medical or surgical intervention.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The sphincter of Oddi is a muscular valve responsible for controlling the flow of bile and pancreatic secretions through the ampulla of Vater into the second part of the duodenum. It is composed of three layers of concentric smooth muscle that surround the common bile duct, the main pancreatic duct, and the ampulla of Vater. The papilla of Vater includes the sphincter of Oddi and its overlying mucosa. So far, the sphincter of Oddi has been identified to have at least three functions:
- To prevent reflux of duodenal contents, thereby preventing the accumulation of sludge and particulate matter in the bile ducts and thus helps to reduce the risk of cholangitis.
- To regulate the flow of bile and pancreatic secretions.
- To allow retrograde filling of the gallbladder to occur.[2][3]
The sphincter of Oddi serves as a great example of the hormonal regulation of smooth muscle. The most important of these hormones is cholecystokinin (CCK). It is released from enteroendocrine cells in response to the digestion of a meal; this stimulates the contraction of the gall bladder, resulting in emptying pancreatic secretions and bile into the common bile duct. CCK has been demonstrated to cause the release of VIP and nitric oxide by intrinsic neurons of the sphincter of Oddi, resulting in a decrease in sphincter tone, ultimately leading to the release of the backed-up contents into the duodenum to aid with digestion. Somatostatin is another noteworthy hormone shown to increase the sphincter of Oddi tone. In contrast, motilin and secretin serve the opposite effect by decreasing the sphincter of Oddi tone and allowing the flow of contents from the gallbladder and pancreas.[4][5][6]
Embryology
The sphincter of Oddi is a concentric ring of muscle originating from the mesenchyme surrounding the pre-ampullary portion of the bile and pancreatic ducts. Initially, it begins as a transverse slit in the circular smooth muscle at the junction between the duodenal wall and the bile and pancreatic ducts. The sequence of the sphincter of Oddi differentiation follows a timeline.[7] It is as follows:
- Week 10: sphincter of Oddi begins differentiation
- Week 12: the bile and pancreatic ducts emerge through the transverse slit of the concentric smooth muscle of the duodenum
- Week 16: the muscularis propria extends outside the fenestra to the upper end of the ampulla
- Week 28: the muscularis propria is differentiated almost to the distal end of the ampulla
Blood Supply and Lymphatics
In about half of individuals, the retroduodenal artery (i.e., the posterior superior and anterior superior pancreaticoduodenal arteries) forms an arterial plexus that provides the primary blood supply to the region of the sphincter of Oddi. The remaining half receives blood supply to the sphincter of Oddi directly from one of the major branches of the retroduodenal artery, with the most likely branch being the posterior superior pancreaticoduodenal artery.[8]
The superior pancreaticoduodenal artery is a branch from the gastroduodenal artery, itself arising from the hepatic artery branch from the celiac trunk. The lymphatic drainage of the sphincter of Oddi follows the drainage of the encompassing ampulla of Vater, which drains into the posterior pancreatoduodenal lymph nodes, followed by the lymph nodes around the superior mesenteric artery, and finally into the para-aortic lymph nodes that follow their well-described path of drainage.[9]
Nerves
The sphincter of Oddi possesses a complex intrinsic set of neural innervation. Currently, two ganglionated plexuses are recognized:
- Myenteric plexus, which lies between the sphincter's muscular layers
- Submucosal plexus
There have also been a few subpopulations discovered in animal models that helped the understanding of the intrinsic innervation of the sphincter of Oddi. One of these described subpopulations was in guinea pigs and was found to be immunoreactive to choline acetyltransferase and served to provide an excitatory effect, thereby increasing the sphincter of Oddi tone. Researchers also found a second, smaller subset of neurons in the sphincter of Oddi that was immunoreactive to nitric oxide (NO) synthase and expressed vasoactive intestinal peptide (VIP) and neuropeptide Y (NPY).[10]
As opposed to the intrinsic neural innervation of the sphincter of Oddi, its extrinsic innervation is not very well understood but is thought to display characteristics similar to that of most of the biliary tract. This neurology includes parasympathetic nervous system innervation originating from the vagus nerve and sympathetic nervous system innervation originating from the superior mesenteric ganglion following the inferior pancreatic duodenal artery.[11]
The sphincter of Oddi is also thought to be part of bidirectional signaling pathways. There seems to be a bidirectional connection between the sphincter of Oddi and the gallbladder; the electrical activity of the gallbladder has been shown to inhibit the sphincter of Oddi contraction reflexively. Similar to the aforementioned bidirectional system, there also exists evidence of a subpopulation of neurons demonstrating bidirectional signaling between the duodenum and sphincter of Oddi ganglia as part of an excitatory cholinergic pathway.[12]
Physiologic Variants
Concerning the sphincter of Oddi musculature, only size variations have been described. Of clinical significance is the existence of hypertrophic sphincter of Oddi smooth muscle in a small proportion of patients, which increases the risk of stenosis. Hypertrophic smooth muscle may also represent a risk factor for chronic obstructive pancreatitis or a cause for biliary-type pain.[13][14]
Surgical Considerations
Surgeons encounter the sphincter of Oddi in many surgical and nonsurgical procedures, so a proper understanding of potential variations in arterial supply is crucial in avoiding undue harm. About 5% of the population's distance between the retroduodenal artery and the papillary orifice is less than 35 millimeters. This proximity creates a small risk of hemorrhage during procedures that require entry through the sphincter of Oddi, such as an ERCP with sphincterotomy, sphincteroplasty, or surgical interventions.[9]
Clinical Significance
Perhaps one of the most clinically significant topics on the sphincter of Oddi is sphincter of Oddi dysfunction (SOD). Sphincter of Oddi dysfunction is defined broadly as the impairment of the sphincter's ability to perform its normal functions of controlling the flow of contents into the duodenum, as mentioned above.[15] The prevalence of sphincter of Oddi dysfunction in the general population is 1.5%.[16] However, it is found in up to 23% of patients with biliary pain and elevated liver enzymes following cholecystectomy - this may be a result of sphincter dysfunction caused by the change in biliary dynamics following cholecystectomy or reflect that sphincter of Oddi dysfunction was the underlying cause of biliary symptoms leading to cholecystectomy.[17]
The main clinical feature of sphincter of Oddi dysfunction is intermittent bouts of epigastric and right upper quadrant pain often precipitated by meals. The pain may last between half an hour and up to several hours and most commonly presents in women between the ages of 20 and 50 years, although it can present at any age and in both sexes.[3] The pain is often associated with a definitive symptomatic profile.[17][18]These symptoms include:
- Nausea and vomiting, which may be mistaken for a gallbladder attack
- Transient increase in liver function tests (LFT) or pancreatic enzymes
- Delay in the emptying of contrast from the CBD or PD while in the supine position
- Radiating pain in the region of the back, shoulder, or scapula
- Dilation of the CBD or PD
While all the above are typical symptoms and findings associated with sphincter of Oddi dysfunction, all of these factors are not present in every patient with sphincter of Oddi dysfunction. SOD is often broken down into two main categories: (1) stenosis and (2) dyskinesia.
Sphincter of Oddi Stenosis
Any process that involves a narrowing of the sphincter of Oddi, excluding dysfunction inherent to the sphincter muscle itself, is described as a sphincter of Oddi stenosis.[19] This condition includes inflammatory or neoplastic processes around and including the sphincter that decreases the caliber of the orifice. Trauma to the sphincter may also lead to stenosis; this includes trauma due to the passage of stones or sludge, as well as intra-operative trauma. Sphincterotomies, performed during ERCP, may also be complicated by stricture formation. As described earlier, patients with congenitally hypertrophic sphincters may also develop SO hypertension, which may lead to stenosis. The result of the sphincter of Oddi stenosis is abnormal motility and increased basal pressures.
Sphincter of Oddi Dyskinesia
While sphincter of Oddi stenosis represents a physical impairment due to damage, inflammation, or neoplastic processes, the sphincter of Oddi dyskinesia represents a less understood motility disorder.[20] SO dyskinesia leads to a transient obstruction of the sphincter, manifesting with the symptoms mentioned above of sphincter of Oddi dysfunction. So far, in the literature, two theories have gained prominence in describing SO dyskinesia:
- A paradoxical response to endogenous hormones due to dysfunctional neurologic pathways may cause sphincter of Oddi dyskinesia.
- The sphincter of Oddi dyskinesia may lie at the hormonal and neurotransmitter level leading to the sphincter of Oddi hypertension.
Sphincter of Oddi Dysfunction Diagnosis and Management
The gold standard test to diagnose sphincter of Oddi dysfunction remains Sphincter of Oddi manometry (SOM), which involves using ERCP techniques and knowledge of manometry to directly examine the motor activity of the sphincter of Oddi.[21] SOM is reproducible, with good interobserver variability, and is stable over repeated measurements in the same patient over time.[17] Manometry can guide treatment decisions in patients with symptoms suggestive of sphincter of Oddi dysfunction. Alternatively, the use of clinical criteria, such as the Milwaukee Classification and Rome IV Criteria, can provide a non-invasive way to stratify patients with suspected sphincter of Oddi dysfunction to allow for appropriate management. In the former system, sphincter of Oddi dysfunction is categorized into three substratifications: patients with biliary pain, abnormal liver function tests, and a dilated common bile duct (Type I SOD), patients with biliary pain, one or more suggestive objective criteria (Type II SOD), and patients with biliary pain only (Type III SOD).[22]
The Milwaukee stratification was used in the EPISOD randomized clinical trial, whose key result was that patients with type III SOD do not benefit from endoscopic sphincterotomy.[23] In fact, remarkably, patients with type III SOD in the trial undergoing sham sphincterotomy reported improved outcomes versus those in the real intervention group (defined as less than six days of pain within the prior 90 days at follow-up).[23] Thus, although Type I and II SOD may be managed with sphincterotomy, it is clear that type III patients do not benefit from this. Key strategies for managing patients with type III SOD include medical therapy with antispasmodics, anti-depressants (e.g., amitriptyline), anti-muscarinics (e.g., trimebutine), calcium-channel blockers (e.g., nifedipine), sublingual nitrites, and oral opiates of varying strengths.[24][25][26][25][24] Further novel therapies that have been trialed include sphincter injection with botulinum toxin.[27][28] These medical therapies have had highly varied reported results, however.[17]
Other tests besides manometry for investigating the function of the sphincter of Oddi include quantitative hepatobiliary scintigraphy (HBS) and fatty meal ultrasonography (FMU). These tests are non-invasive, in contrast to manometry. HBS is a nuclear medicine test in which a tracer is used to demonstrate biliary flow, and the use of intravenous CCK can augment the diagnostic potential of this technique.[29] However, HBS has been found to have a sensitivity of 38% and specificity of 89% for sphincter of Oddi dysfunction, performing poorly compared to manometry.[30] FMU involves performing focussed abdominal ultrasound before and after ingestion of a standardized fatty meal which stimulates CCK and should demonstrate a dynamic change in the diameter of the common bile duct in the setting of obstruction secondary to sphincter of Oddi dysfunction.[31] Compared to SOM, the sensitivity of FMU is 21%, and its specificity is 97%. Thus there may be a role for FMU in patient selection for sphincterotomy in the Type II SOD group, where treatment choice can cause a dilemma.[31]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
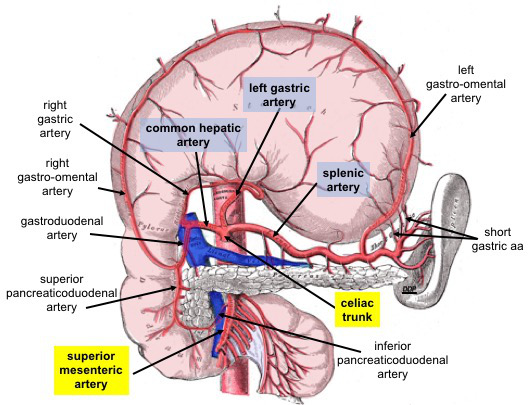
Celiac Trunk Branches. This anterior view shows the stomach reflected superiorly. Celiac trunk branches include the common hepatic, left gastric, and splenic arteries. The common hepatic artery divides into the right and left hepatic, right gastric, and gastroduodenal arteries. The gastroduodenal artery gives rise to the right gastro-omental and anterior and posterior superior pancreaticoduodenal arteries. The left gastric artery supplies the distal esophagus and lesser curvature of the stomach. The splenic artery supplies the spleen and gives rise to the left gastro-omental and short gastric arteries.
The superior mesenteric artery is an abdominal aortic branch inferior to the celiac trunk. The anterior and posterior inferior pancreaticoduodenal arteries arise from this vessel.
Contributed By Dennis M DePace, PhD - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=77732420
References
Loukas M, Spentzouris G, Tubbs RS, Kapos T, Curry B. Ruggero Ferdinando Antonio Guiseppe Vincenzo Oddi. World journal of surgery. 2007 Nov:31(11):2260-5 [PubMed PMID: 17902018]
Leung WD, Sherman S. Endoscopic approach to the patient with motility disorders of the bile duct and sphincter of Oddi. Gastrointestinal endoscopy clinics of North America. 2013 Apr:23(2):405-34. doi: 10.1016/j.giec.2012.12.006. Epub [PubMed PMID: 23540967]
Afghani E, Lo SK, Covington PS, Cash BD, Pandol SJ. Sphincter of Oddi Function and Risk Factors for Dysfunction. Frontiers in nutrition. 2017:4():1. doi: 10.3389/fnut.2017.00001. Epub 2017 Jan 30 [PubMed PMID: 28194398]
Gokin AP, Hillsley K, Mawe GM. Cholecystokinin depolarizes guinea pig sphincter of Oddi neurons by activating CCK-A receptors. The American journal of physiology. 1997 Jun:272(6 Pt 1):G1365-71 [PubMed PMID: 9227471]
Level 3 (low-level) evidenceWyatt AP. The relationship of the sphincter of Oddi to the stomach, duodenum and gall-bladder. The Journal of physiology. 1967 Nov:193(2):225-43 [PubMed PMID: 6065875]
Level 3 (low-level) evidenceChen P, Hu B, Tan Q, Liu L, Li D, Jiang C, Wu H, Li J, Tang C. Role of neurocrine somatostatin on sphincter of Oddi contractility and intestinal ischemia reperfusion-induced acute pancreatitis in macaques. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010 Aug:22(8):935-41, e240. doi: 10.1111/j.1365-2982.2010.01506.x. Epub 2010 May 24 [PubMed PMID: 20497509]
Level 3 (low-level) evidenceAndo H. Embryology of the biliary tract. Digestive surgery. 2010:27(2):87-9. doi: 10.1159/000286463. Epub 2010 Jun 10 [PubMed PMID: 20551648]
Furukawa H, Iwata R, Moriyama N, Kosuge T. Blood supply to the pancreatic head, bile duct, and duodenum: evaluation by computed tomography during arteriography. Archives of surgery (Chicago, Ill. : 1960). 1999 Oct:134(10):1086-90 [PubMed PMID: 10522852]
Bosch A, Peña LR. The sphincter of oddi. Digestive diseases and sciences. 2007 May:52(5):1211-8 [PubMed PMID: 17356921]
Level 3 (low-level) evidenceWells DG, Talmage EK, Mawe GM. Immunohistochemical identification of neurons in ganglia of the guinea pig sphincter of Oddi. The Journal of comparative neurology. 1995 Jan 30:352(1):106-16 [PubMed PMID: 7536219]
Level 3 (low-level) evidenceYi SQ, Ren K, Kinoshita M, Takano N, Itoh M, Ozaki N. Innervation of Extrahepatic Biliary Tract, With Special Reference to the Direct Bidirectional Neural Connections of the Gall Bladder, Sphincter of Oddi and Duodenum in Suncus murinus, in Whole-Mount Immunohistochemical Study. Anatomia, histologia, embryologia. 2016 Jun:45(3):184-8. doi: 10.1111/ahe.12186. Epub 2015 Jul 14 [PubMed PMID: 26179953]
Talmage EK, Hillsley K, Kennedy AL, Mawe GM. Identification of the cholinergic neurons in guinea-pig sphincter of Oddi ganglia. Journal of the autonomic nervous system. 1997 May 12:64(1):12-8 [PubMed PMID: 9188080]
Level 3 (low-level) evidenceDusunceli Atman E, Erden A, Ustuner E, Uzun C, Bektas M. MRI Findings of Intrinsic and Extrinsic Duodenal Abnormalities and Variations. Korean journal of radiology. 2015 Nov-Dec:16(6):1240-52. doi: 10.3348/kjr.2015.16.6.1240. Epub 2015 Oct 26 [PubMed PMID: 26576112]
Cavallini G, Bovo P, Vaona B, Di Francesco V, Frulloni L, Rigo L, Brunori MP, Andreaus MC, Tebaldi M, Sgarbi D, Angelini G, Talamini G, Procacci C, Pederzoli P, Filippini M. Chronic obstructive pancreatitis in humans is a lithiasic disease. Pancreas. 1996 Jul:13(1):66-70 [PubMed PMID: 8783336]
Hyun JJ, Kozarek RA. Sphincter of Oddi dysfunction: sphincter of Oddi dysfunction or discordance? What is the state of the art in 2018? Current opinion in gastroenterology. 2018 Sep:34(5):282-287. doi: 10.1097/MOG.0000000000000455. Epub [PubMed PMID: 29916850]
Level 3 (low-level) evidenceDrossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Digestive diseases and sciences. 1993 Sep:38(9):1569-80 [PubMed PMID: 8359066]
Level 3 (low-level) evidenceBistritz L,Bain VG, Sphincter of Oddi dysfunction: managing the patient with chronic biliary pain. World journal of gastroenterology. 2006 Jun 28; [PubMed PMID: 16804961]
Small AJ, Kozarek RA. Sphincter of Oddi Dysfunction. Gastrointestinal endoscopy clinics of North America. 2015 Oct:25(4):749-63. doi: 10.1016/j.giec.2015.06.009. Epub 2015 Aug 5 [PubMed PMID: 26431602]
Sugawa C, Park DH, Lucas CE, Higuchi D, Ukawa K. Endoscopic sphincterotomy for stenosis of the sphincter of Oddi. Surgical endoscopy. 2001 Sep:15(9):1004-7 [PubMed PMID: 11605112]
Allescher HD. [Sphincter of Oddi dyskinesia]. Der Internist. 2015 Jun:56(6):638, 640-4, 646-7. doi: 10.1007/s00108-014-3605-8. Epub [PubMed PMID: 25995163]
Cheon YK, How to interpret a functional or motility test - sphincter of oddi manometry. Journal of neurogastroenterology and motility. 2012 Apr [PubMed PMID: 22523732]
Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. The New England journal of medicine. 1989 Jan 12:320(2):82-7 [PubMed PMID: 2643038]
Level 1 (high-level) evidenceCotton PB, Durkalski V, Romagnuolo J, Pauls Q, Fogel E, Tarnasky P, Aliperti G, Freeman M, Kozarek R, Jamidar P, Wilcox M, Serrano J, Brawman-Mintzer O, Elta G, Mauldin P, Thornhill A, Hawes R, Wood-Williams A, Orrell K, Drossman D, Robuck P. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: the EPISOD randomized clinical trial. JAMA. 2014 May:311(20):2101-9. doi: 10.1001/jama.2014.5220. Epub [PubMed PMID: 24867013]
Level 1 (high-level) evidenceWilcox CM. Sphincter of Oddi dysfunction Type III: New studies suggest new approaches are needed. World journal of gastroenterology. 2015 May 21:21(19):5755-61. doi: 10.3748/wjg.v21.i19.5755. Epub [PubMed PMID: 26019439]
Vitton V, Ezzedine S, Gonzalez JM, Gasmi M, Grimaud JC, Barthet M. Medical treatment for sphincter of oddi dysfunction: can it replace endoscopic sphincterotomy? World journal of gastroenterology. 2012 Apr 14:18(14):1610-5. doi: 10.3748/wjg.v18.i14.1610. Epub [PubMed PMID: 22529689]
Kalaitzakis E, Ambrose T, Phillips-Hughes J, Collier J, Chapman RW. Management of patients with biliary sphincter of Oddi disorder without sphincter of Oddi manometry. BMC gastroenterology. 2010 Oct 22:10():124. doi: 10.1186/1471-230X-10-124. Epub 2010 Oct 22 [PubMed PMID: 20969779]
Murray W, Kong S. Botulinum toxin may predict the outcome of endoscopic sphincterotomy in episodic functional post-cholecystectomy biliary pain. Scandinavian journal of gastroenterology. 2010 May:45(5):623-7. doi: 10.3109/00365521003615647. Epub [PubMed PMID: 20156033]
Level 2 (mid-level) evidenceWehrmann T,Schmitt TH,Arndt A,Lembcke B,Caspary WF,Seifert H, Endoscopic injection of botulinum toxin in patients with recurrent acute pancreatitis due to pancreatic sphincter of Oddi dysfunction. Alimentary pharmacology & therapeutics. 2000 Nov [PubMed PMID: 11069318]
Jagannath S, Kalloo AN. Efficacy of biliary scintigraphy in suspected sphincter of Oddi dysfunction. Current gastroenterology reports. 2001 Apr:3(2):160-5 [PubMed PMID: 11276385]
Craig AG, Peter D, Saccone GT, Ziesing P, Wycherley A, Toouli J. Scintigraphy versus manometry in patients with suspected biliary sphincter of Oddi dysfunction. Gut. 2003 Mar:52(3):352-7 [PubMed PMID: 12584215]
Rosenblatt ML, Catalano MF, Alcocer E, Geenen JE. Comparison of sphincter of Oddi manometry, fatty meal sonography, and hepatobiliary scintigraphy in the diagnosis of sphincter of Oddi dysfunction. Gastrointestinal endoscopy. 2001 Dec:54(6):697-704 [PubMed PMID: 11726844]