Introduction
Retinitis, characterized by inflammation of the retina, frequently occurs in association with other systemic medical conditions, especially infections and inflammatory diseases, posing a risk of vision loss. The retina, situated at the back of the eye, transmits images as electrical impulses through the optic nerve to the brain, allowing sight.
Infectious agents, inflammatory diseases, and hypersensitivity reactions may all cause inflammation in various areas of the eye. The uvea comprises the iris, ciliary body, and choroid. Anterior uveitis, also known as iritis, involves leukocytes in the anterior chamber, whereas iridocyclitis includes inflammation of the adjacent ciliary body. Posterior uveitis involves inflammation of the choroid or retina and includes chorioretinitis, retinochoroiditis, retinitis, and neuroretinitis.
Microbes such as human herpesvirus 5 (HSV-5) or cytomegalovirus (CMV), Toxoplasma gondii, and Candida are responsible for infectious retinitis. Significant risk factors include active maternal infection during pregnancy or childbirth, exposure to endemic areas, or an immunocompromised state. Retinitis can result from infections in heart valves, the gastrointestinal tract, and the urinary tract, as well as among individuals who use intravenous drugs. Retinitis may be the sole manifestation of autoimmune diseases such as Behçet disease. The consequences of retinitis vary depending on the age, location, and immune status. Affected patients may present with isolated retinitis or in conjunction with choroidal involvement, such as retinochoroiditis or chorioretinitis, requiring clinicians to tailor management strategies based on the specific cause to prevent vision loss and protect the contralateral eye.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Retinitis may be idiopathic or related to infectious, inflammatory, or underlying genetic causes. Posterior uveitis is associated with various human leukocyte antigen (HLA) phenotypes. Birdshot chorioretinitis, linked to HLA-29 in 95% of patients, manifests with chorioretinal lesions resembling birdshot pellets. Uveitis and inflammatory bowel disease co-occur in 45% of patients with HLA-B27. Vogt-Koyanagi-Harada syndrome exhibits a robust HLA class II association, particularly with the HLA-D locus and DR4 allele.
Infectious Causes
Infectious causes include bacterial, spirochetal, viral, fungal, and parasitic diseases.
Parasitic disease: T gondii is a widely distributed intracellular protozoan parasite. Humans and other mammals act as intermediate hosts, whereas cats are the definitive hosts for T gondii. Uveitis caused by T gondii occurs as either a reactivation of a congenitally acquired infection or a new infection.[1]
Viral disease:
- Cytomegalovirus: Posterior uveitis resulting from CMV infection is predominantly observed in immunocompromised patients and may result in blindness.[2] Although uncommon in North America and Europe and more common in Southeast Asia, CMV may rarely cause anterior uveitis in immunocompetent patients.
- Herpes virus: Both HSV-1 and HSV-2 and HSV-3 or varicella zoster virus (VZV) may cause acute retinal necrosis.[3] In addition, ocular HSV may cause keratitis, conjunctivitis, or blepharitis. Neonates may develop chorioretinitis due to disseminated HSV infection. CMV and rarely Epstein-Barr virus (EBV) may also cause acute retinal necrosis. Progressive outer retinal necrosis (PORN) is a clinical variant of necrotizing herpetic retinopathy caused by VZV and occasionally CMV and noted in patients with AIDS.[4]
- West Nile virus: Chorioretinitis and retinal vasculitis are common complications associated with West Nile virus infection. Many affected patients may be asymptomatic.
- Measles virus: Around 7 to 10 years after a measles infection, some patients may develop subacute sclerosing panencephalitis, a fatal neurodegenerative disease. Subacute sclerosing panencephalitis is caused by persistent infection with a genetically altered form of the measles virus, primarily affecting children and young adults.
- Dengue virus: Dengue fever can cause retinitis and foveolitis.
- Chikungunya virus: Infection with the chikungunya virus results in various anterior and posterior segment manifestations, with iridocyclitis and retinitis being the most common.[5]
Bacterial disease:
- Syphilis: Caused by the bacterium Treponema pallidum, syphilis is a leading cause of posterior uveitis. Ocular manifestations can occur in all stages of the disease, although they are more prevalent in secondary and late syphilis.[6]
- Tuberculosis: Mycobacterium tuberculosis is an uncommon cause of posterior uveitis in the United States, but clinicians should consider tuberculosis when uveitis worsens with glucocorticoid therapy or in patients with a history of active tuberculosis, incarceration, immunosuppression, or granulomatous inflammation.
- Cat scratch disease: Trauma from a cat scratch, cat bite, or contact with flea feces may cause an infection with Bartonella henselae, a gram-negative hemotrophic bacillus. Ocular symptoms are generally unilateral.[7]
- Rocky Mountain spotted fever: A zoonosis caused by Rickettsia rickettsii and transmitted to humans by the bite of contaminated ticks, 30% of patients with Rocky Mountain spotted fever (RMSF) develop retinitis. In addition, some may develop retinal vascular involvement and optic disc changes.[8]
- Lyme disease: A vector-borne zoonosis caused by the bite of an Ixodes tick infected by the bacteria Borrelia burgdorferi, retinitis is a rare presentation noted in the late disseminated stage of the disease.[9]
- Histoplasmosis: Patients residing in areas endemic to Histoplasma capsulatum, including states that contain the Ohio and Mississippi river valleys, may develop ocular histoplasmosis syndrome, potentially leading to chorioretinitis.[10]
- Endogenous endophthalmitis or metastatic endophthalmitis: Endogenous microbes are an additional potential infectious source.[11] Approximately 50% are bacterial, and 50% are fungal. The most frequently identified causative bacteria in North America and Europe are Staphylococcus aureus and Streptococcus pneumoniae, whereas Klebsiella pneumoniae is more prevalent in East Asia. Risk factors include diabetes, liver disease, cardiac disease, malignancy, indwelling catheters, and intravenous drug use.
Fungal disease: Candida and Aspergillus are the most common causes of fungal endogenous chorioretinitis and endophthalmitis. Immunocompromised patients are at particular risk of developing the disease. Histoplasma may result in presumed ocular histoplasmosis syndrome, occurs in immunocompetent individuals, and is recognized by the presence of peripapillary atrophy and multiple atrophic chorioretinal scars without vitreous or aqueous humor inflammation.
Helminthic disease: Toxocariasis caused by the nematode Toxocara canis is a rare yet significant contributor to posterior uveitis in infants and young children.[12] Ocular toxocariasis can present as a posterior pole or peripheral granuloma, with few patients developing dense vitritis mimicking endophthalmitis. Diffuse unilateral subacute neuroretinitis is an ocular infectious condition resulting from a worm, the complete understanding of whose etiology remains elusive, potentially resulting in visual impairment and blindness.
Post-Fever Retinitis
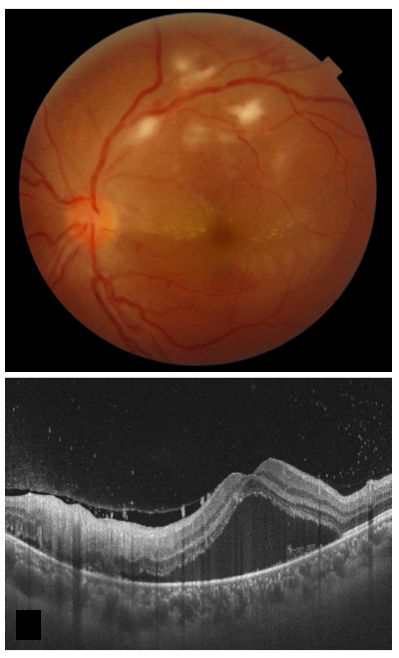
Post-fever retinitis denotes varied retinal manifestations after a systemic febrile illness caused by bacteria, viruses, or protozoa. These manifestations typically appear 2 to 4 weeks after the onset of fever in immunocompetent patients. Symptoms improve with corticosteroids, implying a potential immunological origin. Posterior segment manifestations encompass focal and multifocal patches of retinitis, potential optic nerve engagement, serous macular detachment, macular edema, and localized retinal vessel involvement characterized by vessel beading, tortuosity, and perivascular sheathing.
Noninfective Causes
Systemic inflammatory diseases are also associated with uveitis. The majority of patients with Behçet disease develop uveitis, along with occlusive vasculitis as a characteristic feature. Additional systemic diseases include sarcoidosis, systemic lupus erythematosus, eosinophilic granulomatosis with polyangiitis, psoriatic arthritis, inflammatory bowel disease, Sjögren syndrome, and Vogt-Koyanagi-Harada syndrome.
Epidemiology
Epidemiological information depends on various factors, including the underlying etiology, the patient's immune status, and geographical location.
Cytomegalovirus
CMV retinitis predominantly affects immunocompromised individuals, such as neonates, patients with AIDS, and organ transplant recipients. Around 4% of patients who undergo hematopoietic stem cell transplantation develop CMV retinitis. CMV is a leading opportunistic ocular infection in patients with AIDS, particularly when CD4 T-lymphocyte counts drop below 50 cells/µL.[13] The introduction of highly active antiretroviral therapy has reduced the current prevalence of CMV retinitis from 40% to 20%. The incidence rate of retinitis in the uninvolved eye has decreased by 46%. The incidence rate of CMV retinitis following highly active antiretroviral therapy is 0.36/100 person-years, with an average onset age between 20 and 50.
Toxoplasmosis
T gondii is the most common pathogenic cause of posterior uveitis in immunocompetent hosts. Three genotypes of T gondii exist. Genotype II is most prevalent in Europe and the United States. Approximately 80% to 90% of patients infected with genotype II remain asymptomatic. Genotype I is most prevalent in South and Central America and, more frequently, causes systemic symptoms. In areas such as Brazil, the seropositive rate for toxoplasmosis is nearly 80%, with 6% to 18% of immunocompetent individuals developing ocular symptoms. Toxoplasma retinochoroiditis accounts for 30% to 55% of cases of posterior uveitis in high T gondii endemic regions of the United States and European nations.[14]
Human Herpes Virus
Keratitis is the most prevalent form of ocular disease due to HSV and is a significant cause of blindness worldwide. In neonates, HSV keratitis can lead to complications such as corneal scarring, cataracts, and chorioretinitis. Skin, eye, and mouth disease accounts for approximately 35% to 45% of neonatal HSV cases.
Behçet Disease
Behçet disease is more common in men compared to that in women and typically affects young adults. Men are more likely to be affected by ocular involvement and have a worse visual prognosis. The prevalence of Behçet disease varies geographically, with a higher prevalence observed in countries spanning from eastern Asia to the Mediterranean region.[15] Estimates from Turkey vary from 80 to 370 cases per 100,000 population. In contrast, prevalence estimates from Japan, Korea, China, and the Middle East vary from 13 to 20 cases per 100,000 population. The prevalence in the United States varies from 0.12 to 5 cases per 100,000 persons. Uveitis occurs in 60% to 80% of patients with Behçet disease. Vogt-Koyanagi-Harada syndrome is the second leading cause of uveitis in Japan after Behçet syndrome.
Rocky Mountain Spotted Fever
RMSF is the most common cause of fatal tick-borne disease in the United States and is endemic in the Southeast, the western South Central region, and selected areas of the Northeast parts of the United States and Central and South America.[8] In endemic areas such as North Carolina, the annual incidence among children aged 5 to 9 is estimated at 42 cases per 100,000. Although White patients experience RMSF more frequently compared to their Black counterparts, Black patients have a higher fatality rate. American Indians face the highest risk of RMSF. Males have a higher rate of infection and a higher mortality rate, with the highest incidence of infection observed between 5 to 9 and 60 to 69. The risk of posterior uveitis with RMSF is low.
Lyme Disease
Lyme disease is endemic in North America and Europe.[9] A study involving 160 cases of posterior uveitis reveals that Lyme disease accounts for 4.4% of the cases.
Additional Information
- Acute retinal necrosis affects patients who are immunocompetent or immunosuppressed regardless of age or gender, accounting for 5.5% of uveitis cases over a 10-year period.[16]
- Those at the highest risk of developing ocular histoplasmosis syndrome from H capsulatum are patients who are immunocompromised, infants, and individuals older than 55.
- Approximately 2% to 9% of patients with inflammatory bowel disease and 7% of patients with systemic lupus erythematosus may develop uveitis.
- Ocular toxocariasis primarily affects older children and adolescents.
- Neuroretinitis develops in approximately 1% to 2% of patients affected by cat scratch disease.
Pathophysiology
Cystoid macular edema, neovascularization of the retina or optic disc, disc swelling, epiretinal membranes, and retinal detachment are all potential causes of visual changes associated with posterior uveitis. Permanent changes encompass macular ischemia, optic nerve atrophy, and retinal scarring. Pathogens can enter the eye through bloodstream dissemination or direct introduction, possibly stemming from reactivated or new infections.
Interleukins (IL) and cytokines play a significant role in the pathogenesis and persistence of intraocular inflammation. The eye is an immune-privileged site with various mechanisms to maintain this privilege. The blood-retina barrier and lack of efferent lymphatics prevent free entry and exit from the eye. Suppression of immunocompetent cells occurs through the presence of immunosuppressive factors such as transforming growth factor-beta (TGF-β), alpha-melanocyte–stimulating hormone, and vasoactive intestinal peptide. Finally, the pigmented epithelia of the retina and residential retinal cells inhibit T cells and induce them to become T-regulatory cells by expressing inhibitory cell surface-associated proteins such as TGF-β, FAS/FAS ligand, CD46, and CD59.
The immunosuppressive factors typically maintain control over autoreactive T cells. However, prolonged and intense inflammation can surpass these barriers, and complex genetic–environmental interactions break immune tolerance to generate eye-specific autoreactive T cells. Consequently, autoreactive T cells proliferate and migrate toward the eye. The failure of IL-10 and TGF-β to revert these cells to T-regulatory cells leads to the recruitment of neutrophils and other leukocytes due to IL-17 secretion. Tissue damage occurs due to nonspecific macrophage activation and subsequent cytokine cascades. Some evidence indicates that locally produced cytokines contribute to infectious uveitis, whereas cytokines in non-infectious uveitis appear to originate from both the eye and the peripheral blood.
Cell-mediated immunity may play a significant role in viral uveitis when uveal and corneal tissue cells contain viral-specific antigens on their surface, leading to specific tissue damage such as acute retinal necrosis and CMV retinitis. CMV likely reaches the eye via hematogenous spread. Replication goes unchecked due to impaired CD4 cell function or number. Retinitis induced by CMV, VZV, HSV, and EBV can result in full-thickness retinal necrosis and edema, eventually replaced by atrophic scar tissue. This scar tissue is prone to tearing, leading to retinal detachment. Various immune regulatory cytokines such as IL-6, IL-10, and interferon-gamma (IFN-γ) are present in ocular fluid samples from patients with viral uveitis. Researchers note IL-2, IL-4, IL-6, IL-10, and IFN-γ in samples from patients with HSV-associated acute retinal necrosis.
Fungal uveitis, especially Candida albicans, stimulates interphotoreceptor retinoid-binding protein-mediated Card 9, a subgroup C-type lectin receptor (CLR1) signaling molecule, further activating Dectin-1 and Dectin-2. Both these receptors activate downstream signaling cascades, leading to helper T-cell differentiation into a Th17 phenotype and the secretion of IL-17.
Behçet syndrome is an autoimmune inflammatory vasculitis involving arteries and veins of all sizes. The underlying pathogenesis is multifaceted, involving autoimmune and autoinflammatory features. Altered host bacteria or response to bacteria may play a role in the pathogenesis of Behçet syndrome. Additional potential triggering agents may be viral illnesses or environmental factors such as chemicals and heavy metals. Molecular mimicry due to cross-reactivity of some bacterial antigens with human peptides, altered innate immune function, humoral immune activation, endothelial dysfunction, and activation of polymorphonuclear leukocytes due to increased local expression of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and IL-1β, play a role in Behçet syndrome.[17][18]
Patients with Behçet disease and uveitis have higher levels of IFN-γ, TNF-α, IL-2, IL-6, IL-8, IL-12, and IL-17A in the aqueous humor. Levels of IL-15 are also significantly higher in patients with Behçet disease and uveitis. IL-15 promotes the development, activation, homing, and survival of natural and CD8+ T cells.
T gondii likely reaches the eye through hematogenous spread. Once in the eye, the parasite triggers inflammation, followed by retinitis. The choroid becomes involved secondarily. Host immune responses induce the conversion of tachyzoites to bradyzoites and their encystment, followed by scar formation. The encysted parasite may remain inactive for a prolonged period. When the cyst ruptures, the retinitis may reactivate at the border of old scars, occasionally leading to the formation of new lesions at distant locations from old scars.[1]
Researchers suggest the role of T-cell–mediated pathogenesis with birdshot chorioretinitis, specifically through the Th17 system. Elevated levels of IL-1β, IL-2, IL-6, IL-17, and TNF-α in the aqueous humor and increased serum levels of IL-21, IL-23, and TGF-β1 in patients with active disease suggest Th17-cell–mediated inflammation.[19][20]
Elevated levels of TNF-α, IFN-γ, IL6, IL-8, IL-17, macrophage inflammatory protein (MIP)-1β, and IL-15 in the aqueous humor suggest a Th1/Th17-weighted immune response associated with Vogt-Koyanagi-Harada syndrome. Basu et al propose that the recognition of mycobacterial by macrophages and dendritic cells activates the immune pathway involving Th1 and Th17 cells in the pathogenesis of tuberculosis-related uveitis.
History and Physical
Unlike anterior uveitis, posterior uveitis tends to be painless but can sometimes be accompanied by pain. The symptoms are generally nonspecific and include floaters and decreased visual acuity.
Cytomegalovirus
Typically, CMV retinitis is unilateral but can become bilateral if left untreated. In addition to floaters and decreased visual acuity, patients with CMV retinitis may experience scotomata or photopsia depending on the location of retinal involvement and retinal detachment. Floaters or photopsia are the single most potent symptomatic indicators of CMV retinitis in patients with AIDS. The presence of either of these symptoms warrants a dilated funduscopic examination.
CMV retinitis typically appears as yellow-white retinal lesions with indistinct margins, sometimes with a granular appearance, often located close to retinal vessels and associated with hemorrhage (see Image. Cytomegalovirus Retinitis).[21] The clinical appearance of CMV retinitis may appear in 3 variations as follows:
- The brush-fire type appears as wedge-shaped areas of a perivascular fluffy white lesion with many scattered hemorrhages (see Image. Cytomegalovirus and Associated Hemorrhages).[21]
- The granular type has a more granular-appearing lesion with few associated hemorrhages, often displaying a central area of clearing, with an atrophic retina and stippled retinal pigment epithelium.
- Retinal vasculitis with perivascular sheathing is an atypical manifestation with a clinical appearance similar to frosted branch angiitis.
Toxoplasmosis
In Europe and North America, most patients with ocular disease due to T gondii are asymptomatic, and clinicians note scarring during a routine examination. The retina and choroid are primarily affected, and the vitreous becomes affected secondarily. When symptomatic, floaters alone are generally the presenting symptom. Ophthalmologic examination reveals mild iritis, significant vitreous inflammation, and whitish retinal lesions, often referred to as a headlight in the fog appearance. Visual fields may show an absolute defect with a breakout to the periphery, especially if the retinitis lesions are within 1 disc diameter of the optic disc.[22] Recurrent lesions tend to occur as satellite lesions adjacent to old atrophic lesions (see Image. Toxoplasma Chorioretinitis). Perivasculitis may be present near the active lesion with periarteriolar collections of cells seen as distinct Kyrieleis plaques. Such plaques are also present in acute retinal necrosis, tuberculosis, sarcoidosis, Behçet disease, CMV retinitis, leptospirosis, rickettsial disease, and syphilis.[3][23][24] Patients who are immunocompromised with toxoplasmosis may reveal less profound vitritis with multiple, atypical, or large retinitis lesions, and a pigmented scar may be absent.
Punctate Outer Retinal Toxoplasmosis (PORT) is a subset of ocular toxoplasmosis involving the outer retinal layers and associated with little or no overlying vitreous reaction. Grey-white lesions in the deep retina and retinal pigment epithelium level that resolve as white, punctate tiny dots characterize PORT. Identification of this variant is important as treatment may improve overall outcomes.[25]
Behçet Syndrome
Behçet syndrome clinically manifests with recurrent oral aphthae and various systemic findings, including genital aphthae, ocular complications, skin lesions, gastrointestinal involvement, neurologic disease, vascular disease, or arthritis. Panuveitis is the predominant ocular presentation, often evolving into a chronic, relapsing course and becoming bilateral in most cases. Anterior segment inflammation commonly presents as nongranulomatous acute uveitis, with or without hypopyon. Hypopyon exhibits free movement with shifting head positions. In addition, significant media haze or vitritis may be evident.
In Behçet disease, retinitis is the second most common posterior segment manifestation after retinal vasculitis, exerting a greater influence on veins compared to arteries.[26] The presentation includes patchy areas of focal retinal whitening, histologically linked to retinal vasculitis, choroidal occlusive vasculitis, or primary focal retinal inflammation.[26] Deeper than cotton wool spots, these retinitis areas are more substantial in the posterior pole compared to the periphery, occasionally accompanied by hemorrhage, and typically resolve within 1 to 2 weeks of commencing immunosuppressive therapy. Retinal atrophy may follow once the exudates and hemorrhages resolve. Cystoid macular edema resulting from vascular leakage or optic disc involvement is another noteworthy discovery.
Acute Retinal Necrosis
Caused by HSV and VZV in immunocompetent hosts and CMV in immunocompromised hosts, patients with acute retinal necrosis may present with a red eye and pain in addition to the characteristic findings associated with retinitis. Patchy white-yellow areas that become confluent over time characterize acute retinal necrosis. The disease starts in the periphery with full-thickness discrete necrotizing lesions with scalloped borders. Typically, hemorrhages are less prominent if present. The posterior pole is involved later in the course of the disease. Inflammation of the arterioles can lead to occlusive arteritis, causing more rapid retinal necrosis. Other associated features include vitreal haze, optic disc edema, raised intraocular pressure, and scleritis (see Image. Acute Retinal Necrosis). Although the disease starts unilaterally in the majority, fellow eye involvement may occur as early as 1 to 6 weeks.[27]
Neonatal ocular HSV may initially appear asymptomatic. Early signs include excessive watering of the eye, crying from apparent eye pain, and conjunctival erythema.
PORN, an extreme variant of necrotizing herpetic retinopathy in immunocompromised individuals, presents with early involvement of the posterior pole and rapid progression. Despite extensive retinal involvement, particularly in the deep retina, minimal vitreous inflammation is present.[28] In a large case series by Engstrom et al, two-thirds of eyes with the disease progressed to no light perception within 4 weeks of onset.[29] Features similar to PORN may also be noted in immunocompetent individuals.[30] The whitish retina around the fovea due to retinitis may give rise to a cherry red spot at the macula.[31]
Cat Scratch Disease
Ocular manifestations of cat scratch disease include Parinaud's oculoglandular syndrome, neuroretinitis, papillitis, optic neuritis, and focal retinochoroiditis. Parinaud's oculoglandular syndrome manifests as regional lymphadenopathy and infection of the conjunctiva, eyelid, or adjacent skin surface due to a cat bite or lick near the eye.
Patients with neuroretinitis may present with fever, malaise, and unilateral blurred vision. An afferent pupillary defect is a common finding in physical examinations. Retinal examination reveals hemorrhages, cotton wool spots, and multiple discrete lesions in the deep retina. Some individuals may develop a macular star or a stellate-appearing lesion due to leakage from the optic nerve head. Discrete white retinal and chorioretinal lesions may be a more common finding than the classic macular star. Other posterior segment findings include intermediate uveitis, granulomas, choroiditis, angiomatosis lesions, and serous retinal detachments.[32]
Syphilis
In the ocular context, secondary syphilis can lead to either granulomatous or nongranulomatous anterior uveitis, episcleritis, scleritis, chorioretinitis, vitritis, retinal vasculitis, or papillitis. The later stages of secondary syphilis often affect the posterior segment of the eye. Tertiary syphilis may induce gummas of the eyelid, anterior and posterior uveitis, retinal vasculitis, keratitis, scleritis, or papillitis. In addition, tertiary syphilis can manifest as an Argyll Robertson pupil, characterized by bilaterally small pupils with minimal response to light but normal response to near stimuli.
The typical posterior appearance of syphilis is a large, roundish, yellowish placoid lesion known as acute posterior placoid chorioretinopathy occurring at the level of the retinal pigment epithelium at the macular or paramacular area.[6] An additional hallmark of ocular syphilis is pre-retinal lesions appearing as dots overlying retinitis, resulting in punctuate retinitis. However, syphilis can cause multiple other lesions, including necrotizing chorioretinitis, exudative retinal detachment, retinal vasculitis, papillitis, and neuroretinitis.
Tuberculosis
Clinically, tubercular lesions involve the choroid more than the retina. The features include a small choroidal tubercle appearing as a yellowish choroidal nodule, a large elevated choroidal granuloma or choroidal tuberculoma, and a choroidal or subretinal abscess. Tubercular infection is likely related to serpiginous-like choroiditis. There are two forms of serpiginous choroiditis. The first is a placoid form with a yellowish lesion that occupies the posterior pole. Another presentation is a multifocal serpiginous form characterized by multiple yellow-whitish lesions in the posterior pole and mid-periphery corresponding with areas of choriocapillaritis.[33][34][35]
Rocky Mountain Spotted Fever
Patients with RMSF may also present with eye pain and the characteristic features associated with retinitis. Ocular manifestations of RMSF include conjunctivitis, keratitis, anterior uveitis, panuveitis, retinitis, retinal vascular changes, and optic nerve involvement. RMSF presents as white retinal lesions, typically adjacent to retinal vessels, disc swelling, and macular subretinal fluid with mild to moderate vitreous inflammation.
Toxocariasis
The most common presenting features of ocular toxocariasis are strabismus and decreased visual acuity. Toxocara retinochoroiditis may manifest acutely as a blurred, indistinct white lesion accompanied by overlying vitritis. After inflammation resolution, the lesion appears as a well-defined, elevated white mass, ranging from 0.5 to 4 disc diameters in size, with associated vitreoretinal traction. However, the prevalent ocular characteristic is a granuloma in the posterior pole, observed in 25% to 50% of patients.[36]
Chikungunya Fever
Chikungunya virus presents as an acute febrile polyarthralgia and inflammatory arthritis, in addition to acute cutaneous eruptions and headache, conjunctivitis, and gastrointestinal symptoms. The associated retinitis may morphologically mimic herpetic retinitis, but the chikungunya virus presents with markedly less vitreous reaction and confluent posterior pole retinitis. The absence of a history of fever, joint pains, and skin rash before the onset of visual symptoms can also differentiate chikungunya from acute retinal necrosis.[37] Posterior uveitis can present with no involvement of the anterior segment. Other posterior segment signs include optic neuritis, neuroretinitis, and retrobulbar neuritis.
Endogenous Endophthalmitis
Endogenous endophthalmitis may present with signs of severe endophthalmitis, including fibrin in the anterior chamber, hypopyon, and severe vitritis obscuring the view of the retina.[38] Early cases of fungal endogenous endophthalmitis may present as creamy white or yellow chorioretinal lesions, typically at the posterior pole at or near the fovea, associated with fluffy vitreous opacities. These lesions may form a string of pearls appearance.[39] Advanced cases of endogenous endophthalmitis may present as a subretinal abscess.[40]
Diffuse Unilateral Subacute Neuroretinitis
Patients with diffuse unilateral subacute neuroretinitis present with multifocal gray-white evanescent lesions at the level of the outer retina, typically clustered in 1 segment of the fundus. These evanescent lesions are believed to result from the host's immune response to the nematode in the subretinal space and characteristically migrate depending on the worm's location. In the later stages of the disease, there may be sequelae of degenerative changes in the retinal pigment epithelium and retina, retinal artery narrowing, and optic atrophy.
West Nile Virus
West Nile virus typically manifests as clusters of creamy yellow, deep chorioretinal lesions and scars arranged in a line.[41]
Post-fever retinitis presents as multiple white retinal lesions and macular edema (see Image. Post-Fever Retinitis).
Dengue Fever
Dengue fever can cause retinal vasculitis, yellow subretinal lesions, and foveolitis, or a round yellowish lesion at the fovea.[41] Cerebriform retinitis has been reported in association with parotid swelling.[42]
Sarcoidosis
Sarcoid uveitis-related chorioretinitis commonly presents as multiple small round lesions in the inferior peripheral fundus, which heal with either hyperpigmentation or hypopigmentation. A characteristic manifestation of ocular sarcoidosis is the presence of optic disc granuloma accompanied by multiple arteriolar macroaneurysms.[43]
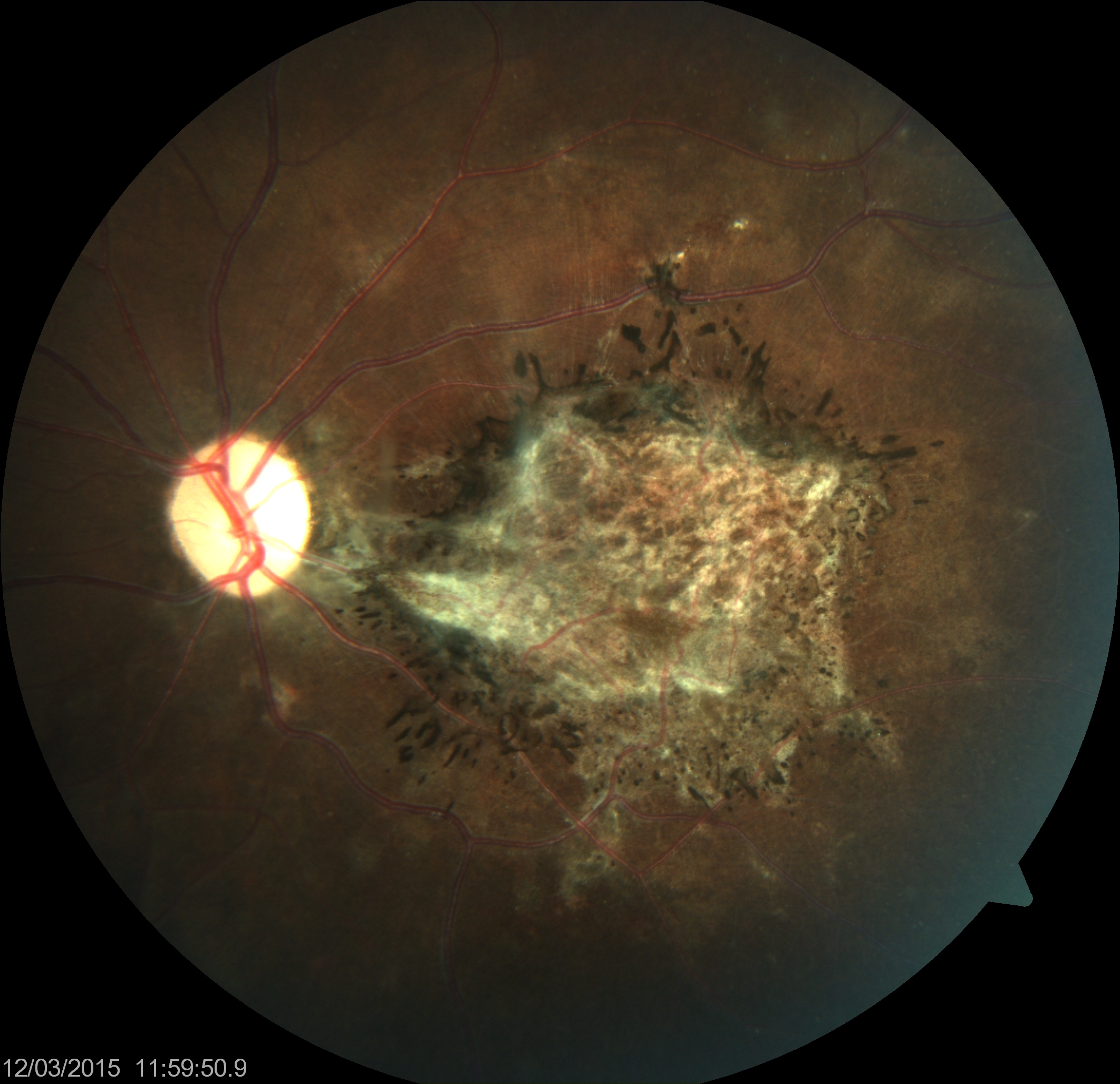
Subacute Sclerosing Panencephalitis
The clinical symptoms of subacute sclerosing panencephalitis begin with neurologic symptoms such as personality changes, lethargy, difficulty in school, and unusual behavior lasting from weeks to years. Myoclonus, worsening dementia, and long-tract motor or sensory disease follow, lasting 3 to 12 months. Finally, further neurologic deterioration with eventual flaccidity or decorticate rigidity and symptoms and signs of autonomic dysfunction followed by a vegetative state and death occur.
Visual symptoms may also precede neurological symptoms. The classic lesion is a focal necrotizing macular retinitis forming a chorioretinal scar with pigmentation in healed lesions. Unlike retinitis associated with toxoplasmosis, vitritis is not a feature. Retinal hemorrhages, edema, and retinal detachment may also occur.[44] Vision loss may be due to chorioretinitis, cortical blindness, or optic disc changes, including papillitis, papilledema, and optic atrophy (see Image. Subacute Sclerosing Panencephalitis).
Evaluation
The primary evaluation of retinitis begins with a thorough ophthalmologic examination, including funduscopic photos.[45] Slit-lamp examination is essential in the evaluation of posterior uveitis. Other potential ocular testing involves the following:
- Fluorescein fundus angiography (FFA)
- Optical coherence tomography (OCT)
- Ultrasound: To exclude retinal detachment and assess the severity of vitreous inflammation when the media is very hazy
- Bacterial, fungal, and viral culture and sensitivity and staining tests, including KOH and gram staining, of the aqueous or vitreous humor
- Polymerase chain reaction (PCR) for bacteria, fungi, Toxoplasma, HSV, HZV, and CMV of the aqueous and vitreous humor depending on the clinical presentation
- Retinal or chorioretinal biopsy or fine-needle aspiration
- Visual field assessment
Patients presenting with posterior uveitis and no evidence of an underlying etiology should undergo a chest radiograph to evaluate for pulmonary sarcoidosis or infections such as tuberculosis. In addition, affected patients should undergo serology for clinically silent syphilis.
Cytomegalovirus
The diagnosis of CMV retinitis is clinical. A thorough funduscopic examination is crucial to differentiate CMV retinitis from benign cotton wool spots and PORN. The key differentiating features include the initial presentation of PORN with multifocal, necrotizing lesions in the peripheral retina, which progresses more rapidly than CMV retinitis. Unlike CMV retinitis, PORN typically spares the retinal vessels. CMV serology is not helpful in the diagnosis of CMV retinitis. Patients with CMV retinitis should also undergo testing for HIV and may require a CD4 count.
Toxoplasmosis
Clinicians diagnose ocular toxoplasmosis based on clinical presentation and detection of anti-Toxoplasma immunoglobulin G (IgG) antibodies. Testing involves enzyme-linked immunosorbent assay (ELISA) for IgM and IgG antibodies. Although acute infections exhibit IgG antibodies, IgM may no longer be detectable since ocular involvement can occur months later. A positive IgG supports but does not confirm diagnosis, especially in immunocompromised patients. Additional tests include aqueous humor sampling for local antibody production, PCR testing for T gondii DNA, and, rarely, vitreal cytopathology. Neuroimaging is crucial to exclude central nervous system involvement in immunocompromised patients. OCT reveals abnormal hyperreflectivity in retinal layers at infection sites, whereas FFA shows early blockage followed by lesion leakage.[46]
Herpes Simplex Virus
Neonates and infants up to 6 weeks with evidence of skin, eye, and mouth disease should undergo a thorough evaluation for CNS and disseminated disease. Clinicians can establish the diagnosis of neonatal HSV infection by isolating HSV in culture, detecting HSV DNA using qualitative or quantitative PCR, or detecting HSV antigens using direct immunofluorescence assays (DFA). Neonates with suspected HSV infection should undergo the following tests:
- Viral culture or HSV PCR on surface swabs of the conjunctivae, mouth, nasopharynx, and rectum
- Viral culture or HSV PCR (with or without DFA) of swabs or scrapings of any skin and mucous membrane lesions
- Cerebrospinal fluid HSV PCR
- Whole blood or plasma HSV PCR
- Viral culture or HSV PCR of tracheal aspirate if intubated
Neonates who have confirmed HSV infection should also undergo additional testing to determine the extent of the infection as follows:
- Complete blood count, including differential and platelet count
- Serum transaminases
- Total and direct bilirubin
- Blood urea nitrogen and creatinine
- Urinalysis
- Lumbar puncture, if not already completed
- Ophthalmologic examination
- Neuroimaging
- Electroencephalogram (EEG) in the presence of additional central nervous system involvement, such as seizures or an abnormal neurological examination
- Chest radiograph in the presence of pulmonary findings
Acute retinal necrosis
The American Uveitis Society defines acute retinal necrosis based on the following clinical characteristics.
- Presence of 1 or more foci of retinal necrosis with discrete borders located in the peripheral retina.
- A rapid progression of the disease in the absence of antiviral therapy.
- Circumferential spread.
- Evidence of occlusive vasculopathy with arterial involvement.
- A prominent inflammatory reaction in the vitreous and anterior chamber.[47]
In cases with an unclear underlying etiology, clinicians should perform PCR testing of the vitreous and aqueous humor for VZV, HSV, CMV, EBV, and toxoplasmosis. In addition, fourth-generation antigen/antibody combination HIV-1/2 immunoassay plus a confirmatory HIV-1/HIV-2 antibody differentiation immunoassay, fluorescent treponemal antibody absorption (FTA-ABS) and rapid plasma reagin (RPR) for syphilis, erythrocyte sedimentation rate (ESR), toxoplasmosis IgG and IgM, purified protein derivative (PPD) skin test, and chest radiograph can help in evaluating for other potential etiologies. OCT reveals fragmentation of retinal tissue.[30] Treatment should not be withheld while awaiting results.
Behçet Disease
For ocular Behçet disease, FFA is the gold standard in evaluating the activity and extent of retinal vasculitis. FFA reveals retinal infiltrates, retinal nerve fiber layer defects, occlusive retinal vasculitis, and a fern-like leakage pattern due to diffuse retinal capillary leakage in the mid-phase of the angiogram.[48] Vessel wall staining and leakage suggest increased vascular permeability caused by the breakdown of the inner blood-retinal barrier. FFA also aids in monitoring response to treatment. OCT and optical coherence tomography angiography (OCTA) help reveal disease activity not otherwise identified and may be beneficial in identifying and monitoring early disease activity.
Rocky Mountain Spotted Fever
Along with the clinical findings, clinicians confirm the diagnosis of RMSF using indirect immunofluorescence assay tests. Initial high antibody titer or a four-fold rise in the convalescent serum titer indicates a positive result. Case confirmation with serology might take 2 to 3 weeks. Serological testing using western blot or detecting rickettsiae in blood or tissue using PCR may be necessary in selected cases.
Syphilis
Clinicians use both treponemal tests, such as the FTA-ABS, and nontreponemal tests, such as the RPR and Venereal Disease Research Laboratory (VDRL), to diagnose syphilis. Screening may start with one and then confirmation of a positive result is necessary with the other. Some labs do have PCR available to detect T pallidum DNA. Patients with suspected neurosyphilis require cerebrospinal fluid-VDRL.
Lyme Disease
Patients presenting with erythema migrans recently traveling to an endemic area do not require further testing. For the remainder of patients, clinicians base the diagnosis on clinical findings and serological data such as an ELISA test for IgM and IgG, which peak during the first month, followed by a western blot test.
Subacute Sclerosing Panencephalitis
Elevated serum anti-measles antibody concentration and cerebrospinal fluid revealing elevated protein concentration and detectable anti-measles antibody characterize subacute sclerosing panencephalitis. Pathognomonic EEG changes during stage II demonstrate bursts of high-voltage complexes of 2 to 3 per second delta waves and sharp waves, followed by a relatively flat pattern. Computed tomography (CT) of the brain may reveal atrophy and scarring.
Tuberculosis
Ocular tuberculosis can be primary or secondary. If secondary, the patient may have active or latent disease. Patients with active disease will show active lesions on chest radiographs, and a sputum sample stained for acid-fast bacilli may confirm the diagnosis. In contrast, patients with latent tuberculosis have a normal chest radiograph but a positive tuberculin skin test or interferon-gamma release assay (IGRA). Diagnosing ocular tuberculosis is challenging as fluid from the eye is typically negative for tuberculosis on both culture and PCR. Directly performing IGRA on fluid from the eye appears to be a promising technique.
Dengue Fever
FFA reveals nonischemic venular occlusion. OCT helps evaluate and monitor the extent of macular edema. Clinicians perform PCR to detect viral genomes during the first week of illness, followed by testing for virus-specific IgM and IgG.
Sarcoidosis
The diagnosis of sarcoidosis begins with a thorough physical examination and appropriate testing to exclude other potential etiologies for the patient's symptoms. Sarcoidosis-specific testing begins with a chest radiograph followed by a high-resolution CT of the chest. Patients with mediastinal lymphadenopathy should also undergo testing for HIV and tuberculosis. Many patients require histopathologic detection of non-necrotizing granulomas unless the clinical presentation overwhelmingly supports the diagnosis.
Cat Scratch Disease
The predominant diagnostic approach is serological testing. An immunofluorescence assay IgG titer exceeding 1:64 suggests a possible B henselae infection, whereas a titer exceeding 1:256 strongly implies a recent or acute infection. PCR testing of ocular fluid is used to confirm the diagnosis.
Treatment / Management
Topical medications exhibit limited effectiveness against posterior uveitis. Periodically, clinicians use difluprednate, a topical corticosteroid with better vitreous humor penetration for inflammation posterior to the lens. Alternatives involve vigilant monitoring, along with periocular or intraocular glucocorticoid injections. Systemic glucocorticoids are considered for severe or bilateral disease, or in individuals with glaucoma when local injections are impractical. Conditions such as Behçet syndrome or serpiginous choroiditis may necessitate systemic treatment. Immunosuppressive agents become essential for cases with bilateral involvement, persistent inflammation, oral glucocorticoid resistance, or severe impairment of daily activities. Patients requiring 10 mg or more of daily prednisone may benefit from steroid-sparing agents such as methotrexate, azathioprine, mycophenolate mofetil, cyclosporine, or tacrolimus. TNF inhibitors are cautiously reserved, primarily for severe Behçet syndrome, due to their potential adverse effects.
Injected or surgically implanted intraocular fluocinolone, a long-acting glucocorticoid, is a treatment option for patients with non-infectious posterior uveitis requiring frequent intraocular injections. Surgically implanted formulations release medication continuously for approximately 2.5 years. At the onset of Vogt-Koyanagi-Harada disease, patients may need intravenous methylprednisolone. Regardless of the therapeutic option chosen, patients must have continued ophthalmologic examinations to monitor treatment response and determine treatment length.
Behçet Syndrome
Treatment for Behçet syndrome aims to control the inflammation, reduce the frequency of recurrences, and avoid complications. Corticosteroids and immunomodulatory therapy are the mainstays of treatment. Topical corticosteroids and cycloplegic agents such as scopolamine or cyclopentolate are the primary treatments for anterior uveitis. If the inflammation continues, systemic glucocorticoids such as prednisone 40mg/d, weaned over 1 month, are appropriate.
Variations in opinions exist regarding initial treatment for posterior uveitis associated with Bechet syndrome. Clinicians utilize systemic glucocorticoids, azathioprine, cyclosporine, IFN-α, or a monoclonal TNF-α antagonist. In many instances, the preferred combination is systemic glucocorticoids plus azathioprine. In the presence of an initial or recurrent episode of severe sight-threatening disease, systemic glucocorticoids plus a monoclonal TNF-α inhibitor such as infliximab is preferred.[49][50] An expert panel from the American Uveitis Society recommends initial treatment with TNF-α inhibitors due to the observed improvement in ocular manifestations compared to other treatments.(A1)
In all cases of infective retinitis, the antimicrobial should be initiated first, followed by steroids. Initiating steroids in the presence of infective retinitis or panuveitis without antimicrobial coverage may result in severe ocular damage and could be life-threatening if systemic disease is present.
CMV Retinitis
Current treatments for CMV retinitis include intravenous and intravitreal ganciclovir and foscarnet, intravenous cidofovir, and oral valganciclovir. Adequate treatment of the underlying immunocompromising condition is essential. Treatment regimens depend on the location of the lesions. Oral valganciclovir, a ganciclovir prodrug with enhanced bioavailability, is first-line therapy for CMV retinitis. Intravenous ganciclovir is appropriate for patients who are unable to tolerate oral medications and involves induction therapy at 5 mg/kg every 12 hours for 14 to 21 days, followed by a maintenance dose of 5 mg/kg/d. Valganciclovir induction therapy consists of 900 mg twice daily, followed by 900 mg/d for maintenance.[51][52] The primary adverse reaction is myelosuppression, necessitating regular blood count monitoring.(B3)
Immediate sight-threatening lesions, those close to the macula or head of the optic nerve, warrant local intravitreal injections and systemic therapy. The remaining patients can use systemic therapy alone. Induction therapy continues for 2 to 3 weeks until the retinitis is inactive, and then the dose is lowered to a maintenance dose. Because ganciclovir is not virucidal, the United States Centers for Disease Control (CDC) guidelines recommend chronic maintenance therapy be continued for AIDS-related CMV retinitis until patients maintain an increase in the CD4+ lymphocyte count greater than 100 to 150 cells/µL for 6 months or longer.[53]
Patients with immediate sight-threatening lesions receive local therapy with an intravitreal injection of ganciclovir 2.5 mg/0.05 mL weekly for 2 to 3 weeks or foscarnet 2.4 mg/injection administered twice weekly for 2 to 4 doses with concurrent systemic therapy.[54] Clinicians can discontinue intravitreal injections once the retinal opacity resolves. If systemic therapy begins within 24 hours of intravitreal injections, subsequent injections may be unnecessary.(B2)
Clinicians can also surgically implant intravitreal ganciclovir implants containing a minimum of 4.5 mg of ganciclovir through the pars plana. These implants act for 6 to 8 months and have a low-risk profile; however, they do not afford any protection for the contralateral eye or other visceral involvement. Immune recovery uveitis is another potential finding after beginning antiretroviral therapy in patients with AIDS with CMV retinitis. Affected patients present with anterior segment inflammation, posterior synechia, cataract, vitritis, cystoid macular edema, and epiretinal membrane.
Toxoplasmosis
Immunocompetent patients with Toxoplasma-related chorioretinitis usually experience a self-limited infection that resolves spontaneously in 4 to 8 weeks. However, treatment is warranted for the following groups of patients.
- Immunocompromised patients
- Pregnant women with acquired disease
- Patients with multiple active lesions
- Patients with lesions within the vascular arcades or adjacent to the optic disk or fovea
- Lesions larger than 1 optic disk diameter
- When visual acuity drops below 20/40 in a previously 20/20 eye
- The patient has sustained a 2-line drop in vision
Trimethoprim-sulfamethoxazole (TMP-SMX) 160 mg TMP/800 mg SMX twice daily in patients with normal renal function for 6 weeks is a first-line therapy in nonpregnant individuals. Traditionally, pyrimethamine combined with sulfadiazine has been the treatment of choice, and both regimens have comparable efficacy. However, TMP-SMX is associated with fewer adverse effects and is more cost-effective, with higher availability in some countries.[55][56][57] An additional alternative is pyrimethamine, sulfadiazine, and leucovorin. Patients who are intolerant to systemic therapy can receive intravitreal clindamycin 1.0 mg/0.1 mL with 1 or more injections administered over 6 weeks. Clinicians determine the number of injections based on the disease activity at the time of diagnosis.(A1)
Patients with significant vitreous inflammation and retinal vasculitis should receive glucocorticoids in addition to antimicrobial therapy. Clinicians initiate steroids 2 to 3 days after the initiation of antimicrobial therapy. The typical starting dose is prednisone 40 mg or 0.5mg/kg/d for a week, followed by a gradual taper. The goal is to have the dose <10 mg/d on discontinuing systemic antibiotics. Patients who receive intravitreal clindamycin may also receive intravitreal dexamethasone 0.4 mg/0.1 mL.
Toxocariasis
Management options for ocular larva migrans include systemic and topical steroids, anthelmintic drugs, and pars plana vitrectomy. For sight-threatening ocular inflammation, patients should receive prednisone 0.5 to 1 mg/kg/d followed by a slow taper. The benefits of adding an anthelmintic are unclear, but adding albendazole 400 mg twice daily for 2 weeks with a fatty meal reduces recurrences. Albendazole is preferred over mebendazole in CNS or ocular involvement because albendazole crosses the blood-brain barrier.
Syphilis
Clinicians manage ocular syphilis similarly to neurosyphilis, utilizing penicillin as the preferred treatment. Acceptable regimens are as follows:
- Aqueous crystalline penicillin G at a dosage of 18 to 24 million units per day, administered as 3 to 4 million units intravenous every 4 hours, or 18 to 24 million units per day daily as a continuous infusion for 10 to 14 days; or
- Procaine penicillin G at a dosage of 2.4 million units intramuscular once daily plus probenecid 500 mg orally 4 times a day, both for 10 to 14 days.
Clinicians often add oral or topical glucocorticoids. Ceftriaxone or doxycycline are alternatives for patients who are allergic to penicillin.
Tuberculosis
The treatment for ocular tuberculosis follows the same approach as pulmonary tuberculosis. Patients with paramacular and optic nerve head tuberculosis also receive systemic glucocorticoids.[58][59] Patients who develop neovascularization may require photocoagulation of the retina. (B3)
Acute Retinal Necrosis
The treatment of acute retinal necrosis should be initiated early to hasten the resolution of the disease in the infected eye and prevent contralateral disease. Initially, patients should be hospitalized and treated with a 10- to 14-day course of intravenous acyclovir 10 to 15 mg/kg every 8 hours, followed by oral acyclovir 800 mg 5 times a day for 6 to 12 weeks.[60] Recently, outpatient treatment with orally administered valacyclovir 2 g 3 times a day has been shown to achieve systemic levels similar to intravenous acyclovir.[61][62] Local treatment with intravitreal injections of ganciclovir 2 mg/0.1 mL is effective in treating sight-threatening herpetic infections with fovea or optic disc involvement.(B3)
Oral steroids should be administered under antiviral cover, starting 24 to 48 hours after antiviral therapy, particularly in cases with optic nerve involvement. The role of prophylactic laser posterior to the area of active retinitis in preventing retinal detachment in the setting of recent acute retinal necrosis remains unclear. Early surgery for an actual retinal detachment associated with acute retinal necrosis is crucial.[63]
Metastatic or Endogenous Endophthalmitis
Affected patients require urgent control of the systemic infection or focus of infection. Ocular management includes intravitreal antimicrobial therapy and vitrectomy. In such cases, The visual prognosis is usually poor.
Subacute Sclerosing Panencephalitis
No cure exists for subacute sclerosing panencephalitis. Most treatment involves symptom management. Some therapies reveal stabilization and prolongation of life, but those effects prove to be only temporary. A study reveals that a combination of intraventricular interferon-alpha treatment and isoprinosine induces remission in 44% to 55% of the cases studied. A long-term follow-up reveals that remission is only temporary. Amantadine improves survival, but isoprinosine is 4 times more effective compared to amantadine and twice as effective as interferon alpha. In addition, healthcare professionals use ribavirin and carbamazepine.
Lyme Disease
Doxycycline, ceftriaxone, and amoxicillin can treat any stage of Lyme disease, with ceftriaxone preferred for CNS involvement. These medications are also effective in treating ocular disease, with some studies suggesting that cephalosporins have better ocular penetration. Affected patients also likely require glucocorticoid therapy to control inflammation.
Dengue Fever
Most patients with ocular manifestations of dengue fever recover fully without therapeutic interventions. However, patients with bilateral involvement or severe ocular symptoms may receive systemic glucocorticoids or immunoglobulins.
Cat Scratch Disease
The optimal treatment regimen for cat scratch disease is unclear. Experts recommend doxycycline and rifampin for patients aged 8 or older. For patients younger than 8 or unable to tolerate doxycycline, the regimen includes rifampin plus azithromycin or TMP-SMX. Clinicians administer either choice for 4 to 6 weeks. In addition, patients receive 1 mg/kg/d of oral steroids for 2 weeks, followed by a gradual 4-week taper.
Sarcoidosis
The initial management of the ocular symptoms of sarcoidosis involves systemic oral or intravenous glucocorticoids in doses of 1 to 1.5 mg/kg/d. Methotrexate and mycophenolate mofetil are often utilized early in ocular disease as steroid-sparing agents. Azathioprine and cyclosporine are additional alternatives. Clinicians may consider adalimumab and other systemic immunomodulators if other immunosuppressive medications fail.
Rocky Mountain Spotted Fever
Doxycycline or chloramphenicol are the antimicrobials of choice to treat RMSF. Corticosteroids and cycloplegics are also utilized to treat ocular symptoms.
Differential Diagnosis
The list of differential diagnoses is extensive, including distinguishing between the various diagnoses discussed throughout this topic. Additional diagnoses to consider in patients with Behçet disease are as follows:
- Systemic lupus erythematosus
- Reactive arthritis
- Inflammatory bowel disease
- Celiac disease
- Stevens-Johnson syndrome
- Pemphigoid
- Pemphigus
- Lichen planus
- Linear IgA bullous dermatosis
- Medication effects, including those of methotrexate, other chemotherapy agents, and nicorandil
- Nutritional deficiencies such as vitamin B12, iron, and folic acid
- Recurrent aphthous stomatitis
- Cyclic neutropenia
- Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome
- Hyperimmunoglobulinemia D syndrome
- A20 haploinsufficiency
Additional diagnoses to consider in patients with CMV include benign cotton wool spots and immune recovery uveitis.
Pseudotumor cerebri can also mimic the much less frequent bilateral cat scratch disease.
Children with retinoblastoma and ocular toxocariasis can present with strabismus, poor vision, and leukocoria. Clinicians should consider ocular toxoplasmosis and tuberculosis when considering the diagnosis of toxocariasis.
Potential causes of granulomatous uveitis are as follows:
- Sarcoidosis
- Candida
- Histoplasmosis
- Cryptococcosis
- Tuberculosis
- Post-streptococcal infections
- Multiple sclerosis
- Vogt-Koyanagi-Harada disease
- Sympathetic ophthalmia
- Lymphoma
- Blau syndrome
- Histiocytosis
- Granuloma annulare
- Lens-induced uveitis
- Common variable immune deficiency
- Juvenile idiopathic arthritis
Prognosis
Visual outcomes vary based on the infecting microbe, the patient's immune status, and specific retinal involvement sites. Generally, the prognosis is favorable with appropriate treatment. Vision loss may result from foveal or optic disc involvement, choroidal neovascularization, macular scarring, or optic atrophy. Rhegmatogenous retinal detachment due to breaks in the thin, necrotic retina can also cause visual loss. Poor prognostic factors for retinitis include the following:
- Progressive outer retinal necrosis: PORN tends to progress or recur, leading to a dismal prognosis. Most affected patients subsequently experience total retinal detachments and minimal-to-no light perception.[4]
- Behçet syndrome: If not treated, Behçet syndrome causes blindness.
- Neonatal HSV: Skin, eye, and mouth disease may appear benign at the onset of the illness but is associated with a high risk of progression to the central nervous system or disseminated disease if not treated.
- Acute retinal necrosis: Visual outcomes associated with acute retinal necrosis are generally poor, primarily due to retinal detachment, but may also be due to chronic vitritis, epiretinal membrane, macular ischemia, and optic neuropathy.[53]
- Subacute sclerosing panencephalitis: Subacute sclerosing panencephalitis is a lethal disease associated with a very poor prognosis in terms of salvage of vision.
- Cat scratch disease: Most patients with neuroretinitis have a good long-term prognosis. Patients with macular exudates may experience residual defects such as optic disk pallor, diminished contrast sensitivity, and altered color vision. Additional complications are macular holes with posterior vitreous detachment.
Complications
The complications of retinitis include blindness, retinal breaks, rhegmatogenous retinal detachment, optic neuropathy, optic atrophy, macular scar, epiretinal membrane/vitreomacular traction, cystoid macular edema, choroidal neovascularization, complicated cataract, chronic vitritis, macular ischemia, glaucoma, vitreous hemorrhage, and phthisis.[64]
Deterrence and Patient Education
Retinitis comprises various inflammatory disorders affecting the retina, often resulting in symptoms such as floaters and diminished visual acuity. Recognizing the signs and dangers of retinitis is crucial, as untreated cases can lead to irreversible vision impairment. The causes of retinitis are diverse, including viral infections such as CMV or HSV and parasitic and inflammatory conditions. Patients should remain vigilant for changes in their vision, such as new floaters or flashes of light, as these may indicate retinal inflammation. Timely detection and treatment are vital for preventing vision loss and managing retinitis-related complications. Regular eye examinations are essential for individuals at higher risk, such as those with compromised immune systems or a history of ocular inflammation. Patients need to adhere to prescribed treatment regimens and attend follow-up appointments to optimize management and minimize the impact of retinitis on their vision and overall well-being.
Enhancing Healthcare Team Outcomes
Retinitis is an inflammatory condition affecting the retina, often characterized by symptoms such as floaters and decreased visual acuity. This condition can have various underlying causes, including viral infections such as CMV or HSV, parasitic infections, and inflammatory diseases. Retinitis can lead to irreversible vision loss without prompt recognition and management, underscoring the importance of early diagnosis and treatment of both retinitis and the underlying condition.
Retinitis is not generally responsive to topical treatment. The initial management of noninfectious uveitis posterior to the lens may include observation, and periocular and intraocular glucocorticoid injections. Oral glucocorticoids or additional anti-inflammatory or immunosuppressive agents may be necessary for patients with treatment-resistant symptoms. Infectious retinitis requires initiation of the appropriate antiviral or antibiotic medications prior to the initiation of glucocorticoids.
In cases where retinitis is secondary to systemic conditions such as AIDS, syphilis, or autoimmune diseases, primary care physicians, advanced care practitioners, and infectious disease specialists collaborate with ophthalmologists to diagnose and manage the underlying condition effectively. Optometrists also contribute significantly to enhancing healthcare team outcomes in retinitis management. They assist in monitoring patients for vision changes, evaluating treatment responses, and providing supportive care, such as prescribing corrective lenses or low-vision aids to optimize visual function. Pharmacists help monitor potential drug interactions and aid in patient education regarding medications. This interdisciplinary approach ensures comprehensive care that addresses both the ocular and systemic aspects of retinitis, improving overall patient outcomes and quality of life.
Media
(Click Image to Enlarge)
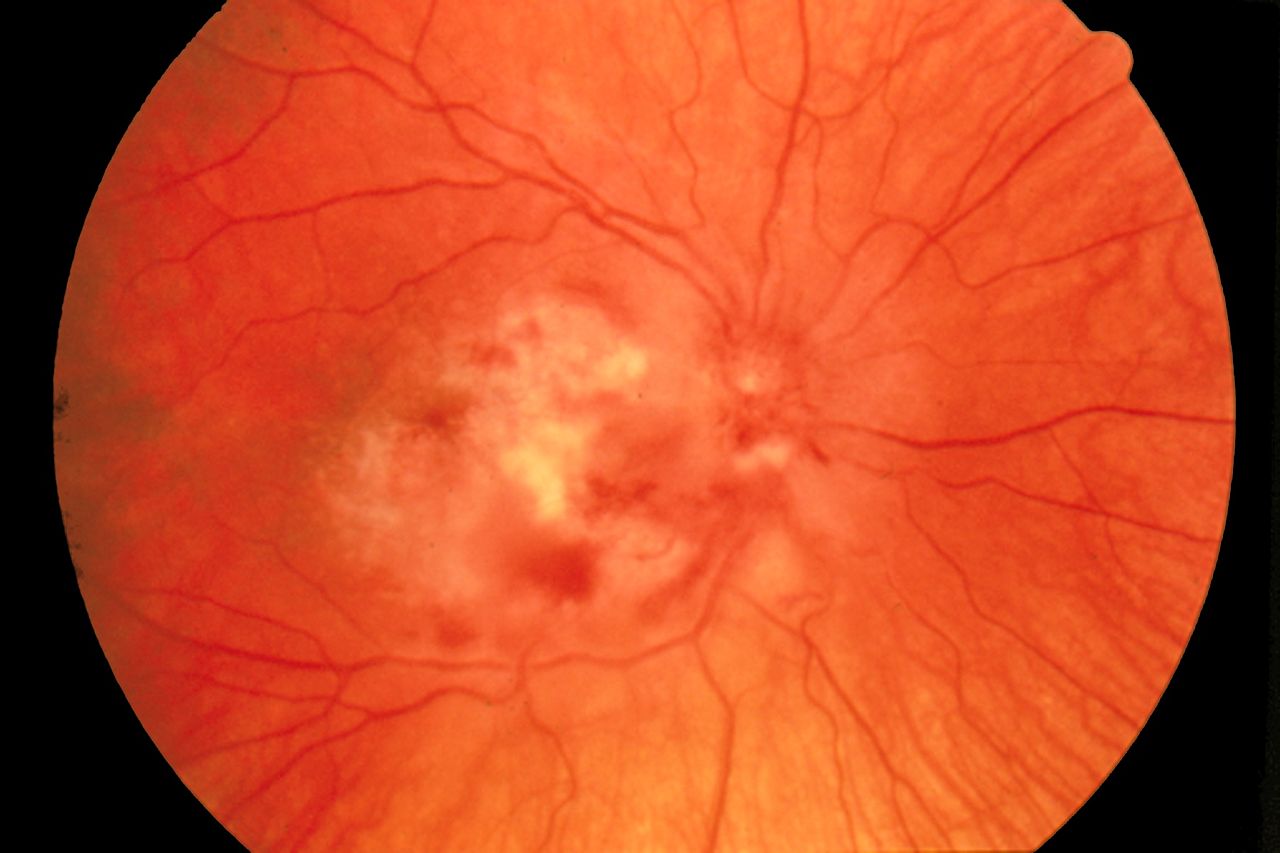
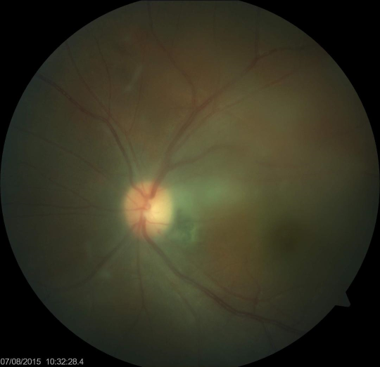
Cytomegalovirus Retinitis. The image depicts a view of a fundus affected by cytomegalovirus retinitis.
National Eye Institute, Public Domain, via Wikimedia Commons.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
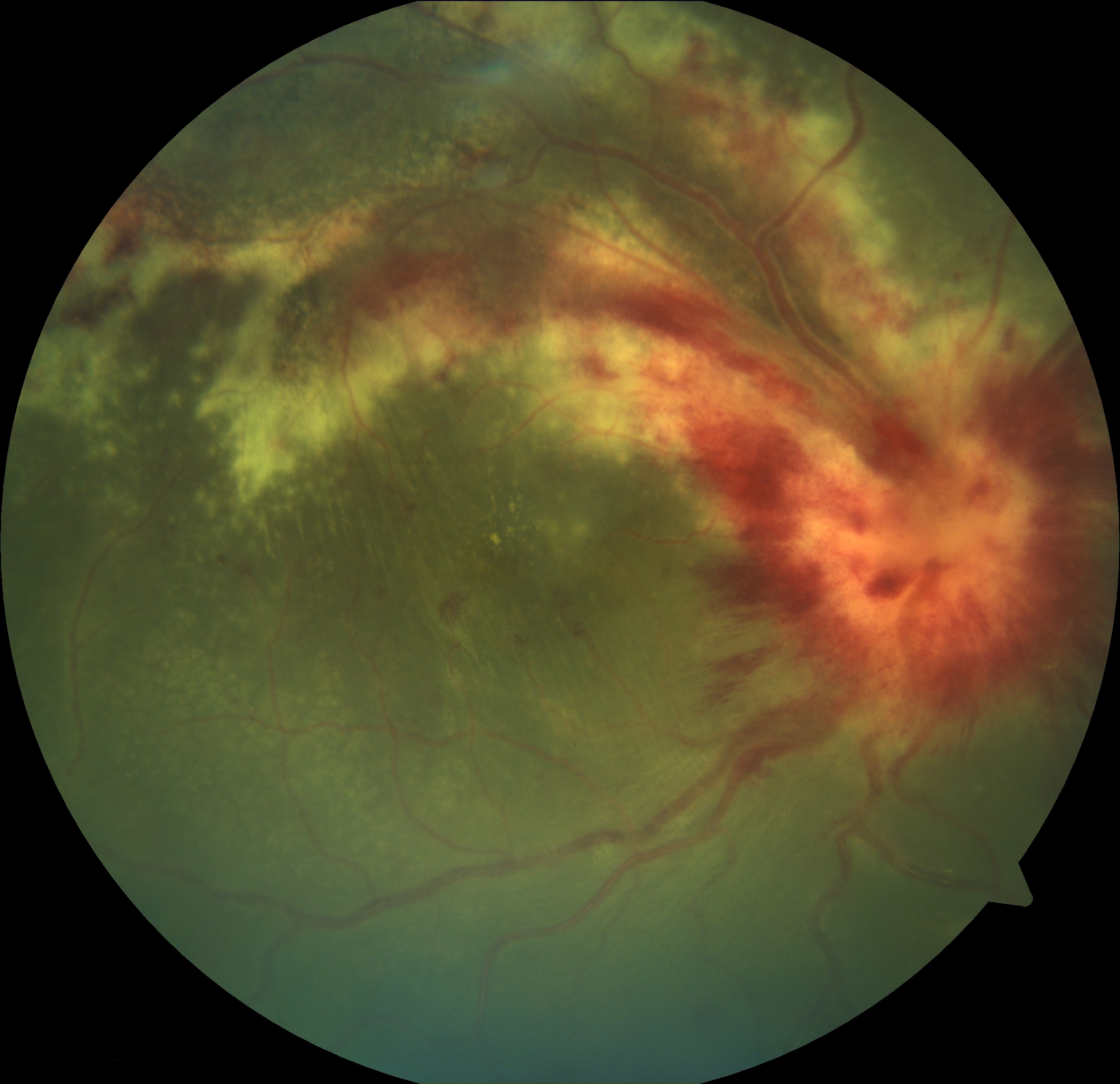
Acute Retinal Necrosis. Retinitis is demonstrated by the peripheral whitish retinal lesions. Typical additional findings include vitreous involvement and a paucity of hemorrhages, as opposed to cytomegalovirus retinitis, which presents with no vitreous involvement and a prominence of hemorrhages.
Contributed by K Tripathy, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Park YH, Nam HW. Clinical features and treatment of ocular toxoplasmosis. The Korean journal of parasitology. 2013 Aug:51(4):393-9. doi: 10.3347/kjp.2013.51.4.393. Epub 2013 Aug 30 [PubMed PMID: 24039281]
Level 3 (low-level) evidenceCarmichael A. Cytomegalovirus and the eye. Eye (London, England). 2012 Feb:26(2):237-40. doi: 10.1038/eye.2011.327. Epub 2011 Dec 16 [PubMed PMID: 22173076]
Chawla R, Tripathy K, Sharma YR, Venkatesh P, Vohra R. Periarterial Plaques (Kyrieleis' Arteriolitis) in a Case of Bilateral Acute Retinal Necrosis. Seminars in ophthalmology. 2017:32(2):251-252. doi: 10.3109/08820538.2015.1045153. Epub 2015 Jul 10 [PubMed PMID: 26161821]
Level 3 (low-level) evidenceHolland GN. The progressive outer retinal necrosis syndrome. International ophthalmology. 1994:18(3):163-5 [PubMed PMID: 7852023]
Mahendradas P, Ranganna SK, Shetty R, Balu R, Narayana KM, Babu RB, Shetty BK. Ocular manifestations associated with chikungunya. Ophthalmology. 2008 Feb:115(2):287-91 [PubMed PMID: 17631967]
Level 2 (mid-level) evidenceKoundanya VV, Tripathy K. Syphilis Ocular Manifestations. StatPearls. 2024 Jan:(): [PubMed PMID: 32644383]
Jafri SK, Kumar R, Ibrahim SH. Subacute sclerosing panencephalitis - current perspectives. Pediatric health, medicine and therapeutics. 2018:9():67-71. doi: 10.2147/PHMT.S126293. Epub 2018 Jun 26 [PubMed PMID: 29985487]
Level 3 (low-level) evidenceKhairallah M, Jelliti B, Jenzeri S. Emergent infectious uveitis. Middle East African journal of ophthalmology. 2009 Oct:16(4):225-38. doi: 10.4103/0974-9233.58426. Epub [PubMed PMID: 20404989]
Hatchette TF, Davis I, Johnston BL. Lyme disease: clinical diagnosis and treatment. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2014 May 29:40(11):194-208 [PubMed PMID: 29769842]
Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Survey of ophthalmology. 2015 Jul-Aug:60(4):279-95. doi: 10.1016/j.survophthal.2015.02.005. Epub 2015 Mar 5 [PubMed PMID: 25841248]
Level 3 (low-level) evidenceShrader SK, Band JD, Lauter CB, Murphy P. The clinical spectrum of endophthalmitis: incidence, predisposing factors, and features influencing outcome. The Journal of infectious diseases. 1990 Jul:162(1):115-20 [PubMed PMID: 2355187]
Level 2 (mid-level) evidenceGupta A,Tripathy K, Ocular Toxocariasis StatPearls. 2022 Jan [PubMed PMID: 35015409]
Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL, Studies of the Ocular Complications of AIDS Research Group. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. American journal of ophthalmology. 2012 Jun:153(6):1016-24.e5. doi: 10.1016/j.ajo.2011.11.014. Epub 2012 Feb 4 [PubMed PMID: 22310076]
Level 2 (mid-level) evidenceMcCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. American journal of ophthalmology. 1996 Jan:121(1):35-46 [PubMed PMID: 8554079]
Bonfioli AA, Orefice F. Behçet's disease. Seminars in ophthalmology. 2005 Jul-Sep:20(3):199-206 [PubMed PMID: 16282155]
Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. The British journal of ophthalmology. 2007 Nov:91(11):1452-5 [PubMed PMID: 17504853]
Chawla R, Tripathy K. Panuveitis simulating ocular Behçet's in cases of chronic myelogenous leukaemia in remission. BMJ case reports. 2017 Nov 23:2017():. pii: bcr-2017-222238. doi: 10.1136/bcr-2017-222238. Epub 2017 Nov 23 [PubMed PMID: 29170182]
Level 3 (low-level) evidenceTakeno M, Kariyone A, Yamashita N, Takiguchi M, Mizushima Y, Kaneoka H, Sakane T. Excessive function of peripheral blood neutrophils from patients with Behçet's disease and from HLA-B51 transgenic mice. Arthritis and rheumatism. 1995 Mar:38(3):426-33 [PubMed PMID: 7880197]
Level 3 (low-level) evidenceKuiper JJ, Mutis T, de Jager W, de Groot-Mijnes JD, Rothova A. Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. American journal of ophthalmology. 2011 Aug:152(2):177-182.e1. doi: 10.1016/j.ajo.2011.01.031. Epub 2011 May 13 [PubMed PMID: 21570674]
Ahn JK, Yu HG, Chung H, Park YG. Intraocular cytokine environment in active Behçet uveitis. American journal of ophthalmology. 2006 Sep:142(3):429-34 [PubMed PMID: 16935587]
Tripathy K, Venkatesh P, Chawla R. Cytomegaloviral retinitis. The National medical journal of India. 2016 Jan-Feb:29(1):35 [PubMed PMID: 27492038]
Stanford MR, Tomlin EA, Comyn O, Holland K, Pavesio C. The visual field in toxoplasmic retinochoroiditis. The British journal of ophthalmology. 2005 Jul:89(7):812-4 [PubMed PMID: 15965156]
Level 2 (mid-level) evidenceChawla R, Tripathy K, Meena S, Behera AK. Subretinal Hypopyon in Presumed Tubercular Uveitis: A Report of Two Cases. Middle East African journal of ophthalmology. 2018 Jul-Dec:25(3-4):163-166. doi: 10.4103/meajo.MEAJO_187_17. Epub [PubMed PMID: 30765956]
Level 3 (low-level) evidenceTripathy K, Chawla R, Sharma YR, Vohra R. Rickettsia retinitis cases in India: a few comments. Journal of ophthalmic inflammation and infection. 2016 Dec:6(1):7. doi: 10.1186/s12348-016-0076-1. Epub 2016 Feb 27 [PubMed PMID: 26920002]
Level 3 (low-level) evidenceDoft BH, Gass DM. Punctate outer retinal toxoplasmosis. Archives of ophthalmology (Chicago, Ill. : 1960). 1985 Sep:103(9):1332-6 [PubMed PMID: 4038125]
Level 3 (low-level) evidenceDominguez LN, Irvine AR. Fundus changes in Behcet's disease. Transactions of the American Ophthalmological Society. 1997:95():367-82; discussion 382-6 [PubMed PMID: 9440180]
Level 3 (low-level) evidenceYoung NJ, Bird AC. Bilateral acute retinal necrosis. The British journal of ophthalmology. 1978 Sep:62(9):581-90 [PubMed PMID: 708676]
Level 3 (low-level) evidenceGuex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocular immunology and inflammation. 1997 Dec:5(4):259-65 [PubMed PMID: 9455742]
Level 3 (low-level) evidenceEngstrom RE Jr, Holland GN, Margolis TP, Muccioli C, Lindley JI, Belfort R Jr, Holland SP, Johnston WH, Wolitz RA, Kreiger AE. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994 Sep:101(9):1488-502 [PubMed PMID: 8090452]
Level 2 (mid-level) evidenceChawla R, Tripathy K, Gogia V, Venkatesh P. Progressive outer retinal necrosis-like retinitis in immunocompetent hosts. BMJ case reports. 2016 Aug 10:2016():. doi: 10.1136/bcr-2016-216581. Epub 2016 Aug 10 [PubMed PMID: 27511757]
Level 3 (low-level) evidenceTripathy K, Patel BC. Cherry Red Spot. StatPearls. 2024 Jan:(): [PubMed PMID: 30969663]
Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Current opinion in ophthalmology. 1999 Jun:10(3):209-16 [PubMed PMID: 10537781]
Level 3 (low-level) evidenceChawla R, Venkatesh P, Tripathy K, Chaudhary S, Sharma SK. Successful Management of Proliferative Diabetic Retinopathy and Multiple Choroidal Tubercles in a Patient with Miliary Tuberculosis. Journal of ophthalmic & vision research. 2018 Apr-Jun:13(2):210-211. doi: 10.4103/jovr.jovr_203_16. Epub [PubMed PMID: 29719654]
Tripathy K, Chawla R. Choroidal tuberculoma. The National medical journal of India. 2016 Mar-Apr:29(2):106 [PubMed PMID: 27586221]
Dutta Majumder P, Biswas J, Gupta A. Enigma of serpiginous choroiditis. Indian journal of ophthalmology. 2019 Mar:67(3):325-333. doi: 10.4103/ijo.IJO_822_18. Epub [PubMed PMID: 30777946]
Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Annals of tropical medicine and parasitology. 2010 Jan:104(1):3-23. doi: 10.1179/136485910X12607012373957. Epub [PubMed PMID: 20149289]
Level 3 (low-level) evidenceBergstrom R, Tripathy K. Acute Retinal Necrosis. StatPearls. 2024 Jan:(): [PubMed PMID: 29262034]
Simakurthy S, Tripathy K. Endophthalmitis. StatPearls. 2024 Jan:(): [PubMed PMID: 32644505]
Mandelcorn ED. Infectious causes of posterior uveitis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2013 Feb:48(1):31-9. doi: 10.1016/j.jcjo.2012.11.013. Epub [PubMed PMID: 23419296]
Venkatesh P,Temkar S,Tripathy K,Chawla R, Intralesional antibiotic injection using 41G needle for the management of subretinal abscess in endogenous endophthalmitis. International journal of retina and vitreous. 2016; [PubMed PMID: 27847635]
Khairallah M, Kahloun R, Ben Yahia S, Jelliti B, Messaoud R. New infectious etiologies for posterior uveitis. Ophthalmic research. 2013:49(2):66-72. doi: 10.1159/000344009. Epub 2012 Dec 18 [PubMed PMID: 23258387]
Level 3 (low-level) evidenceTrehan HS, Sheth SS, Mathai A, Reddy RK, Moorthy RS. Diagnostic and therapeutic challenge. Retina (Philadelphia, Pa.). 2010 Jan:30(1):180-3. doi: 10.1097/IAE.0b013e3181b8ce2f. Epub [PubMed PMID: 19823105]
Level 3 (low-level) evidenceSingh D, Tripathy K. Retinal Macroaneurysm. StatPearls. 2024 Jan:(): [PubMed PMID: 35015432]
Tripathy K, Chawla R, Mittal K, Farmania R, Venkatesh P, Gulati S. Ophthalmic examination as a means to diagnose Subacute Sclerosing Panencephalitis: an optical coherence tomography and ultrawide field imaging evaluation. Eye and vision (London, England). 2017:4():1. doi: 10.1186/s40662-016-0066-2. Epub 2017 Jan 19 [PubMed PMID: 28116334]
Tripathy K, Sharma YR, Gogia V, Venkatesh P, Singh SK, Vohra R. Serial ultra wide field imaging for following up acute retinal necrosis cases. Oman journal of ophthalmology. 2015 Jan-Apr:8(1):71-2. doi: 10.4103/0974-620X.149896. Epub [PubMed PMID: 25709284]
Level 3 (low-level) evidenceGupta V, Gupta A. Ancillary investigations in uveitis. Indian journal of ophthalmology. 2013 Jun:61(6):263-8. doi: 10.4103/0301-4738.114093. Epub [PubMed PMID: 23803477]
Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. American journal of ophthalmology. 1994 May 15:117(5):663-7 [PubMed PMID: 8172275]
Level 1 (high-level) evidenceNussenblatt RB. Uveitis in Behçet's disease. International reviews of immunology. 1997:14(1):67-79 [PubMed PMID: 9203027]
Alpsoy E, Yilmaz E, Başaran E. Interferon therapy for Behçet's disease. Journal of the American Academy of Dermatology. 1994 Oct:31(4):617-9 [PubMed PMID: 8089288]
Level 1 (high-level) evidenceAlpsoy E, Durusoy C, Yilmaz E, Ozgurel Y, Ermis O, Yazar S, Basaran E. Interferon alfa-2a in the treatment of Behçet disease: a randomized placebo-controlled and double-blind study. Archives of dermatology. 2002 Apr:138(4):467-71 [PubMed PMID: 11939808]
Level 1 (high-level) evidenceBiron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral research. 2006 Sep:71(2-3):154-63 [PubMed PMID: 16765457]
Level 3 (low-level) evidenceTripathy K, Mittal K, Venkatesh P, Bakhshi S, Chawla R. Treatment of unilateral zone I cytomegalovirus retinitis in acute lymphoblastic leukemia with oral valganciclovir and intravitreal ganciclovir. Oman journal of ophthalmology. 2017 Sep-Dec:10(3):250-252. doi: 10.4103/ojo.OJO_190_2016. Epub [PubMed PMID: 29118508]
Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, Centers for Disease Control and Prevention (CDC), National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2009 Apr 10:58(RR-4):1-207; quiz CE1-4 [PubMed PMID: 19357635]
Vishnevskia-Dai V, Shapira Y, Rahav G, Shimoni A, Somech R, Moisseiev J. Cytomegalovirus retinitis in HIV-negative patients: a practical management approach. Ophthalmology. 2015 Apr:122(4):866-868.e3. doi: 10.1016/j.ophtha.2014.11.010. Epub 2014 Dec 31 [PubMed PMID: 25556113]
Level 2 (mid-level) evidenceZhang Y, Lin X, Lu F. Current treatment of ocular toxoplasmosis in immunocompetent patients: a network meta-analysis. Acta tropica. 2018 Sep:185():52-62. doi: 10.1016/j.actatropica.2018.04.026. Epub 2018 Apr 25 [PubMed PMID: 29704469]
Level 1 (high-level) evidencede-la-Torre A, Stanford M, Curi A, Jaffe GJ, Gomez-Marin JE. Therapy for ocular toxoplasmosis. Ocular immunology and inflammation. 2011 Oct:19(5):314-20. doi: 10.3109/09273948.2011.608915. Epub [PubMed PMID: 21970662]
Level 1 (high-level) evidenceSoheilian M, Sadoughi MM, Ghajarnia M, Dehghan MH, Yazdani S, Behboudi H, Anisian A, Peyman GA. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005 Nov:112(11):1876-82 [PubMed PMID: 16171866]
Level 3 (low-level) evidenceSaxena R, Singh D, Phuljhele S, Kalaiselvan V, Karna S, Gandhi R, Prakash A, Lodha R, Mohan A, Menon V, Garg R. Ethambutol toxicity: Expert panel consensus for the primary prevention, diagnosis and management of ethambutol-induced optic neuropathy. Indian journal of ophthalmology. 2021 Dec:69(12):3734-3739. doi: 10.4103/ijo.IJO_3746_20. Epub [PubMed PMID: 34827033]
Level 3 (low-level) evidenceKokkada SB, Barthakur R, Natarajan M, Palaian S, Chhetri AK, Mishra P. Ocular side effects of antitubercular drugs - a focus on prevention, early detection and management. Kathmandu University medical journal (KUMJ). 2005 Oct-Dec:3(4):438-41 [PubMed PMID: 16449853]
Tam PM, Hooper CY, Lightman S. Antiviral selection in the management of acute retinal necrosis. Clinical ophthalmology (Auckland, N.Z.). 2010 Feb 2:4():11-20 [PubMed PMID: 20169044]
Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007 Feb:114(2):307-12 [PubMed PMID: 17123607]
Level 3 (low-level) evidenceHuynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. American journal of ophthalmology. 2008 Apr:145(4):682-6. doi: 10.1016/j.ajo.2007.11.016. Epub 2008 Jan 28 [PubMed PMID: 18226802]
Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986 Mar:93(3):296-300 [PubMed PMID: 3703498]
Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Seminars in ophthalmology. 2018:33(6):788-803. doi: 10.1080/08820538.2018.1477966. Epub 2018 Jun 14 [PubMed PMID: 29902388]
Level 3 (low-level) evidence