Introduction
A sinus of Valsalva aneurysm (SOVA) is a rare cardiac condition that includes an abnormal dilatation of the aortic root between the aortic valve annulus and the sinotubular junction caused by weakness in the elastic lamina at the junction of the aortic media and the annulus fibrosis. Usually, the sinuses prevent the coronary artery ostia occlusion during systole when the aortic valve opens. The normal sinus diameter is less than 4 cm for men and 3.6 cm for women (see Image. Sinus of Valsalva Aneurysm).
While SOVAs are usually isolated, rare cases involve aneurysms in 2 or 3 sinuses. A SOVA most commonly occurs in the right coronary sinus (70%) and typically remains asymptomatic until it ruptures into nearby structures. The aneurysmal sinus can rupture into an adjacent heart chamber, and the specific location of the rupture depends on the SOVA's position: the right, left, and noncoronary sinuses are adjacent to the interventricular septum, left ventricular free wall, and interatrial septum, respectively. These anatomical proximities help explain the rupture's origin based on the SOVA's location.[1] This rupture creates a communication between the aorta and the heart chamber, leading to progressive heart failure. Without treatment, patients with a ruptured SOVA face poor prognoses and high mortality rates, making timely intervention essential. Any diagnosis of SOVA should prompt an urgent referral to a cardiothoracic surgeon for consideration of repair.[2][3][4][5] SOVAs occur in approximately 0.2% to 0.9% of patients who had cardiac surgery. SOVA can be either congenital or acquired; congenital SOVA, most commonly seen in men of Asian descent, results from abnormal development of the bulbus cordis and often coexists with anomalies like ventricular septal defect (VSD), aortic insufficiency, and bicuspid aortic valve. Acquired SOVA can be linked to prior surgeries, atherosclerosis, endocarditis, syphilis, and other forms of trauma.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
As previously mentioned, SOVAs can be either congenital or acquired. Congenital SOVAs result from abnormal development of the bulbus cordis during embryogenesis, leading to structural weaknesses in the aortic wall. This type of SOVA is often linked to various other cardiac anomalies, with a VSD being present in 30% to 60% of cases. Other common associations include aortic valve regurgitation (20%), bicuspid aortic valve (10%), pulmonic stenosis, coarctation of the aorta, atrial septal defect (ASD), and occasionally, coronary artery anomalies. Additionally, subvalvular aneurysms affecting the aortic and mitral annuli are known associations.
Acquired SOVAs, on the other hand, develop due to factors that weaken the aortic wall over time, including connective tissue issues and infectious diseases. Embryologically, the sinus of Valsalva forms as a blind diverticulum due to pressure forces on the aortic root. Aneurysmal dilation of the sinus of Valsalva can occur due to connective tissue weaknesses from disorders such as Marfan syndrome and Ehlers-Danlos syndrome and is historically associated with the presence of a bicuspid aortic valve.[6][7][8][9] Other connective tissue causes include chronic atherosclerotic changes leading to cystic medial necrosis, chest trauma, and iatrogenic injury during aortic valve replacement surgery. Additionally, vasculitides involving the proximal aorta, such as Takayasu arteritis and inflammatory aortitis, can contribute to the formation of SOVA. Infectious causes that weaken elastic tissue and lead to acquired SOVA include syphilis, tuberculosis, and infective endocarditis.
Epidemiology
SOVAs are rare, occurring in approximately 0.2% to 0.9% of patients undergoing cardiac surgery. The incidence is even lower in the general population, with many cases remaining undetected until a significant event, such as a rupture, occurs. SOVAs comprise up to 3.5% of all congenital heart defects. Congenital SOVAs are 4 times more common in men and particularly prevalent among Asian populations, suggesting a genetic predisposition.[9][10][11] They are typically identified in younger individuals due to their congenital nature. Acquired SOVAs tend to present in older adults, reflecting the cumulative effects of risk factors like atherosclerosis and past infections. About 70% of SOVAs occur in the right coronary sinus. The condition is often asymptomatic until rupture, which can lead to severe complications and high mortality rates without prompt treatment.
Pathophysiology
The pathophysiology of SOVA involves a complex interplay of anatomical and physiological factors that lead to the formation and potential rupture of the aneurysm. SOVAs occur at the sinuses of Valsalva, the 3 dilated portions of the aortic root situated between the aortic valve annulus and the sinotubular junction. These sinuses are normally reinforced by the elastic lamina, a structural layer providing integrity and resilience to the aortic wall. SOVAs form when there is a weakness or defect in this lamina, particularly at the junction of the aortic media and the annulus fibrosis. In congenital cases, the abnormal development of the bulbus cordis during embryogenesis results in a structurally weak aortic wall, predisposing the sinuses to aneurysmal dilatation. This congenital defect is often associated with other congenital heart anomalies, such as VSD, aortic insufficiency, and bicuspid aortic valve.
Acquired SOVAs result from degenerative changes in the aortic wall due to aging, atherosclerosis, or connective tissue disorders, leading to gradual weakening and susceptibility to aneurysm formation. Infectious and inflammatory processes, such as endocarditis and syphilis, can cause inflammatory damage to the aortic wall, further contributing to SOVA development. Trauma and complications from cardiac surgeries can also precipitate SOVA by physically damaging the aortic wall. The aortic root is subject to significant hemodynamic stress due to the high-pressure blood flow from the left ventricle. This stress is particularly pronounced at the sinuses of Valsalva, where the aortic valve cusps attach. In the presence of a structural defect or weakness, this stress can exacerbate the dilatation of the aortic wall, leading to aneurysm formation.
The most critical complication of SOVA is rupture. As the aneurysm enlarges, the thin wall can eventually give way, causing blood to spill into adjacent cardiac chambers or the pericardial space, creating a pathological communication between the aorta and the heart chambers and leading to hemodynamic instability and heart failure. The consequences of rupture typically depend on the anatomical location of the aneurysm. Rupture of the right and noncoronary sinuses typically results in communication between the aorta and either the right atrium or the right ventricular outflow tract, thus creating a left-to-right shunt, which can lead to right ventricular overload and right-sided heart failure. A left SOVA rupture is clinically less significant, causing communication to the left atrium or left ventricular outflow tract.[12][13][14] Chronic aortic regurgitation can develop if the aneurysm distorts the aortic valve, leading to volume overload and subsequent heart failure. Thus, the pathophysiology of SOVA involves congenital structural weaknesses, degenerative changes, and hemodynamic stress, making understanding and managing this condition critical for patient outcomes.
Histopathology
The histopathology of SOVA reveals distinct structural changes in the aortic wall that contribute to the development and progression of the aneurysm. Key features include degeneration of the elastic lamina, where elastic fibers show significant fragmentation and loss, leading to weakened aortic walls predisposed to aneurysmal dilatation. Medial degeneration, characterized by cystic medial necrosis with loss of smooth muscle cells and the presence of mucoid extracellular matrix material, is often evident. Inflammatory changes are also common, with infiltration of lymphocytes and macrophages indicating ongoing inflammation, which can further weaken the aorta. Atherosclerotic changes, such as lipid-laden macrophages, intimal thickening, and calcification, are observed in acquired SOVAs.
Chronic inflammatory processes can lead to fibrosis and scarring within the aortic wall, with fibrous tissue replacing normal elastic fibers, reducing the aorta's elasticity and resilience. The intimal layer may become thickened and fibrotic in response to chronic stress and injury. In congenital SOVA, structural abnormalities in the vascular architecture reflect the underlying developmental defect, including irregular arrangement of smooth muscle cells and elastic fibers, often associated with other congenital anomalies like VSD or bicuspid aortic valve. Thrombus formation is another histopathological feature, especially where blood stasis within the dilated sinus occurs, with thrombi potentially organizing into the wall. Damage to the endothelial lining, often due to turbulent blood flow within the aneurysm, can lead to thrombus formation and embolization risk.
History and Physical
History
The clinical history of a patient with a SOVA may reveal a range of symptoms depending on whether the aneurysm is intact or has ruptured. Many patients with an intact SOVA are asymptomatic, and the condition is often discovered incidentally during imaging studies for other reasons. When symptoms do occur, they may include:
- Chest pain: Nonspecific and may be mistaken for angina or other cardiac conditions
- Dyspnea: Difficulty breathing due to pressure on adjacent cardiac structures or, in severe cases, heart failure
- Palpitations: Irregular heartbeats caused by the aneurysm's effect on the heart's electrical conduction system
In cases where the SOVA has ruptured, the history may include more acute and severe symptoms:
- Sudden severe chest pain: Often described as tearing or ripping
- Acute dyspnea: Sudden shortness of breath due to rapid onset of heart failure
- Syncope: Fainting or loss of consciousness due to severe hemodynamic instability
Patients may also report a history of previous cardiac surgery, trauma, or conditions predisposing to acquired SOVA, such as endocarditis or syphilis.
Physical Exam
The physical examination of a patient with SOVA can vary widely, from unremarkable in asymptomatic cases to dramatic findings in cases of rupture. Key physical exam findings include:
- Heart murmurs: Continuous or diastolic murmur, particularly if the SOVA has ruptured into a cardiac chamber, creating abnormal blood flow patterns
- Continuous
- Best heard along the left sternal border if there is a communication between the aorta and the right atrium or ventricle
- Diastolic
- Indicative of aortic regurgitation caused by the aneurysm affecting the aortic valve
- Continuous
- Signs of heart failure: Elevated jugular venous pressure, peripheral edema, and pulmonary rales, which indicate fluid overload
- Pulse pressure: A widened pulse pressure may be noted for significant aortic regurgitation.
In the event of a ruptured SOVA, additional findings might include:
- Hypotension: Low blood pressure due to acute blood loss and hemodynamic collapse
- Tachycardia: Rapid heart rate as a compensatory mechanism for low blood pressure
- Cyanosis: Bluish discoloration of the skin and mucous membranes due to inadequate oxygenation
Evaluation
The evaluation and workup of a SOVA involves a combination of clinical assessment, imaging studies, and diagnostic tests to confirm the diagnosis, understand the extent of the aneurysm, and plan appropriate management. The following outlines a comprehensive approach:
Imaging Studies
- Echocardiography
- Transthoracic echocardiography
- Can visualize the aortic root, identify the aneurysm, assess the size, and detect associated anomalies like aortic regurgitation or VSD
- Transesophageal echocardiography
- Provides more detailed images, especially useful for evaluating the extent of the aneurysm and its effect on adjacent structures
- Color Doppler can reveal continuous flow during both systole and diastole in cases of ruptured SOVA.
- Transthoracic echocardiography
- Computed tomography angiography
- Offers high-resolution images of the aortic root and sinuses of Valsalva
- Test of choice for quantifying the size and morphology of the SOVA
- Particularly useful for surgical planning and assessing the relationship of the aneurysm with surrounding cardiac and vascular structures
- Cine cardiac magnetic resonance imaging
- Considered the gold standard for diagnosis but is not required if other imaging modalities sufficiently give the diagnosis and pertinent anatomic and physiologic details [15]
- Provides excellent soft tissue contrast and detailed anatomical information
- Useful for patients who cannot undergo computed tomography due to contrast allergy or renal insufficiency
Cardiac Catheterization
- Angiography
- Invasive procedure providing detailed images of the coronary arteries and the aortic root
- Can help in the assessment of the hemodynamic impact of the aneurysm and is particularly useful if surgical intervention is planned
Patients with intermediate or high risk for coronary artery disease will usually undergo cardiac catheterization to assess possible bypass grafting at the time of cardiac surgery.
Laboratory Tests
- Blood tests
- Complete blood count: This test checks for signs of infection or anemia, which could complicate the management of SOVA.
- Inflammatory markers: Elevated markers like C-reactive protein or erythrocyte sedimentation rate could suggest ongoing inflammation or infection.
- Syphilis serology: Given the historical association between syphilis and aortic aneurysms, testing for syphilis may be warranted.
Additional Testing
- Electrocardiogram: To assess for any electrical abnormalities or evidence of ischemia that might be secondary to the aneurysm’s effect on coronary perfusion
Treatment / Management
Medical Management
Medical management of SOVA is primarily a temporary measure to stabilize patients until definitive surgical or transcatheter intervention can be performed, as it is insufficient for definitive treatment. For unruptured SOVA, medical management involves regular monitoring with echocardiography or other imaging modalities to track aneurysm size and progression. Blood pressure control is essential, typically achieved with antihypertensive medications such as beta-blockers, angiotensin-converting enzyme inhibitors, or calcium channel blockers to reduce stress on the aortic wall. Patients are also advised to modify their activities, avoiding strenuous physical exertion and heavy lifting to prevent increases in intrathoracic pressure that could precipitate rupture. In cases of ruptured SOVA, immediate medical stabilization is critical, involving fluid resuscitation, blood pressure control, and managing heart failure or shock symptoms until surgical treatment can be performed.
Surgical Repair
Surgical repair of SOVA is a critical intervention, particularly for symptomatic, large, or rapidly enlarging unruptured aneurysms and all ruptured aneurysms. The primary goal is to prevent rupture and restore normal aortic and cardiac function. An unruptured SOVA should be surgically repaired if it is associated with significant symptoms or rapidly enlarges. The 2010 American Guidelines for Thoracic Aortic Disease recommend considering surgical repair in those with aneurysms greater than 5.5 cm, greater than 5 cm in those with bicuspid valves, and greater than 4.5 cm in the setting of connective tissue disease. SOVA repair should be considered when a yearly growth rate exceeds 0.5 cm. Surgical repair involves cardiopulmonary bypass, cardioplegia, and primary or patch closure.
The surgical procedure typically involves a median sternotomy, providing direct access to the heart. Various surgical techniques are employed depending on the aneurysm's size, location, and involvement of the aortic valve. Aortic root replacement, where the aneurysmal portion of the aortic root is replaced with a synthetic graft, is a common approach. This may include valve-sparing techniques or valve replacement if the aortic valve is affected. Alternatively, patch repair, which involves reinforcing the aneurysm with a patch to preserve native aortic tissue, may be performed for less extensive aneurysms.
In cases of ruptured SOVA, urgent cardiothoracic surgical evaluation and immediate surgical repair are essential due to the high risk of mortality.[16][17] This emergency procedure often involves similar techniques but under more urgent conditions, requiring rapid stabilization of the patient. Any associated cardiac issues, such as VSDs or aortic valve dysfunction, are also addressed during surgery. Postoperative care in an intensive care unit is crucial for monitoring and managing complications like bleeding, infection, or heart failure. Long-term follow-up with imaging studies is necessary to ensure the integrity of the repair and monitor for potential recurrence or other complications. Surgical repair of SOVA, while invasive, remains a definitive treatment with a high success rate, significantly improving patient outcomes when performed promptly and effectively. Surgical mortality ranges from 1.9% to 3.6%, while survival rates are close to 90% after 15 years.
Transcatheter Closure
Transcatheter closure of SOVA is an emerging, minimally invasive technique used to treat ruptured and unruptured aneurysms. Over the past 2 decades, enhanced transcatheter techniques have expanded the range of nonsurgical repair options. This approach offers a viable alternative to traditional open-heart surgery, especially for high-risk surgical candidates due to age, comorbidities, or other factors. Indications for the procedure include emergency intervention for ruptured SOVA causing hemodynamic instability or heart failure and elective treatment for symptomatic, enlarging, or high-risk unruptured aneurysms.
The procedure involves a thorough pre-procedural assessment, including imaging studies like echocardiography, computed tomography angiography, or magnetic resonance imaging to delineate the aneurysm's anatomy. Vascular access is typically obtained through the femoral artery or vein, and the procedure is performed under fluoroscopic and echocardiographic guidance. A catheter is navigated to the aneurysm site, and a closure device, such as an Amplatzer occluder or vascular plug, is deployed to seal the aneurysm, eliminating the abnormal communication between the aorta and adjacent cardiac chambers. Post-deployment imaging confirms the proper placement and function of the device.
Transcatheter closure offers several advantages, including avoiding open-heart surgery, reducing surgical risks, and shortening recovery time and hospital stay. Patients typically experience faster recovery and can resume normal activities within a few days to weeks. Studies have demonstrated high success rates for transcatheter closure, with effective aneurysm occlusion and relief of symptoms. Potential complications, such as device malposition, residual shunt, or embolization, are generally low and manageable with prompt intervention. Regular follow-up with imaging is necessary to monitor the closure's integrity and detect any device-related complications early. With advancements in device technology and procedural techniques, transcatheter closure is increasingly preferred in managing SOVA, offering significant benefits over traditional surgical approaches.
A recent study of the transcatheter closure selection criteria was documented in 256 cases of SOVA. The most common criterion, reported in 77% of the cases, was the absence of other cardiac anomalies besides a ruptured SOVA that would necessitate surgery. All the patients studied underwent transcatheter closure of ruptured SOVA, achieving a total success rate of 95.6%, with various device types employed. Patent ductus arteriosus occluders were the most frequently used devices in 172 cases (41.7%), followed by the Amplatzer ductal occluder in 85 cases (20.6%). The device's size was determined based on the defect size assessed by transesophageal echocardiography or other imaging techniques.[1][18] Ultimately, randomized trial data will be necessary to optimize the management of these rare conditions.[19](A1)
Differential Diagnosis
The differential diagnoses for SOVA include:
- Aortic stenosis
- Atrial septal defect
- Atrioventricular block
- Dilated cardiomyopathy
- Hypertrophic cardiomyopathy
- Isolated coronary artery anomalies
- VSD surgery
Prognosis
The prognosis of SOVA is generally favorable, particularly when surgical intervention is performed for unruptured aneurysms. Results from a recent study demonstrated that most surgeries for unruptured SOVAs were uneventful, achieving a 96% success rate, with 51 out of 53 cases being successfully managed. However, in-hospital mortality occurred in 4% of cases, with 2 patients succumbing: 1 during the operation and another within 48 hours after the operation due to multiorgan failure. The length of hospital stay following surgical management ranged from 4 to 21 days, reflecting varying recovery periods.
Follow-up data for 31 patients, spanning from 5 days to 9 years, indicated that most patients were asymptomatic and exhibited complete aneurysm obliteration, full restoration of aortic root anatomy, and normal valvular function in 94% of cases (29 out of 31). Despite the generally positive outcomes, complications did arise in a minority of patients. One patient required percutaneous intervention during follow-up due to a detected leak, with only partial thrombosis of the aneurysm after surgical repair. At a 2-month follow-up after this intervention, a computed tomography angiogram showed almost complete thrombosis of the aneurysm lumen. Additionally, 1 patient experienced a recurrence of SVA at 9 years post-surgery, necessitating further surgical intervention.[20] Overall, the prognosis for patients with SOVA, particularly those undergoing surgery for unruptured aneurysms, is optimistic, with most patients achieving long-term stability and normal function.
The prognosis of ruptured SOVA is generally poor without prompt surgical intervention. The rupture of a SOVA leads to the sudden onset of severe symptoms, including acute chest pain, dyspnea, and hemodynamic instability. The rupture creates an abnormal communication between the aorta and adjacent cardiac chambers, often resulting in significant blood loss, rapid development of heart failure, and potential multiorgan failure due to compromised perfusion. Without immediate surgical repair, the mortality rate is high. The acute nature of the condition necessitates urgent medical attention, as delays in treatment can lead to fatal outcomes. Even with surgical intervention, the complexity of the condition means there are inherent risks, and the patient's overall health and the presence of comorbidities can significantly influence outcomes.
Study data indicate that surgical repair of a ruptured SOVA can stabilize the patient's condition and improve long-term outcomes, but the immediate postoperative period remains critical. The success of surgery depends on various factors, including the size and location of the rupture, the patient's hemodynamic status at presentation, and the presence of any concurrent cardiac conditions. Following surgical repair, patients typically require close monitoring in an intensive care setting to manage potential complications such as bleeding, infection, and residual or recurrent aneurysms. Long-term follow-up is essential to monitor for recurrence, assess heart function, and ensure the integrity of the repair. Despite the risks, successful surgical intervention can significantly improve the prognosis for patients with ruptured SOVA, enabling many to achieve stable, long-term health.
Complications
The complications of SOVA can be severe and life-threatening, particularly if the aneurysm ruptures. The key complications include:
- Rupture
- This is the most critical and life-threatening complication.
- Rupture creates an abnormal communication between the aorta and adjacent cardiac chambers or the pericardial space, leading to acute hemodynamic instability.
- This presents with sudden severe chest pain, dyspnea, hypotension, and potentially cardiac tamponade if blood accumulates in the pericardial space.
- Rupture poses a high mortality rate without prompt surgical intervention.
- Heart failure
- Chronic volume overload due to aortic regurgitation or acute overload from rupture can lead to heart failure.
- Symptoms include dyspnea, fatigue, edema, and reduced exercise tolerance.
- Aortic insufficiency
- The aneurysm can distort the aortic valve, leading to valvular insufficiency.
- Symptoms include shortness of breath, fatigue, and palpitations due to blood regurgitating from the aorta into the left ventricle.
- Endocarditis
- The aneurysm's abnormal structure can be a nidus for bacterial infection, leading to infective endocarditis.
- Symptoms include fever, chills, night sweats, and signs of systemic embolization.
- Arrhythmias
- The aneurysm can disrupt the normal electrical conduction pathways of the heart.
- Arrhythmia can present with palpitations, dizziness, syncope, and potentially sudden cardiac death (if a severe arrhythmia occurs).
- Thromboembolism
- The stasis of blood within the aneurysm can lead to clot formation.
- These clots can embolize the systemic circulation, causing strokes or other ischemic events.
- Pressure on adjacent structures
- A large aneurysm can compress adjacent cardiac structures or coronary arteries.
- This presents as angina due to coronary artery compression or dyspnea and dysphagia due to compression of other structures.
- Recurrence
- There is a risk of aneurysm recurrence even after surgical repair, necessitating ongoing monitoring.
- Regular follow-up imaging should be done to detect and manage any recurrence early.
Deterrence and Patient Education
Deterrence and patient education are crucial in managing SOVA to prevent complications and ensure optimal outcomes. Regular monitoring, including routine echocardiograms and follow-up appointments with a cardiologist, is essential for assessing the aneurysm's size and progression. Controlling risk factors, such as strict blood pressure management and infection prevention with prophylactic antibiotics, is vital. Patients should avoid strenuous activities, such as contact sports and heavy lifting, that increase intrathoracic pressure and risk rupture. Genetic counseling is recommended for those with a congenital SOVA, especially if there is a family history of connective tissue disorders or aortic aneurysms.
Patient education involves helping patients understand the nature of SOVA, including its causes, symptoms, and potential complications. Emphasizing the importance of early detection and management and teaching patients to recognize symptoms indicating aneurysm progression or rupture is key. Ensuring medication adherence, particularly for antihypertensives, and promoting a heart-healthy diet, smoking cessation, and stress management techniques support overall cardiovascular health. Developing an emergency plan outlining steps to take if acute symptoms occur is also critical.
For patients who have undergone surgical repair, clear instructions on postoperative care, signs of complications, and the importance of follow-up appointments are necessary. Discussing any activity restrictions during recovery and gradually reintroducing activities as their clinician recommends helps ensure a smooth recovery. Encouraging participation in patient support groups and involving family members in educational sessions can create a supportive home environment and help patients adhere to management plans. Effective deterrence and patient education enable proactive management of SOVA, helping to prevent complications and improve patient outcomes and quality of life.
Pearls and Other Issues
SOVAs may remain asymptomatic until they rupture acutely, most frequently into the right atrium or right ventricle, although simultaneous rupture into both chambers is rare. Physical examination plays a crucial role in assessing these patients; the emergence of a new continuous murmur is always a significant finding and should prompt immediate echocardiography for timely diagnosis and intervention. Transthoracic echocardiogram and transesophageal echocardiogram are highly effective imaging techniques for investigating SOVAs and related cardiac abnormalities. Further, the ruptured SOVA is a surgical urgency with a high rate of mortality if left untreated; surgical repair remains the preferred treatment method, although transcatheter closure devices are acceptable for patients with suitable anatomy and high risk for cardiac surgery.[21]
Enhancing Healthcare Team Outcomes
Effective management of sinus of Valsalva aneurysm (SOVA) requires a multidisciplinary approach involving physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals. Physicians and advanced clinicians need to possess a high level of expertise in diagnosing and managing SOVA, including performing and interpreting advanced imaging studies, understanding the indications for surgical versus transcatheter intervention, and providing acute care in emergency situations. Nurses are crucial in patient education, pre and postoperative care, and complication monitoring. They ensure patient comfort and adherence to treatment plans while coordinating care among different specialties. Pharmacists contribute by managing medication therapy, ensuring appropriate anticoagulation, and addressing drug interactions and side effects.
Interprofessional communication is essential for enhancing patient-centered care and outcomes. Regular case conferences and multidisciplinary team meetings facilitate sharing information, aligning treatment plans, and making collaborative decisions. Care coordination involves seamless transitions between different levels of care, from acute settings to rehabilitation and long-term follow-up. This requires clear communication and documentation, electronic health records, and patient navigation services. By fostering a collaborative environment, healthcare teams can enhance patient safety, improve outcomes, and optimize team performance in managing SOVA.
Media
(Click Image to Enlarge)
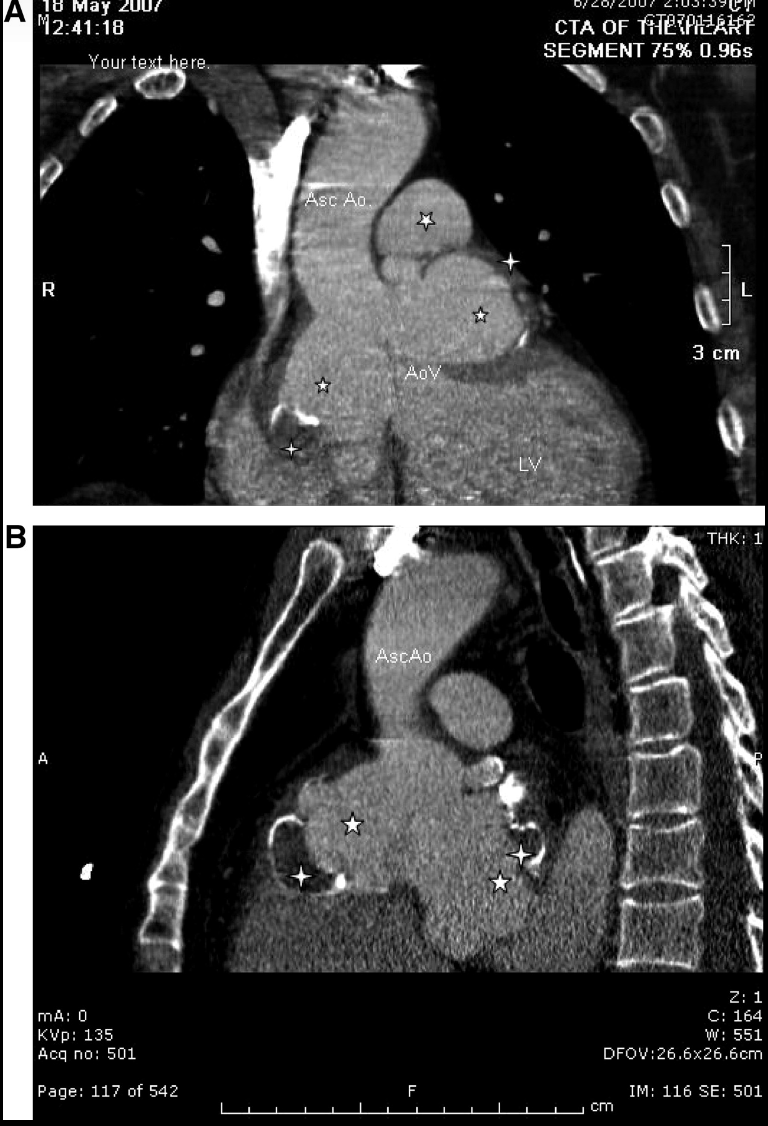
Sinus of Valsalva Aneurysm. Computed tomographic coronal (A) and sagittal (B) views demonstrating the named finding. Shows layered thrombus within the aneurysms of sinuses of Valsaalva; ☆, dilated sinuses of Valsalva. Asc Ao indicates ascending thoracic aorta; AoV, aortic valve; and LV, left ventricle.
Contributed by Dr. Joaquin B. Gonzalez. Obtained with informed consent.
References
Ayati A, Toofaninejad N, Hosseinsabet A, Mohammadi F, Hosseini K. Transcatheter closure of a ruptured sinus of valsalva: a systematic review of the literature. Frontiers in cardiovascular medicine. 2023:10():1227761. doi: 10.3389/fcvm.2023.1227761. Epub 2023 Aug 25 [PubMed PMID: 37727309]
Level 1 (high-level) evidenceSerban AM, Bătrâna N, Cocoi M, Ianoș R, Moț Ș, Kovacs E, Axente DD, Man C, Molnar A. The role of echocardiography in the diagnosis and management of a giant unruptured sinus of Valsalva aneurysm. Medical ultrasonography. 2019 May 2:21(2):194-196. doi: 10.11152/mu-1741. Epub [PubMed PMID: 31063525]
Galeczka M, Glowacki J, Yashchuk N, Ditkivskyy I, Rojczyk D, Knop M, Smerdzinski S, Cherpak B, Szkutnik M, Bialkowski J, Fiszer R, Lazoryshynets V. Medium- and long-term follow-up of transcatheter closure of ruptured sinus of Valsalva aneurysm in Central Europe population. Journal of cardiology. 2019 Oct:74(4):381-387. doi: 10.1016/j.jjcc.2019.03.012. Epub 2019 Apr 23 [PubMed PMID: 31023567]
Wierda E, Koolbergen DR, de Mol BAJM, Bouma BJ. Rupture of a giant aneurysm of the sinus of Valsalva leading to acute heart failure: a case report demonstrating the excellence of echocardiography. European heart journal. Case reports. 2018 Sep:2(3):yty090. doi: 10.1093/ehjcr/yty090. Epub 2018 Aug 2 [PubMed PMID: 31020167]
Level 3 (low-level) evidenceHajizeinali A, Hosseinsabet A. Percutaneous device closure of a ruptured aortic sinus of Valsalva aneurysm in a patient with a mechanical bileaflet aortic valve. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2019 Apr:47(3):243. doi: 10.5543/tkda.2018.91650. Epub [PubMed PMID: 30982825]
Wingo M, de Angelis P, Worku BM, Leonard JR, Khan FM, Hameed I, Lau C, Gaudino M, Girardi LN. Sinus of Valsalva aneurysm repairs: Operative technique and lessons learned. Journal of cardiac surgery. 2019 Jun:34(6):400-403. doi: 10.1111/jocs.14041. Epub 2019 Apr 6 [PubMed PMID: 30953447]
Ohno N, Watanabe K, Maeda T, Kato O, Ueno G, Yoshizawa K, Fujiwara K. A rare case of unruptured extracardiac multiple sinus of Valsalva aneurysms originating from the orifices with partial aortic wall defects. Surgical case reports. 2019 Mar 27:5(1):47. doi: 10.1186/s40792-019-0608-7. Epub 2019 Mar 27 [PubMed PMID: 30919117]
Level 3 (low-level) evidenceUrbanski PP, Hirao S, Irimie V. Root repair in patient with huge sinus Valsalva aneurysm and severe aortic regurgitation. General thoracic and cardiovascular surgery. 2020 May:68(5):530-533. doi: 10.1007/s11748-019-01104-8. Epub 2019 Mar 13 [PubMed PMID: 30868369]
Haseeb Ul Rasool M, Swaminathan G, Hosna AU, Bhutta Z, Foster A, Ahammed MR, Collura G. Sinus of Valsalva Aneurysm: An Atypical Etiology of Recurrent Syncope. Cureus. 2023 Aug:15(8):e43325. doi: 10.7759/cureus.43325. Epub 2023 Aug 11 [PubMed PMID: 37700985]
Seo KW, Park JS. Sinus of Valsalva Aneurysm and Multiple Aortic Aneurysms Provoked by Viral Myocarditis. Korean circulation journal. 2019 Feb:49(2):194-196. doi: 10.4070/kcj.2018.0309. Epub [PubMed PMID: 30693683]
Stöger G, Borger MA, Misfeld M. A giant aneurysm of the left sinus of Valsalva. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2019 Sep 1:56(3):627. doi: 10.1093/ejcts/ezz010. Epub [PubMed PMID: 30689795]
Diwakar A, Patnaik SS, Hiremath CS, Chalam KS, Dash P. Rupture of sinus of valsalva - A 15 years single institutional retrospective review: Preoperative heart failure has an impact on post operative outcome? Annals of cardiac anaesthesia. 2019 Jan-Mar:22(1):24-29. doi: 10.4103/aca.ACA_243_17. Epub [PubMed PMID: 30648675]
Level 2 (mid-level) evidenceChen J, Liang HN, Wu L, Dong SH, Li JH. Right sinus of Valsalva aneurysm spontaneously dissecting into the interventricular septum in a rare case of Behcet's disease. European heart journal. Cardiovascular Imaging. 2019 May 1:20(5):601. doi: 10.1093/ehjci/jey218. Epub [PubMed PMID: 30629150]
Level 3 (low-level) evidenceStróżyk A, Kołaczkowska M, Fijałkowska J, Siondalski P, Fijałkowski M. Sinus of Valsalva rupture in a patient with a mechanical aortic prosthesis: aneurysm dissecting into the interventricular septum. Kardiologia polska. 2018:76(12):1742. doi: 10.5603/KP.2018.0235. Epub [PubMed PMID: 30566219]
Ghosh S, Bootla D, Barward P, Sharma A. Multilobulated Sinus of Valsalva Aneurysm Dissecting into the Interventricular Septum (DAIS) and Rupturing into Left Ventricle: A Case Report. European heart journal. Case reports. 2022 Feb:6(2):ytac019. doi: 10.1093/ehjcr/ytac019. Epub 2022 Feb 7 [PubMed PMID: 35233482]
Level 3 (low-level) evidenceSarkar M, Wehman B, Mukherjee R, Taylor BS. Left sinus of Valsalva aneurysm presenting as myocardial ischemia. The Journal of thoracic and cardiovascular surgery. 2019 Mar:157(3):e167-e168. doi: 10.1016/j.jtcvs.2018.09.070. Epub 2018 Oct 10 [PubMed PMID: 30527717]
Chan N, Charalambous M, Fuschetto DP, Fuschetto O, Makaryus JN. Severe compression of the left circumflex coronary artery by a large sinus of Valsalva aneurysm. Journal of cardiovascular computed tomography. 2020 Sep-Oct:14(5):e26-e28. doi: 10.1016/j.jcct.2018.11.001. Epub 2018 Nov 3 [PubMed PMID: 30482470]
Kassab K, Jolly N, Vij A, Kattoor AJ. Percutaneous closure of ruptured sinus of Valsalva: A review. Cardiovascular revascularization medicine : including molecular interventions. 2024 Mar:60():91-94. doi: 10.1016/j.carrev.2023.09.006. Epub 2023 Sep 28 [PubMed PMID: 37777419]
Weinreich M, Yu PJ, Trost B. Sinus of valsalva aneurysms: review of the literature and an update on management. Clinical cardiology. 2015 Mar:38(3):185-9. doi: 10.1002/clc.22359. Epub 2015 Mar 10 [PubMed PMID: 25757442]
Nguyen Q, Vervoort D, Phan K, Luc JGY. Surgical management for unruptured sinus of Valsalva aneurysms: a narrative review of the literature. Journal of thoracic disease. 2021 Mar:13(3):1833-1850. doi: 10.21037/jtd-20-2682. Epub [PubMed PMID: 33841972]
Level 3 (low-level) evidenceDoost A, Craig JA, Soh SY. Acute rupture of a sinus of Valsalva aneurysm into the right atrium: a case report and a narrative review. BMC cardiovascular disorders. 2020 Feb 18:20(1):84. doi: 10.1186/s12872-020-01383-7. Epub 2020 Feb 18 [PubMed PMID: 32070284]
Level 3 (low-level) evidence