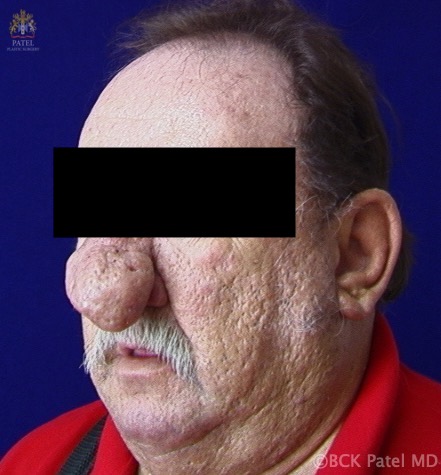
Introduction
Rhinophyma is a disfiguring nasal deformity due to the proliferation of sebaceous glands and underlying connective tissue. The name itself is broken down into "rhis," derived from Greek meaning nose, and "phyma," also Greek, for skin tumor. While rhinophyma is the most common subtype of these tumor-like swellings, this type of presentation can manifest at other sites.[1] These include the following:
- Mentophyma (chin)
- Metophyma (forehead)
- Gnatophyma (chin)
- Otophyma (ears)
- Bleharophyma (eyelids)
Rosacea is the precursor condition to the later development of rhinophyma and was linked to this disease as the ultimate expression of rosacea in 1846 by Ferdinando Hebra Von (1816-1880).[2] Historically, the condition has been known to exist, as seen in a 15th Century painting by Domenico Ghirlandaio (1490), "An Old Man and His Grandson," which shows a man with a large rhinophyma (See Figure).
This disfiguring disorder is essential to treat, as patients are subject to psychological distress and respiratory issues when alar thickening can obstruct the external nasal valves. An additional social challenge is a commonly presumed link to excessive alcohol use. The direct causal relationship between rhinophyma and alcohol has not been substantiated and has promoted a social stigma, as colloquial names for this condition include "whiskey nose" and "gin blossom."[3][4]"Potato nose" is another common lay description of this type of nose. Hollywood has used rhinophyma to indicate distasteful or villainous characters: in "Snow White and the Seven Dwarfs" (1937), the villain Queen was given a rhinophyma, besides deep rhytids and periorbital swelling.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Rosacea has previously been thought to progress sequentially through various subtypes, including erythematotelangiectatic rosacea, papulopustular rosacea, and phymatous rosacea. However, given the high frequency of overlapping clinical features and etiologies within subtypes, the classification of rosacea has now taken a phenotype-based diagnostic approach to accurately characterize patients.[5] Rosacea often starts between the ages of 20 to 30 with telangiectasias and excessive facial flushing exacerbated by vasoactive substances, including but not limited to caffeine, alcohol, and ultraviolet light. The initial cause of dysregulation of inflammatory cells, vasculature, and lymphatics underlying rosacea is multifactorial and has not been completely elucidated. Increased activation of the innate immune system is partially due to overexpression of toll-like receptors leading to activation of NF-kB with subsequent production of inflammatory cytokines, mast cells, and macrophages. Mast cells additionally increase vasodilation and angiogenesis through the production of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), allowing for elevated vascular permeability, edema, and cutaneous erythema.
The vasculature is further dysregulated by the histamine-induced vasodilation and vasoconstriction from local angiotensin II produced subsequently to chymase release. Cutaneous fibrosis of connective tissue and hyperplasia of sebaceous glands result from persistent inflammation and is characteristic of phymatous change. [6] While the underlying bony structure remains unchanged, the surrounding nasal skin hypertrophies with the preferential expansion of the nasal tip and alae.[7] The skin mite Demodex folliculorum, is known to live within the sebaceous unit and has been linked to this condition with unclear timing of a primary or secondary disease manifestation.[8][9]
Epidemiology
Rosacea is more common in females. However, rhinophyma is most commonly found in white men over the age of 50, with a male-to-female ratio of 5 to 1 to 30 to 1. Occurrence in Asian or African American men has been reported in the literature but is uncommon. There is a hypothesis that androgenic influences predispose rhinophyma development in males.[9]
Pathophysiology
Mechanisms of the pathogenesis of rosacea, and in turn rhinophyma, have not been completely elucidated and are thought to be multifactorial, involving a combination of neurovascular dysregulation and innate immune responses. Initially, vasodilation leads to fluid leakage into the dermal interstitium and matrix, predisposing to inflammation, fibrosis, and characteristic erythematous skin due to telangiectatic growth. Increased mast cell presence has been appreciated in all forms of rosacea and appears in the highest density when papules and pustules begin to develop.[10]
Histopathology
Histopathologic examination reveals hypertrophic sebaceous glands and thickened dermis containing fibrovascular myxoid stroma and lymphatic cells. Expansion of the sebaceous unit leads to sebum plugging. In observed cases of severe rhinophyma, sebaceous glands become destroyed by edema and fibrosis, producing a lymphoedema-type histologic picture.[2]
History and Physical
Rhinophyma is diagnosed clinically with erythema, telangiectasias, and skin thickening in the nasal region. The lower two-thirds of the nose is affected more than the upper third. There is no specific test for rhinophyma. Diagnostic confirmation is by histology.[1]
Rosacea was divided into four stages by Wilkin in 1994: pre-rosacea, vascular rosacea, inflammatory rosacea, and late rosacea. Rhinophyma belongs to the "late rosacea" stage.[11]
Further refinement of rosacea in 2002 by the National Rosacea Society (NRS) allowed standardization of rosacea.[12] The diagnosis of rosacea requires the presence of one or more primary features and one or more secondary features.
Primary Features
- Transient erythema (blushing)
- Non-transient erythema (persistent redness)
- Papules
- Pustules
- Telangiectasia
Secondary Features
- Facial skin hypersensitivity (burning or stinging)
- Plaques
- Edema
- Ocular manifestations
- Non-facial involvement
- Phymatous changes
The Global Rosacea Consensus (ROSCO) 2019 panel has additionally divided the features of rosacea into similar categories for phenotypic classification.[5]
Diagnostic Features
- Phymatous changes
- Persistent erythema
Major Features
- Flushing and transient erythema
- Papules and pustules
- Telangiectasia
Minor Features
- Burning sensation
- Stinging or painful sensation
- Dry sensation
- Localized facial swelling
Evaluation
No laboratory or radiographic tests are necessary for this condition. Clinical evaluation of the patient determines the type and grade of rhinophyma. The severity and sub-types of rhinophyma get classified in different ways.
The first is a division into four clinical variants: glandular, fibrous, fibroangiomatosis, and actinic:
- Glandular rhinophyma: is primarily due to the expansion of sebaceous glands
- Fibrous rhinophyma is due to hyperplasia of connective tissue
- Fibroangiomatous rhinophyma contains fibrosis, telangiectasias, and inflammatory lesions
- Actinic rhinophyma: elastic fibers grow in nodular masses, producing disfigurement[13]
An additional severity scale, defined by Freeman et al. in 1970, is also helpful. Beginning with early vascular type, rhinophyma progresses to:
- Moderate diffuse enlargement
- Early localized tumor
- Extensive diffuse enlargement
- Extensive with localized tumor diffuse enlargement[14]
This condition can also be broken down by a grading system into minor, moderate, or major rhinophyma, used by researcher El-Azhary et al. in 1991 - under this system, rhinophyma is:
- Minor when presenting with telangiectasias accompanied by minor thickening of the skin
- Moderate if skin thickening is accompanied by lobules
- Major is appropriate when prominent nodules and nasal hypertrophy are appreciated[15]
The NRS established a standard grading for rosacea and phymatous changes:
Severity level:
- 0: Absent
- 1: Follicles are patulous, but no contour changes
- 2: contour change without nodularity
- 3: change in contour with the nodular component
Once the rhinophyma has undergone evaluation, a discussion of options is next. Viable choices always include the possibility of not performing any surgical intervention. In the presence of severity levels 1 or 2, the patient may elect to continue to observe. It is wise to follow these patients by taking clinical photographs, including the chin-up position and the lateral views.
Treatment / Management
Management of Rosacea
While rosacea responds well to treatment, this does not prevent or treat rhinophyma—rosacea treatment centers around antibiotics and oral isotretinoin, best utilized in mild to moderate severity. Isotretinoin aids symptom reduction by lowering sebum production and sebaceous gland size. Conversely, topical Retin-A can potentially worsen rosacea due to enhanced irritation and sensitivity of increasingly erythematous and heavy telangiectatic skin.
Management of Rhinophyma
Patients must be off oral isotretinoin before surgery as it can prevent re-epithelialization.[16] The research proposes that topical metronidazole decreases skin inflammation by inhibiting the generation of reactive oxygen species as opposed to the reduction of skin bacteria.[17] While tetracyclines are known for their bacteriostatic effects, their anti-inflammatory properties benefit rosacea patients but do little for rhinophyma.[17] Historically, X-ray therapy was utilized in the 1920s as it was shown to decrease sebaceous gland size and promote the atrophy of the pilosebaceous unit. However, this treatment is out of favor due to the causation of secondary skin malignancies.
Advanced rhinophyma treatment requires surgical measures to remove tissue. The principal aim of surgery is to reduce the hypertrophied sebaceous glands and re-contour the nose. Secondarily, techniques are used to promote re-epithelialization of the nose. Surgery requires careful steps as rhinophyma occurs in a noticeable central region of the face.[18] These include:
- Reduction of hypertrophied tissue
- Re-contouring of the nasal region
- Controlling excessive bleeding
- Preventing postoperative complications.
Utilizing multiple modalities during surgery is common. Excisional methods carry an additional advantage of providing a specimen for pathologic evaluation, an option not available in ablative techniques due to complete tissue destruction.
Excisional Treatment
Full-thickness resection with reconstruction via flap or graft: this approach offers the benefits of immediate coverage of the open wound, avoids excessive heat damage, and eliminates recurrence risk by removing all pathologic tissue, including underlying cancer. However, this approach has fallen out of favor as flaps and grafts risk failure; grafts can have an incomplete skin color match and also require more operative wounds.
Partial-thickness tissue removal: this method preserves the pilosebaceous unit. By only excising superficial tissue and retaining the underlying adnexal structures, re-epithelialization occurs by secondary intention. It can be done with a standard scalpel and disposable razor blade, making this method cost-effective. Difficulties of this method include the risk of scarring with unintentional removal of the sebaceous unit and achieving hemostasis for a clear visual field. The use of electrocautery is often necessary to control bleeding and add additional nasal refinement.[19] Using a heated Shaw scalpel for partial thickness excision can lower the heavy bleeding seen in cold scalpel excision. Operating at 160 to 200 degrees C, it provides bulky tissue reduction and thermal energy for hemostasis. Risks of heated scalpel excision include mild scarring, burns, and the potential for slight nasal alar collapse.
Cryosurgery excisions: this is no longer widely performed. While its benefits entail relatively low intraoperative pain and lack of cartilage damage, this procedure often requires more than one visit with additional risks of scarring, dyschromia, and difficulty contouring the nose.
Dermabrasion: dermabrasion is used primarily as an adjuvant treatment post bulk removal to allow for precise nasal contouring.[7]
Ablative Treatment
Many different ablative approaches are options in treating rhinophyma:
Electrosurgery: this approach can both coagulate and cut using a wire loop or epilating needle.
Electrocautery: heat is utilized from an outside source, while electrosurgery uses radiofrequency electricity to generate heat within the tissue. Both methods have proven beneficial in providing a nearly bloodless cost-effective procedure.[4] Caution is necessary when employing these methods, especially when cauterizing large blood vessels, as thermal energy can heat surrounding tissue and underlying cartilaginous tissue producing texturization of the skin, high risk of postoperative scarring, and necrosis.
Coblation: "cold ablation" is an ablative method driven by radiofrequency to excite electrolytes within a conductive medium. Charged ions form a field used to de-epithelialize tissue at low generated temperature (less than 90 degrees C), decreasing thermal damage risk.[20]
Laser Treatment
Early treatments were with the argon laser: although selective coagulation of capillaries is possible, the depth of destruction of tissue is not predictable.
CO2 laser treatment has the ability to both cut and vaporizes the skin. Utilizing a 10600 nm wavelength, the laser is primarily absorbed by water, allowing for a lower depth of penetration with the ability to reach up to .5mm beyond the visibly burned layer. As the laser is obliterating the sebaceous glands, sebum gets expressed, which serves as a surrogate depth marker as squeezing the skin during surgery without apparent sebum excretion indicates an appropriate stopping point to avoid scarring. It requires less thermal energy than electrocautery and electrosurgery, allows for a bloodless surgical field, necessitates simple postoperative care, and has an overall low scarring risk. The surgeon can utilize CO2 laser along with bulk scalpel removal for more precise contouring. Initial scalpel reduction before using the CO2 laser also allows tissue for histopathologic assessment. Common disadvantages include hypopigmentation, dilated pores due to the destruction of prior sites of follicular cysts and fibrosed sebaceous glands, expensive equipment cost, and lengthy procedure time. Complete reepithelization takes approximately three weeks.[20][21]
The Er: YAG laser is an alternative laser option. Operating at the ideal wavelength for water absorption, 2940 nm, it has a smaller heat damage zone of fewer than 50 micrometers allowing for a reduced time to re-epithelialization of only 1 to 2 weeks. However, it can only provide minimal hemostasis.[22](B2)
Other lasers with a successful track record include the diode laser (808 nm), the Nd: YAG laser (1064 nm), and the KTP laser (532 micrometers).
Plasma and Radiofrequency: more recently, we have had success using Helium plasma in combination with radiofrequency: this is a unique energy-delivery mode that causes instantaneous heating but also instant cooling in tissues. Whereas bulk heating for more than 152 seconds is needed to a temperature of more than 65 degrees centigrade when using other devices like radiofrequency or ultrasound, helium plasma-RF energy raises tissue temperature to 85 degrees C within 0.044 seconds with the minimal transmission of heat to surrounding tissues; this allows us to ablate the nodules on the nose sequentially with little bleeding. The healing is similar to that seen after the CO2 laser.[23] Redness and pinkness of the skin can take longer to abate than seen with the CO2 laser.
Non-surgical Treatments Undergoing Research
Fibrotic cytokines TGFb1 and TGFb2 are proposed to play a role in the skin thickening in rhinophyma. Payne et al. have researched the in vitro outcomes of the anti-estrogen medication tamoxifen on fibroblasts with TGFb2 production and secretion in cultured rhinophymatous skin. Results included both decreased fibroblast function and TGFb2 down-regulation. Thus far, this medication has also demonstrated fibrosis inhibition in Dupuytren contracture.[24](B2)
Differential Diagnosis
Despite the unique look of rhinophyma, it can be mimicked by other conditions. Keffe et al. reported an assumed rhinophyma later pathologically diagnosed as entirely composed of basal cell carcinoma.[25] Basal cell carcinoma is suggested to develop in 3 to 10% of rhinophyma patients and can easily hide within the deformed nodular skin. Adenoid squamous cell carcinoma, squamous cell carcinoma, sebaceous adenoma, sebaceous carcinoma, and angiosarcoma have also been histologically diagnosed within rhinophyma. Sarcoidosis, lymphoma, metastatic lung cancer, and granuloma eosinophilicum have all posed clinical similarities to rhinophyma.[2]
In areas with endemic leishmaniasis, any patient presenting with progressive nasal swelling resembling rhinophyma should arouse suspicion of having the Leishmania parasite. Diagnosis is via a smear and a PCR test. Treatment is generally with intramuscular meglumine antimoniate.[26]
Prognosis
Short-term results of surgical treatment of rhinophyma provide patients with an improved cosmetic appearance. Few long-term studies show the probability of recurrence. One follow-up survey of 52 patients receiving CO2 laser treatment within the past five years showed all responders verified that the "beneficial response from laser treatment had been maintained."[21] An additional study analyzing the recurrence of rhinophyma post-shave excision showed that 47% of 21 patients, with a mean follow-up time of 13.2 months, reported a slow recurrence of rhinophyma.[22] Recurrence is potentially due to the maintenance of the pilosebaceous unit during partial excisions combined with a relapse in the condition. More data is necessary to draw further conclusions.
Complications
Common complications of surgical treatments include scarring and prolonged healing time. The nasal tip and alae have the highest scarring risk as the cartilage resides closer to the dermis.[24] Using heated scalpels, ablation therapy, or laser treatments also risks thermal damage that can induce additional scarring, burns, and skin texturization. Inadvertently deep excisions complicate wound healing as the underlying adnexal structures are necessary for re-epithelialization.[19] Care should be taken not to perforate the nasal cartilages when using any excision technique.
Postoperative and Rehabilitation Care
Classic post-operative care for the excisional methods described here includes applying antibiotic ointment, vitamin A and D ointment, and petroleum jelly to promote healing and moisture retainment. Non-adherent dressings and hemostatic agents can be utilized for 7 to 10 days to promote continued hemostasis, reduce infection risk, and accelerate wound healing. The literature reports the use of specialty dressings, including calcium alginate, oxidized cellulose polymer saturated with fibrin sealant, and xeroform petroleum gauze with microfibrillar collagen or triple antibiotic ointment.[27][28]
Fibrin glue after dermabrasion has been reported with satisfactory healing outcomes.[29] Finally, reports of sprayable skin technology employed the processing of autologous cells into a cell suspension and applied below an occlusive dressing to speed up the reepithelialization process.[30]
Deterrence and Patient Education
Patients should receive counsel on the proper management of early-stage rosacea as well as phymatous rosacea. While progression to rhinophyma is not preventable, adequate management can lessen symptoms to reduce the psychological burden. More pertinent to the earlier stages of rosacea, patients should learn to avoid certain stimuli to decrease vasodilatory skin flushing and persistent erythema. Patients should avoid the following: environmental stimuli such as extreme hot and cold temperatures and sunburns, emotional stimuli from excessive stress and anxiety, physiological stimuli such as caffeine and alcohol in addition to spicy foods and heavy exercise, and exogenous stimuli including chemical peels and other facial irritants. Due to increased transient epidermal water loss, moisturizer use is increasingly important in these patients.
Regular sunscreen application is also essential to avoid disease exacerbation from sunburns and prevent increased production of free oxygenated radicals that can lead to rosacea aggravation. Patients with progressing rosacea can additionally start a topical metronidazole treatment that has proved effective in moderate-severe rosacea to help reduce papules and pustules. Additionally, oral tetracyclines, or erythromycin in intolerant patients, provide anti-inflammatory benefits. Patients with phymatous rosacea should be advised on the utility of oral isotretinoin to ameliorate the growth of sebaceous gland hypertrophy and reduce superficial blood flow. Patients should understand that surgical treatment is necessary for tissue debulking, and there is a possibility of disease recurrence after tissue removal.[31]
Pearls and Other Issues
Rhinophyma is considered the final and most severe stage of rosacea, primarily affecting white males over the age of 50.
Hypertrophy of the sebaceous glands and fibrosis of underlying connective tissue is the pathologic basis of this condition.
Diagnosis of rhinophyma is typically a clinical diagnosis.
Treatment of rosacea does not prevent rhinophyma development. Definitive treatment requires surgery composed of excisional, ablative, or laser methods.
Enhancing Healthcare Team Outcomes
In the less severe stages of rosacea, dermatologists can help optimize the treatment of this condition. Upon reaching the point of requiring surgical treatment, the advantages and disadvantages of the necessary procedure(s) require a consult with experienced plastic surgeons, ear, nose, and throat (ENT) surgeons, or dermatologists trained to perform these procedures. Depending on the surgical treatment, anesthesiologists must have adequate sedation and pain control during the operation. Pathologists can be employed to analyze biopsy specimens to rule out malignancy. Nurses are an integral part of the team for post-operational monitoring in the healthcare facility. Lastly, pharmacists play a role in confirming the proper antibiotic coverage to prevent postoperative infection.
The National Rosacea Society Expert Committee updated its guidelines in 2017 on the standard classification and pathophysiology of rosacea. Last updated in 2002, current guidelines were developed after an assessment of articles from peer-reviewed journals and expert advice to guide practitioners on proper investigations, aid in the diagnosis, and provide treatment recommendations.[32] [Level 5] No current consensus exists on the ideal surgical treatment as the option chosen largely depends on the combined decision of the patient and provider based on cost, disease severity, and provider experience.
In summary, rhinophyma requires an interprofessional team approach, including physicians, specialists, specialty-trained nurses, and pharmacists, all collaborating across disciplines to achieve optimal patient results. All team members must maintain accurate records of their interactions and interventions so that all interprofessional teammates can access updated and accurate information regarding the case. Nurses, pharmacists, and other providers must openly communicate with the treating clinicians and specialists so that any necessary interventions can be implemented promptly. [Level 5]
Media
(Click Image to Enlarge)
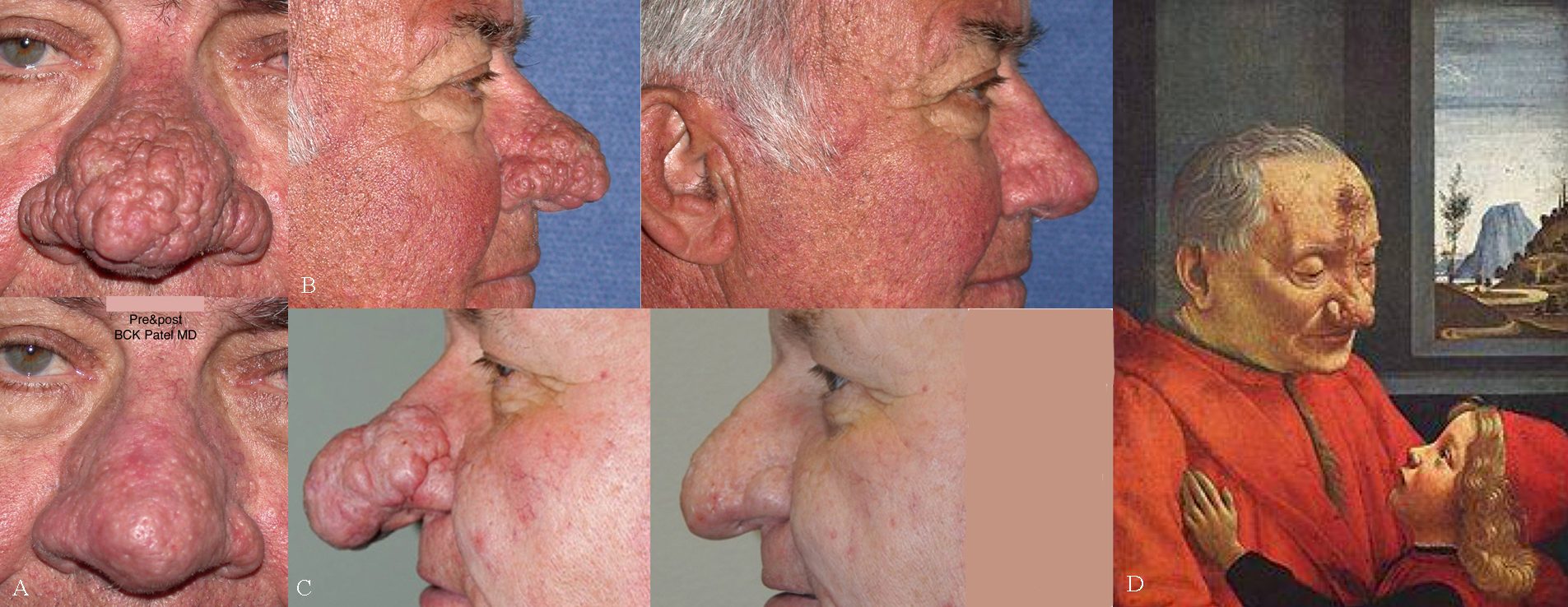
Rhinophyma. A & B: Before and after photographs of a patient who underwent CO2 laser ablative treatment. C. Patient underwent treatment with the plasma ablation. D. 15th Century painting by Domenico Ghirlandaio (1490), "An Old Man and His Grandson", which shows a man with a large rhinophyma Contributed by Prof. Bhupendra C. K. Patel MD, FRCS
(Click Image to Enlarge)
(Click Image to Enlarge)

58-year-old gentleman undersent scalpel resection of the rhinophyma and immediate CO2 laser resurfacing and shrinkage of the surrounding tissues with healing by secondary intention. Photos show the appearance 4 months after resection of the rhinophyma. Contributed by Prof. Bhupendra C. K. Patel MD, FRCS
References
Vishwas KV, Raju BP, Nagaraju U. Managing Rhinophyma by Trimodal Therapy-Novel Approach. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2017 Jun:69(2):176-180. doi: 10.1007/s12070-017-1052-2. Epub 2017 Jan 5 [PubMed PMID: 28607886]
Rohrich RJ, Griffin JR, Adams WP Jr. Rhinophyma: review and update. Plastic and reconstructive surgery. 2002 Sep 1:110(3):860-69; quiz 870 [PubMed PMID: 12172152]
Level 3 (low-level) evidenceLaun J, Gopman J, Elston JB, Harrington MA. Rhinophyma. Eplasty. 2015:15():ic25 [PubMed PMID: 25987948]
Fink C,Lackey J,Grande DJ, Rhinophyma: A Treatment Review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2018 Feb; [PubMed PMID: 29140869]
Schaller M, Almeida LMC, Bewley A, Cribier B, Del Rosso J, Dlova NC, Gallo RL, Granstein RD, Kautz G, Mannis MJ, Micali G, Oon HH, Rajagopalan M, Steinhoff M, Tanghetti E, Thiboutot D, Troielli P, Webster G, Zierhut M, van Zuuren EJ, Tan J. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. The British journal of dermatology. 2020 May:182(5):1269-1276. doi: 10.1111/bjd.18420. Epub 2019 Oct 16 [PubMed PMID: 31392722]
Level 3 (low-level) evidenceRodrigues-Braz D, Zhao M, Yesilirmak N, Aractingi S, Behar-Cohen F, Bourges JL. Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models. Molecular vision. 2021:27():323-353 [PubMed PMID: 34035646]
Sadick H, Goepel B, Bersch C, Goessler U, Hoermann K, Riedel F. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Annals of plastic surgery. 2008 Jul:61(1):114-20. doi: 10.1097/SAP.0b013e31815f12d2. Epub [PubMed PMID: 18580161]
Ayers S Jr, Mihan R, Marks R, Harcourt-Webster JN. Demodex folliculorum in rosacea. Archives of dermatology. 1970 Jun:101(6):706-7 [PubMed PMID: 4246518]
Little SC, Stucker FJ, Compton A, Park SS. Nuances in the management of rhinophyma. Facial plastic surgery : FPS. 2012 Apr:28(2):231-7. doi: 10.1055/s-0032-1309304. Epub 2012 May 6 [PubMed PMID: 22562574]
Liu A, Al-Lami A, Kapoor K. Rhinophyma: when Red Nose Day is no laughing matter. The British journal of general practice : the journal of the Royal College of General Practitioners. 2019 Mar:69(680):137. doi: 10.3399/bjgp19X701585. Epub [PubMed PMID: 30819745]
Wilkin JK. Rosacea. Pathophysiology and treatment. Archives of dermatology. 1994 Mar:130(3):359-62 [PubMed PMID: 8129416]
Saleem MD. Revisiting Rosacea Criteria: Where Have We Been, Where Are We Going, and How Will We Get There? Dermatologic clinics. 2018 Apr:36(2):161-165. doi: 10.1016/j.det.2017.11.011. Epub 2017 Nov 29 [PubMed PMID: 29499799]
Jansen T, Plewig G. Clinical and histological variants of rhinophyma, including nonsurgical treatment modalities. Facial plastic surgery : FPS. 1998:14(4):241-53 [PubMed PMID: 11816064]
Freeman BS, Reconstructive rhinoplasty for rhinophyma. Plastic and reconstructive surgery. 1970 Sep; [PubMed PMID: 4247302]
el-Azhary RA, Roenigk RK, Wang TD. Spectrum of results after treatment of rhinophyma with the carbon dioxide laser. Mayo Clinic proceedings. 1991 Sep:66(9):899-905 [PubMed PMID: 1921499]
Goldstein JA, Comite H, Mescon H, Pochi PE. Isotretinoin in the treatment of acne: histologic changes, sebum production, and clinical observations. Archives of dermatology. 1982 Aug:118(8):555-8 [PubMed PMID: 6213204]
McClellan KJ, Noble S. Topical metronidazole. A review of its use in rosacea. American journal of clinical dermatology. 2000 May-Jun:1(3):191-9 [PubMed PMID: 11702300]
Somogyvári K, Battyáni Z, Móricz P, Gerlinger I. Radiosurgical excision of rhinophyma. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2011 May:37(5):684-7. doi: 10.1111/j.1524-4725.2011.01965.x. Epub 2011 Apr 1 [PubMed PMID: 21457393]
Krausz AE, Goldberg DJ, Ciocon DH, Tinklepaugh AJ. Procedural management of rhinophyma: A comprehensive review. Journal of cosmetic dermatology. 2018 Dec:17(6):960-967. doi: 10.1111/jocd.12770. Epub 2018 Sep 17 [PubMed PMID: 30225926]
Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic plastic surgery. 2001 Sep-Oct:25(5):372-7 [PubMed PMID: 11692253]
Madan V,Ferguson JE,August PJ, Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. The British journal of dermatology. 2009 Oct; [PubMed PMID: 19624541]
Wetzig T, Averbeck M, Simon JC, Kendler M. New rhinophyma severity index and mid-term results following shave excision of rhinophyma. Dermatology (Basel, Switzerland). 2013:227(1):31-6. doi: 10.1159/000351556. Epub 2013 Aug 30 [PubMed PMID: 24008235]
Level 2 (mid-level) evidenceGentile RD. Cool Atmospheric Plasma (J-Plasma) and New Options for Facial Contouring and Skin Rejuvenation of the Heavy Face and Neck. Facial plastic surgery : FPS. 2018 Feb:34(1):66-74. doi: 10.1055/s-0037-1621713. Epub 2018 Feb 6 [PubMed PMID: 29409106]
Payne WG, Ko F, Anspaugh S, Wheeler CK, Wright TE, Robson MC. Down-regulating causes of fibrosis with tamoxifen: a possible cellular/molecular approach to treat rhinophyma. Annals of plastic surgery. 2006 Mar:56(3):301-5 [PubMed PMID: 16508362]
Level 2 (mid-level) evidenceKeefe M, Wakeel RA, McBride DI. Basal cell carcinoma mimicking rhinophyma. Case report and literature review. Archives of dermatology. 1988 Jul:124(7):1077-9 [PubMed PMID: 2968781]
Level 3 (low-level) evidenceYoussef M, Soua Y, Belhadjali H. Rhinophyma-like cutaneous leishmaniasis. Indian journal of dermatology, venereology and leprology. 2014 Nov-Dec:80(6):537-8. doi: 10.4103/0378-6323.144175. Epub [PubMed PMID: 25382513]
Level 3 (low-level) evidenceZide MF. Surgical removal of rhinophyma. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2008 Oct:66(10):2168-77. doi: 10.1016/j.joms.2008.01.036. Epub [PubMed PMID: 18848122]
Greaney L,Singh NP,Roberts DN, Surgical management of rhinophyma. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology [PubMed PMID: 20500598]
Level 3 (low-level) evidenceClarós P, Sarr MC, Nyada FB, Clarós A. Rhinophyma: Our experience based on a series of 12 cases. European annals of otorhinolaryngology, head and neck diseases. 2018 Feb:135(1):17-20. doi: 10.1016/j.anorl.2017.08.005. Epub 2017 Sep 21 [PubMed PMID: 28943211]
Level 3 (low-level) evidenceTaghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2008:61(3):330-3. doi: 10.1016/j.bjps.2006.11.022. Epub 2007 Feb 2 [PubMed PMID: 18267312]
Level 3 (low-level) evidenceAbokwidir M, Feldman SR. Rosacea Management. Skin appendage disorders. 2016 Sep:2(1-2):26-34 [PubMed PMID: 27843919]
Gallo RL,Granstein RD,Kang S,Mannis M,Steinhoff M,Tan J,Thiboutot D, Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. Journal of the American Academy of Dermatology. 2018 Jan; [PubMed PMID: 29089180]