Introduction
Serotonin syndrome (serotonin toxicity) is a serious and potentially life-threatening condition that results from excessive serotonergic activity throughout the central nervous system. Serotonin is a neurotransmitter that regulates mood, behavior, and other physiological functions. The neurotransmitter is produced naturally in the body and can also be found in certain medications, illicit substances, and supplements. Serotonin syndrome may be a consequence of therapeutic medication use, accidental interactions between medications and recreational drugs, or an intentional overdose.[1] The clinical severity of serotonin syndrome varies broadly, as some cases are relatively mild while others are fatal. Symptoms classically include altered mental status, autonomic dysfunction, and neuromuscular excitation.[2][3]
The diagnosis of serotonin syndrome is clinical, which provides challenges in rapid and accurate diagnosis. Several criteria exist for this clinical diagnosis, but the Hunter criteria are generally the most broadly used. Healthcare professionals must take a detailed medical history and assess the patient's medication regimen to arrive at an accurate diagnosis.[4] Treatment for serotonin syndrome involves discontinuing the offending medications and providing supportive care.
Mild cases are under-recognized, while severe cases generally require hospitalization. Drugs to counteract serotonin toxicity may be administered in severe cases. Serotonin syndrome generally precipitates within 1 day of a serotonergic change, either from the addition of a medication, change in dose, or use of other substances. Preventing serotonin syndrome involves careful monitoring of medication use, avoiding the concurrent use of multiple serotonin-increasing drugs, and consulting with healthcare professionals if concerns about drug interactions are apparent.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine neurotransmitter with multifaceted cellular and physiological functions in humans. Most serotonin is produced by intestinal enterochromaffin cells and in the peripheral nervous system, where it regulates gastrointestinal motility, uterine contractions, vasoconstriction, and bronchoconstriction.[6] The raphe nuclei in the brain stem produce serotonin in the central nervous system, CNS. In the CNS, serotonin modulates attention, behavior, and thermoregulation. Additionally, serotonin is produced in the skin's Merkel cells, pulmonary neuroendocrine cells, and taste receptor cells of the tongue. Further, serotonin is stored in blood platelets and promotes aggregation. Notably, platelets lack enzymes to synthesize serotonin independently and rely on serotonin uptake from the plasma to store serotonin.[7] Serotonin production is 90% from gastrointestinal enterochromaffin cells, 8% from platelets, and 2% from the CNS.[8]
The serotonin signaling pathway in the CNS begins at the production source, in the brainstem's raphe nuclei. The raphe nuclei are centered in the reticular formation, and their axons extend to other brain areas. The rostral group of nuclei extends into cortical and subcortical structures, and these pathways are anticipated to regulate wakefulness, attention, mood, sexual behavior, thermoregulation, and appetite. The caudal raphe nuclei extend into the spinal regions and regulate motor tone and nociception.[9] The peripheral serotonin signaling pathway generally begins with platelet activation, leading to serotonin secretion from stores back into the plasma, which has downstream effects on target organ serotonin receptors.[10]
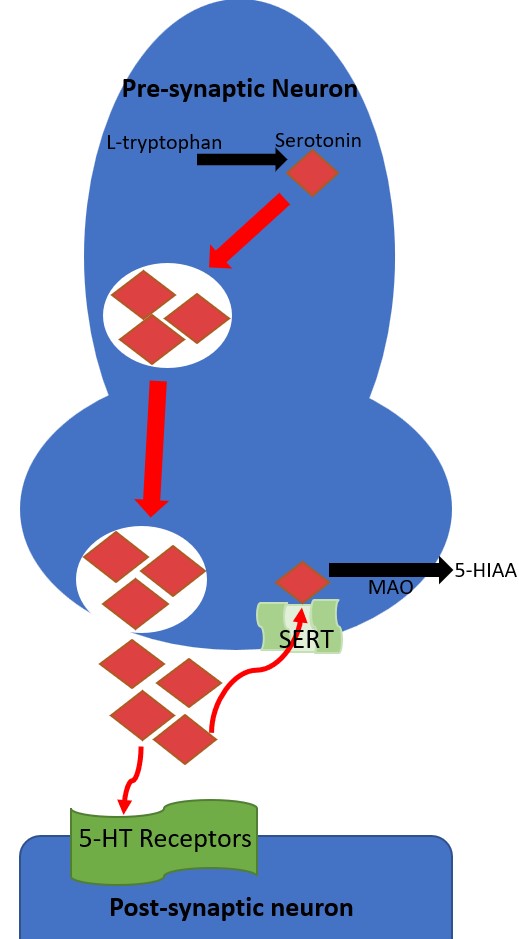
The serotonin signaling pathway's receptor is located in pre- and post-synaptic neurons. In a reuptake process, the pre-synaptic serotonin transporter (SERT) transports serotonin from the synaptic cleft back into the pre-synaptic neuron. SSRIs and tricyclic antidepressants bind SERT, inhibiting their ability to transport serotonin and increasing the amount of serotonin in the synaptic cleft available to activate post-synaptic serotonin receptors. When serotonin is transported by SERT back into the pre-synaptic neuron, it can be metabolized by monoamine oxidase. Monoamine oxidase inhibitors are a class of antidepressants that inhibit the metabolism of serotonin and, when implicated in serotonin syndrome, tend to cause the most severe cases.[6]
Seven known families of post-synaptic 5-HT receptors are recognized, named 5-HT1 through 5-HT7, and each has multiple subtypes resulting in pharmacologically distinct receptors (see Image. The Neurotransmission of Serotonin).[11] Black arrows indicate a chemical change and red arrows indicate serotonin transport. The enzyme tryptophan hydroxylase converts L-tryptophan hydroxylase into serotonin and is stored in pre-synaptic intracellular vesicles. Through exocytosis, serotonin is released into the synaptic cleft, where it can activate post-synaptic 5-HT receptors. Serotonin is taken back up into the pre-synaptic neuron by the SERT transporter. Serotonin is metabolized into 5-HIAA (5-hydroxyindoleacetic acid).
The 5-HT1 receptors are inhibitory G-protein-coupled receptors and have 5-subtypes: 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F. Several medications are prescribed to target these receptor subtypes. Buspirone targets 5-HT1A, and the antimigraine agents sumatriptan and zolmitriptan target 5-HT1B and 5-HT1D.[9] The 5-HT2 receptor family are excitatory G-protein-coupled receptors with 3 subtypes: 5-HT2A, 5-HT2B, and 5-HT2C. The 5-HT2A receptor is a common target of most second-generation antipsychotic medications that antagonize the receptor. Notably, 5-HT2A antagonism is suspected to shunt serotonin to available 5-HT1A receptors not blocked by antipsychotics, which may be a risk factor for precipitating serotonin syndrome.[12] 5-HT2C is targeted by the weight-loss drug lorcaserin (withdrawn from the United States market in 2020).[13] The 5-HT3 receptor is an excitatory ligand-gated ion channel most relevant for initiating emesis when activated. Ondansetron is a 5-HT3 receptor antagonist that directs antiemetic properties. The 5-HT4 receptor is a stimulatory G-coupled-protein receptor that increases gastric motility. Prucalopride is a 5-HT4 agonist that treats constipation. The 5-HT5 receptors are G-protein-coupled receptors comprised of 2 subtypes, 5-HT5A, and 5-HT5B, but have little known function and are unknown targets of medications. The 5-HT6 and 5-HT7 receptors are also stimulatory G-protein-coupled receptors, with some antidepressants and antipsychotics modulating activity through off-targeting, but no medications currently target 5-HT6 or 5-HT7. The etiology of serotonin syndrome results from cellular toxicity of excess target post-synaptic 5-HT stimulation.[9]
Epidemiology
Serotonin syndrome occurs in all ages, without sex preference.[9] The exact incidence of serotonin syndrome is difficult to measure for several reasons. First, it is a relatively uncommon condition. Second, mild cases are likely significantly underdiagnosed (or misdiagnosed). Third, mild cases may not require medical evaluations; fourth, many clinicians are unfamiliar with the condition.[4][14][15]
In the United States, the percentage of adults taking serotonergic antidepressants has significantly increased in the past 2 decades.[9] Specifically, estimates were 6% of adults in 1999 and 10.4% in 2010, which continued to increase into the 2020s.[16] The Toxic Exposure Surveillance System (TESS) receives case reports from medical practices in the United States.[9] TESS data from 2016 revealed over 54,000 incidences of toxic exposures to selective serotonin reuptake inhibitors (SSRIs) and 102 attributable deaths to these agents.[17] Compared to 2002 TESS data, an 18% increase in cases was detected through 2016 and an 8% increase in deaths.[9][18] A study reviewing serotonin syndrome reports to The United States Food and Drug Administration Adverse Event Reporting System revealed that nearly half of serotonin syndrome cases involved using a single serotonergic agent.[9][19]
Pathophysiology
Serotonin syndrome results when a clinical manifestation results from modification to the serotonergic transmission system.[9] The imbalance is usually seen within 24 hours of either the intake of a new serotonergic agent, dose change of a prior serotonergic agent, or any other change impacting the metabolism of prior serotonergic homeostasis. Serotonin syndrome may occur as a consequence of therapeutic medication use, illicit drug use, or intentional overdose. Multiple substances may precipitate serotonin toxicity by a variety of mechanisms.
Broadly, these can be considered by the following categories: 1) increased serotonin synthesis, 2) inhibiting serotonin metabolism or breakdown, 3) increased exocytosis of serotonin from presynaptic neurons, 4) increased activation of postsynaptic serotonin receptors, 5) antagonism of 5-HT2A receptors, 6) inhibiting serotonin reuptake from the synaptic cleft (see Table 1. Major Mechanisms of Common Substances and Medications That Modulate the Serotonergic System).[9][20]
Table 1. Major Mechanisms of Common Substances and Medications That Modulate the Serotonergic System [20][9]
| Increased Serotonin Synthesis | Inhibit Serotonin Metabolism | Increased Serotonin Exocytosis | Increased 5-HT1 Activation | 5-HT2A Antagonism | Reuptake Inhibition |
| *Tryptophan | MAO-I | Cocaine | Buspirone | Atypical antipsychotics | SSRI |
| *St. John's Wort | Ecstasy (MDMA) | Triptans | Chlorpromazine | SNRI | |
| Linezolid | Dextromorphan | Ergot Derivatives | |||
| Tedizolid | Opiates | ||||
| Methylene Blue | LSD | ||||
| Procarbazine | Mirtazapine | ||||
| *Syrian Rue | Trazodone | ||||
| Lithium |
MAO-I, monoamine oxidase inhibitor; MDMA, 3,4-Methylenedioxymethamphetamine; LSD, Lysergic acid diethylamide; SSRI, selective serotonin reuptake inhibitor; SNRI, selective serotonin and norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant. *indicates a supplement or herbal remedy. Note, mechanisms of action are not exclusive, and many listed substances have minor-moderate mechanisms of other categories.
Although the use of a single agent precipitates many cases of serotonin syndrome, drug interactions can perpetuate risk and potentially increase the severity of serotonin syndrome. The cytochrome P450 pathway metabolizes many serotonergic medications, specifically CYP2D6 and CYP3A4.[21] Serotonergic and nonserotonergic agents can inhibit the cytochrome P450 pathway, which can cause an increased serotonergic drug concentration, leading to serotonin syndrome. Common examples of non-serotonergic agents impacting CYP450 metabolism include ciprofloxacin and fluconazole.[22] Serotonin agent tolerability varies from person to person due to many factors, including differing medication doses, medication combinations, administration timings, illicit substance use, phenotypic differences in the CYP540 pathway, SERT receptor, and 5-HT receptors.[21][9][21][23]
When serotonin syndrome is present, excessive stimulation of 5-HT receptors leads to pathological changes in the affected target organs. Mental status changes are expected, including severity through delirium. Autonomic dysfunction can occur due to the loss of the ability to self-thermoregulate, heart rate changes, and blood pressure changes. Gastrointestinal symptoms are common, mainly vomiting and diarrhea.[24] A classic feature of serotonin syndrome is neuromuscular hyperactivity, which typically presents as hyperreflexia and myoclonus. In addition, tremor Babinski signs can be present. Severe cases are associated with hyperthermia and seizures.[9]
History and Physical
Serotonin syndrome describes a broad array of clinical findings that are generally reflective of the degree of severity of serotonin toxicity. Further, the diagnosis is clinical and requires a thorough history and physical examination. Laboratory studies are helpful, especially in ruling out other conditions with overlapping clinical features.[25][9]
History
A detailed history of medications, supplements, and illicit substance use should be obtained if serotonin syndrome is suspected. This may present challenges in obtaining a reliable history if the patient presents with altered mental status, which is a common feature of serotonin syndrome.[9] In addition to the substance names, formulations, doses, and schedules, any information related to medication changes or the use of new substances is critical in determining the likelihood of serotonin syndrome. This information, combined with the onset of symptoms and the rate of symptom progression, is essential in diagnosing serotonin syndrome. Most serotonin syndrome cases present within 6 to 24 hours of a serotonergic agent dose change or intake of a new substance.[26]
A particular area of concern is related to intentional overdoses of serotonergic agents. Intentional overdoses that lead to serotonin syndrome are more severe than accidental exposures leading to the condition. Because of the difference in severity, it is essential to directly ask the patient if the intent to overdose was premeditated.[23]
Physical Examination
The physical examination is crucial in differentiating serotonin syndrome from other acute syndromes with overlapping symptoms. Serotonin syndrome has a broad array of possible presentations, and not all of the listed symptoms may be present in each case. Vital sign abnormalities generally reveal tachycardia and hypertension, but fluctuations in these vital signs are due to autonomic instability. Further, hyperthermia may occur due to loss of temperature regulation controls from serotonin toxicity. Physical exam findings may include altered mental status (with agitation), ocular clonus, dilated pupils, akathisia, tremor, deep tendon hyperreflexia, inducible (or spontaneous) muscle clonus, muscle rigidity, positive Babinski signs, flushed skin, diaphoresis, increased bowel activity (see Image. Common Signs of Serotonin Syndrome). The neuromuscular findings are generally more observable in the lower extremities, with hyperreflexia and muscle clonus being the most common findings (see Image. Clinical Examination of Ankle Clonus).[4][9]
Evaluation
Serotonin syndrome is a clinical diagnosis, and no diagnostic laboratory studies are indicated. Still, laboratory evaluations can assist in clarifying the clinical picture, assessing the severity of serotonin syndrome, and directing the treatment plan. Common nonspecific laboratory findings include increased white blood cell count, decreased sodium bicarbonate, and elevated creatine phosphokinase.[24] Severe serotonin syndrome may lead to intravascular coagulation with platelet abnormalities, rhabdomyolysis with highly elevated creatine kinase, and metabolic acidosis; for patients who present with intentional overdose, acetaminophen levels, salicylate concentrations, and electrocardiogram should be obtained. Head computed tomography is helpful to rule out acute head injuries.[9]
Several diagnostic criteria are available, including Sternbach, Radomski, and Hunter. However, the Hunter Toxicity Criteria Decision Rules are accepted as the most accurate. The Hunter Criteria is recommended as it has been demonstrated to be the most accurate compared to the gold standard diagnosis of a medical toxicologist, with 84% sensitivity and 97% specificity.[9][15][25]
Hunter Toxicity Criteria Decision Rules
- History of recent exposure to a serotonergic drug
- At least one of the following:
- Spontaneous clonus
- Inducible clonus with agitation or diaphoresis
- Ocular clonus with agitation and diaphoresis
- Tremor and hyperreflexia
- Hypertonia and temperature over 38 °C with ocular or inducible clonus
Patients with a history of stable dosing of a serotonergic agent and a history of tolerating that medication are unlikely to develop serotonin syndrome spontaneously. Therefore, Hunter Criteria has outlined clarifications for criteria 1 (history of recent exposure to a serotonergic agent) as follows:
- Initiation or increase in the dose of a serotonergic agent
- Pharmacologic change that decreases the metabolism of a serotonergic agent
- Overdose with a serotonergic agent unless the only agent exposed is a serotonin receptor agonist (Table 1, 5-HT activation)
- Drug-drug interactions only involve direct serotonin receptor agonists (5-HT activation) [15]
Treatment / Management
Discontinue Serotonergic Agents
The first step in managing serotonin syndrome is accurately and promptly recognizing the condition. Misdiagnosis is a common pitfall that has the potential to worsen outcomes, particularly if serotonin agents are continued. Thereby, patients with hyperthermia of unknown origin are not recommended to continue serotonergic agents. Discontinuation of all serotonergic agents is the first step in treating serotonin syndrome. As serotonin syndrome is a toxicity, the condition resolves with the removal of the offending agents. However, specific serotonergic agents, such as fluoxetine, have long half-lives, which may cause symptoms to persist for multiple days. [27]
Supportive Care
After discontinuation of serotonergic agents, supportive care to normalize the vital signs is recommended during the serotonergic agent's detox from the body. Supportive care includes administering intravenous fluids and oxygen (goal of saturation ≥94%), cardiac monitoring, and correction of vital signs in autonomic instability. Intravenous crystalloids can be given to assist in treating volume depletion in hyperthermia.[9](B3)
Sedation
Delirious agitation with muscle contractions contributes significantly to hyperthermia and creatine kinase elevation. Chemical restraint (sedation) is highly preferred in serotonin syndrome, as physical restraints may still result in significant muscle contractions and worsen the condition.[4] Benzodiazepines are the preferred agents, as they have no anticholinergic or serotonergic properties, and they are not dopamine receptor antagonists. Additionally, they assist in controlling high blood pressure and tachycardia. No consensus is apparent on which benzodiazepines are preferable in treating serotonin syndrome; however, diazepam has been shown to prolong survival in rat models with serotonin syndrome.[28](B3)
Vital Sign Management in Autonomic Instability
Autonomic instability in serotonin can present challenges, given the potential for dramatic fluctuations in blood pressure and heart rate. Because of this, short-acting agents, including esmolol, nicardipine, or nitroprusside, are preferred.[4] Longer-acting agents such as propranolol should be avoided, given the unpredictability of autonomic instability.[9] Hypotension resulting from MAO-I's should be treated with direct-acting sympathomimetic agents such as epinephrine, phenylephrine, or norepinephrine. Dopamine should be avoided because it is metabolized into epinephrine and norepinephrine; when a MOA-I is present, it is difficult to predict cellular regulation of this conversion, which can lead to an exaggerated hemodynamic response.[4][9](B3)
Hyperthermia
A significant contributor to resolving hyperthermia involves adequate chemical sedation to prevent excessive heat production from agitation-related muscle activity. Patients who have very high temperatures of 41.1 °C and above require sedation and intubation in conjunction with intensive care services. Antipyretics such as acetaminophen are ineffective because the increased muscular activity is the main contributor to hyperthermia (along with central deregulation) in serotonin syndrome. In refractory cases, external cooling measures are supplemental.[9](B3)
Serotonin Antagonists
The administration of an antidote (serotonin antagonists) is not required in all cases of serotonin syndrome. However, if supportive measures, vital signs management, and chemical sedation do not adequately treat serotonin syndrome, then antidotes can be considered the next step. Cyproheptadine has nonspecific 5-HT1A and 5-HT2A antagonistic properties and some anticholinergic properties, but its major mechanism functions as a histamine-1 receptor antagonist. The histamine-1 receptor antagonism also assists with sedation. Notably, cyproheptadine is only available as an oral pill but can be crushed and administered via nasogastric or orogastric tube. The initial dose recommended is 12 mg, with 2 mg administered every 2 hours until adequate clinical response.[9] Notably, inconclusive evidence exists for the efficacy of cyproheptadine, with reports documenting different outcomes.[29][30][31][32](B3)
Differential Diagnosis
Neuroleptic Malignant Syndrome
Serotonin syndrome and neuroleptic malignant syndrome (NMS) are 2 serious conditions of commonly prescribed psychiatric medications, with some overlapping clinical features, which can lead to difficulty in establishing an accurate clinical diagnosis. However, with close history and physical examinations, the 2 conditions can be distinguished. Serotonin syndrome is only precipitated by agents that impact the serotonergic system, while NMS is precipitated by dopamine receptor antagonists (namely, antipsychotics). This demonstrates the importance of obtaining an accurate medication history. The syndrome develops rapidly within a few hours of a serotonergic agent change, while NMS develops subacutely within days to weeks.[33] Physical examination findings also differ in serotonin syndrome, which has neuromuscular hyperreactivity, most easily demonstrated by hyperreflexia, clonus, and tremors. In contrast, the physical examination findings for NMS show a slowed neuromuscular response, most easily demonstrated by rigidity.
Both conditions improve with the removal of the offending agent, but serotonin syndrome resolves rapidly (within 1 day generally), and NMS resolves slowly (1 to 2 weeks). Overlapping findings of the 2 conditions include the potential for hyperthermia, delirium, leukocytosis, elevated creatine phosphokinase, and metabolic acidosis.[33][9]
Malignant Hyperthermia
Serotonin syndrome and malignant hyperthermia are both life-threatening conditions. Serotonin syndrome may cause hyperthermia, but it is not present in all cases and results after exposure to serotonergic agents. Malignant hyperthermia results from exposure to halogenated volatile anesthetics and depolarizing muscle relaxants (succinylcholine). Generally, these exposures are from surgery, an electroconvulsive therapy session, or another session that utilized anesthetics. Malignant hyperthermia also presents with severe muscle rigidity (often likened to the severity of rigor mortis).[34]
Anticholinergic Toxicity
Shared features of anticholinergic toxicity and serotonin syndrome include hyperthermia and delirium—however, anticholinergic toxicity presents in the context of anticholinergic medications. The anticholinergic properties of most serotonergic agents are generally limited, except TCAs and paroxetine. Still, other psychiatric medications commonly have anticholinergic properties, and the potential for a mixed serotonin syndrome with anticholinergic toxicity is possible, particularly in the context of an intentional overdose of psychiatric medications. Anticholinergic toxicity also presents with dry mucous membranes, urinary retention, and decreased bowel sounds. In contrast, increased bowel sounds are a common feature of isolated serotonin syndrome.[9]
Delirium of Other Etiology
Altered mental status, with or without agitation, is a common nonspecific clinical finding. Serotonin syndrome presents with neuromuscular hyperactivity (tremor, clonus, hyperreflexia), which is uncommon in most causes of delirium.[9]
Prognosis
The prognosis of serotonin syndrome varies on the degree of serotonin toxicity, which is primarily attributable to the dose and type of serotonergic agent. Notably, MAO-I's are associated with the most severe cases of serotonin syndrome, but intentional overdoses of any serotonergic drug can result in severe presentations. Mild cases may only require observation, while severe cases require intensive care. After serotonin syndrome resolves, a comprehensive assessment is needed to assess the risks and benefits of resuming the previous serotonergic regimen.[9]
Complications
Serotonin syndrome typically resolves rapidly and safely when quickly treated. Generally, full recovery from serotonin syndrome is expected after stabilization. The duration of serotonin syndrome depends on a few factors but is most impacted by the half-life of the culprit medications. When the altered mental status resolves, vital signs are normal, and a normal neurological examination is elicited (no clonus or hyperreflexia), the patient is safe for discharge. The patient's clinicians consider medication reconciliation to prevent another episode of serotonin syndrome.[4]
Deterrence and Patient Education
Serotonin syndrome may not always be described as a risk when obtaining consent for prescribing a serotonergic agent. Although serotonin syndrome is more likely to occur when multiple serotonergic agents are present, it can still precipitate from the use of a single drug. Therefore, informing patients of the risk of serotonin syndrome is essential when initiating a serotonergic agent.[9] Further, physical examinations during outpatient visits can help monitor for signs of serotonin syndrome. Specifically, performing a neurological exam on patients prescribed serotonergic agents can quickly identify subclinical symptoms of serotonin syndrome.
Enhancing Healthcare Team Outcomes
Serotonin syndrome can be challenging to recognize, but the interdisciplinary team can assist in prompt diagnosis and management. Emergency technicians, nurses, and nursing assistants often obtain medication histories, which are brought to the clinician's attention if adverse reactions to serotonergic agents are a concern. Toxicologists provide expert recommendations in severe cases. Additionally, psychiatric medications are a ubiquitous source of serotonergic agents. After serotonin syndrome resolves, they may assist in determining the risks and benefits of continuing the previous medication regimen or suggesting alternative treatments. Serotonin syndrome was more intensely studied in prior decades, particularly after the emergence of MAO-I's, TCAs, and SSRIs. Still, evidence for the general management of serotonin syndrome is relatively consistent, with the most important emphasis being prompt recognition.
Media
(Click Image to Enlarge)
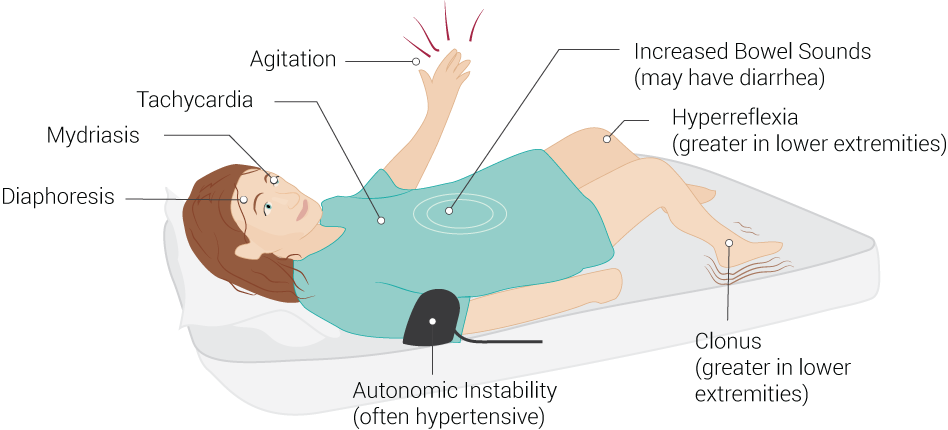
Common Signs of Serotonin Syndrome. Serotonin syndrome signs include increased bowel sounds (may have diarrhea), hyperreflexia (greater in lower extremities), clonus (greater in lower extremities), autonomic instability (often hypertensive), diaphoresis, mydriasis, tachycardia, and agitation.
Contributed by B Palmer
(Click Video to Play)
Clinical Examination of Ankle Clonus. Clinical examination of ankle clonus is typically demonstrated in upper motor neuron lesions or serotonin syndrome.
Contributed by RS Menon, MD
(Click Image to Enlarge)
References
Francescangeli J, Karamchandani K, Powell M, Bonavia A. The Serotonin Syndrome: From Molecular Mechanisms to Clinical Practice. International journal of molecular sciences. 2019 May 9:20(9):. doi: 10.3390/ijms20092288. Epub 2019 May 9 [PubMed PMID: 31075831]
Duma SR, Fung VS. Drug-induced movement disorders. Australian prescriber. 2019 Apr:42(2):56-61. doi: 10.18773/austprescr.2019.014. Epub 2019 Apr 1 [PubMed PMID: 31048939]
Srivastava A, Singh P, Gupta H, Kaur H, Kanojia N, Guin D, Sood M, Chadda RK, Yadav J, Vohora D, Saso L, Kukreti R. Systems Approach to Identify Common Genes and Pathways Associated with Response to Selective Serotonin Reuptake Inhibitors and Major Depression Risk. International journal of molecular sciences. 2019 Apr 23:20(8):. doi: 10.3390/ijms20081993. Epub 2019 Apr 23 [PubMed PMID: 31018568]
Boyer EW, Shannon M. The serotonin syndrome. The New England journal of medicine. 2005 Mar 17:352(11):1112-20 [PubMed PMID: 15784664]
Birmes P, Coppin D, Schmitt L, Lauque D. Serotonin syndrome: a brief review. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2003 May 27:168(11):1439-42 [PubMed PMID: 12771076]
Level 3 (low-level) evidenceBerger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual review of medicine. 2009:60():355-66. doi: 10.1146/annurev.med.60.042307.110802. Epub [PubMed PMID: 19630576]
Level 3 (low-level) evidenceBamalan OA, Moore MJ, Al Khalili Y. Physiology, Serotonin. StatPearls. 2024 Jan:(): [PubMed PMID: 31424752]
Schlienger RG, Meier CR. Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction? American journal of cardiovascular drugs : drugs, devices, and other interventions. 2003:3(3):149-62 [PubMed PMID: 14727927]
Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. International journal of tryptophan research : IJTR. 2019:12():1178646919873925. doi: 10.1177/1178646919873925. Epub 2019 Sep 9 [PubMed PMID: 31523132]
Level 3 (low-level) evidenceNi W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clinical and experimental pharmacology & physiology. 2006 Jul:33(7):575-83 [PubMed PMID: 16789923]
Level 3 (low-level) evidenceBarnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999 Aug:38(8):1083-152 [PubMed PMID: 10462127]
Level 3 (low-level) evidenceCelada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. Journal of psychiatry & neuroscience : JPN. 2004 Jul:29(4):252-65 [PubMed PMID: 15309042]
Tak YJ, Lee SY. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Current obesity reports. 2021 Mar:10(1):14-30. doi: 10.1007/s13679-020-00422-w. Epub 2021 Jan 6 [PubMed PMID: 33410104]
Werneke U, Jamshidi F, Taylor DM, Ott M. Conundrums in neurology: diagnosing serotonin syndrome - a meta-analysis of cases. BMC neurology. 2016 Jul 12:16():97. doi: 10.1186/s12883-016-0616-1. Epub 2016 Jul 12 [PubMed PMID: 27406219]
Level 1 (high-level) evidenceChiew AL, Buckley NA. The serotonin toxidrome: shortfalls of current diagnostic criteria for related syndromes. Clinical toxicology (Philadelphia, Pa.). 2022 Feb:60(2):143-158. doi: 10.1080/15563650.2021.1993242. Epub 2021 Nov 22 [PubMed PMID: 34806513]
Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. The Journal of clinical psychiatry. 2014 Feb:75(2):169-77. doi: 10.4088/JCP.13m08443. Epub [PubMed PMID: 24345349]
Level 2 (mid-level) evidenceGummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 34th Annual Report. Clinical toxicology (Philadelphia, Pa.). 2017 Dec:55(10):1072-1252. doi: 10.1080/15563650.2017.1388087. Epub 2017 Nov 29 [PubMed PMID: 29185815]
Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Youniss J, Rose SR, Borys D, May ME. 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. The American journal of emergency medicine. 2003 Sep:21(5):353-421 [PubMed PMID: 14523881]
Culbertson VL, Rahman SE, Bosen GC, Caylor ML, Echevarria MM, Xu D. Implications of Off-Target Serotoninergic Drug Activity: An Analysis of Serotonin Syndrome Reports Using a Systematic Bioinformatics Approach. Pharmacotherapy. 2018 Sep:38(9):888-898. doi: 10.1002/phar.2163. Epub 2018 Jul 29 [PubMed PMID: 29972695]
Level 1 (high-level) evidenceRacz R, Soldatos TG, Jackson D, Burkhart K. Association Between Serotonin Syndrome and Second-Generation Antipsychotics via Pharmacological Target-Adverse Event Analysis. Clinical and translational science. 2018 May:11(3):322-329. doi: 10.1111/cts.12543. Epub 2018 Mar 25 [PubMed PMID: 29575568]
Mitchell PB. Drug interactions of clinical significance with selective serotonin reuptake inhibitors. Drug safety. 1997 Dec:17(6):390-406 [PubMed PMID: 9429838]
Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. General hospital psychiatry. 2008 Jul-Aug:30(4):372-7. doi: 10.1016/j.genhosppsych.2008.03.008. Epub [PubMed PMID: 18585543]
Level 3 (low-level) evidenceIsbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clinical neuropharmacology. 2005 Sep-Oct:28(5):205-14 [PubMed PMID: 16239759]
Level 3 (low-level) evidenceMason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine. 2000 Jul:79(4):201-9 [PubMed PMID: 10941349]
Level 3 (low-level) evidenceDunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM : monthly journal of the Association of Physicians. 2003 Sep:96(9):635-42 [PubMed PMID: 12925718]
Level 2 (mid-level) evidenceRamsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. British journal of pharmacology. 2007 Nov:152(6):946-51 [PubMed PMID: 17721552]
Martin TG. Serotonin syndrome. Annals of emergency medicine. 1996 Nov:28(5):520-6 [PubMed PMID: 8909274]
Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochemistry international. 2003 Jul:43(2):155-64 [PubMed PMID: 12620284]
Level 3 (low-level) evidenceKapur S, Zipursky RB, Jones C, Wilson AA, DaSilva JD, Houle S. Cyproheptadine: a potent in vivo serotonin antagonist. The American journal of psychiatry. 1997 Jun:154(6):884 [PubMed PMID: 9167527]
Level 3 (low-level) evidenceGraudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. The Journal of emergency medicine. 1998 Jul-Aug:16(4):615-9 [PubMed PMID: 9696181]
Level 3 (low-level) evidenceBaigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. European journal of anaesthesiology. 2003 Jul:20(7):586-8 [PubMed PMID: 12884999]
Level 3 (low-level) evidenceHorowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatric emergency care. 1999 Oct:15(5):325-7 [PubMed PMID: 10532660]
Level 3 (low-level) evidenceMills KC. Serotonin syndrome. A clinical update. Critical care clinics. 1997 Oct:13(4):763-83 [PubMed PMID: 9330840]
Ali SZ, Taguchi A, Rosenberg H. Malignant hyperthermia. Best practice & research. Clinical anaesthesiology. 2003 Dec:17(4):519-33 [PubMed PMID: 14661655]