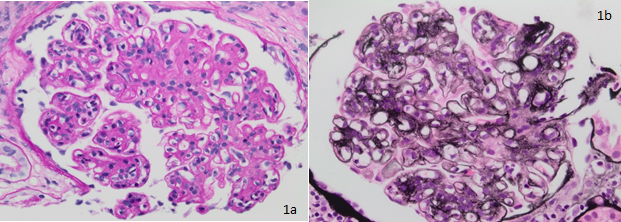
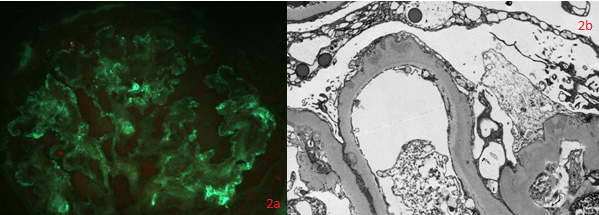
Introduction
Renal biopsy is a crucial diagnostic tool used to evaluate kidney diseases by obtaining tissue samples for microscopic examination. There are 2 main types of renal biopsies—targeted and non-targeted biopsies. Targeted biopsies help diagnose the pathologic nature of a mass lesion. In contrast, non-targeted biopsies sample the renal cortex to diagnose systemic diseases affecting the kidneys, such as kidney dysfunction after transplant, complications from medications, diabetes mellitus, or other chronic diseases.
Renal biopsies can also be classified based on the method used to obtain the specimen, including:
- Percutaneous biopsy
- Transvenous biopsy
- Laparoscopic biopsy
- Open surgical biopsy
This activity primarily focuses on percutaneous image-guided targeted and non-targeted biopsies, which is the preferred approach due to its minimally invasive nature, high diagnostic yield, and relatively low complication rate.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The normal native (non-transplant) kidney is situated in the retroperitoneal space. Biopsies of native kidneys should be performed whenever possible, ensuring that the needle only enters this space to minimize complications from transgressing the peritoneal cavity. For non-targeted biopsies, sampling the renal cortex is almost always preferred over the renal medulla because the cortex contains all glomeruli, Bowman's space, the efferent and afferent renal arterioles, proximal tubules, distal convoluted tubules, and early portions of the collecting tubules. In contrast, the medulla consists of distal convoluted tubules and loops of Henle. Medullary sampling is rarely required, with exceptions including suspected tumors; nephronophthisis, characterized by renal cysts at the corticomedullary junction; or medullary cystic kidney disease, although the latter 2 are almost always diagnosed through imaging rather than biopsy.[1][2]
The standard or majority configuration of the renal arterial vasculature typically begins with a single artery that divides into anterior and posterior branches. All renal arteries are end arteries without significant collateral arteries supplying the same vascular bed. The distal arterioles end where the anterior and posterior supply regions meet. This area, typically located along the posterolateral aspect of the kidney and referred to as Hyrtl's or Brodel's line, is the theoretically safest place to obtain renal tissue samples for non-targeted biopsies; however, this relatively hypovascularity area is not visible on imaging and must be approximated.[3]
Before performing a renal biopsy, imaging studies such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) should be conducted. This imaging helps plan the needle tract used to perform the procedure, and a standard anatomic configuration should not be assumed for every patient. Anatomic variants, such as ectopic or horseshoe-shaped kidneys, require a different approach from typical anatomy.
Indications
Noninvasive laboratory and diagnostic imaging tests should be utilized first to minimize the patient's risks, discomfort, and expenses of a renal biopsy.
Guidelines from several professional medical societies regarding when to perform renal biopsies are summarized below.
Targeted Biopsies for Renal Tumors
- The 2024 National Comprehensive Cancer Network (NCCN) guidelines (available on the society's website) state that the recommended abdominal imaging studies provide high diagnostic accuracy. Therefore, a needle biopsy is not always necessary before surgery. In selected individuals, a needle biopsy may be considered for small lesions to establish the diagnosis of renal cell carcinoma and guide active surveillance strategies, cryosurgery, radiofrequency, and ablation strategies. In addition, if imaging reveals a central lesion or a homogeneous infiltration of renal parenchyma, a biopsy may be warranted to differentiate urothelial carcinoma or lymphoma, respectively.
- The 2021 American Urological Association (AUA) guidelines state that a biopsy can be considered when it is necessary to determine whether a mass is hematologic, metastatic, inflammatory, or infectious.[4]
- The 2020 joint American College of Radiology (ACR)/Society of Interventional Radiology (SIR) guidelines (available on the ACR website) address targeted and non-targeted biopsies. For targeted biopsies, the guidelines recommend that biopsy is appropriate when renal function is normal, but the diagnosis remains indeterminate after completing all three of the following tests—CT without and with contrast, MRI without and with contrast, and ultrasound with duplex or Doppler. All of these tests assess for lesion vascularity. In individuals with renal insufficiency—where intravenous contrast is deemed too risky based on a nephrologist's assessment—a biopsy can be considered the third most appropriate test, after ultrasound with duplex or Doppler and MRI without contrast, thereby avoiding CT without contrast. The ACR guidelines also suggest that masses with intermediate levels of enhancement from 10 to 20 Hounsfield units (HU) may be appropriate for biopsy as the next step versus other options, such as surveillance, based on work by Heilbrun,[5] Vikram,[6] and Jacobs.[7]
- Guidelines from the SIR in 2019 and 2020 address some of the technical aspects of renal biopsy but do not address clinical decision-making issues for kidney biopsy. However, they classify kidney biopsy as the highest risk category for bleeding and discuss such procedures collectively.[8][9]
- The 2018 Cardiovascular and Interventional Radiological Society of Europe guidelines on biopsy do not address particular issues for kidney biopsy other than placing kidney biopsy in the highest category or bleeding risk.[10]
- The 2014 European Association of Urology (EAU) guidelines state that due to the high diagnostic accuracy of current imaging, a biopsy is not necessary for the setting of localized or locally advanced disease before surgical treatment in fit patients with a long life expectancy and a highly suspicious, contrast-enhancing renal mass on CT or MRI.[11]
Pre- and post-contrast CT or MRI (with or without contrast subtraction technology) are needed to confidently ascertain whether a kidney lesion is enhanced. The differential diagnosis for an enhancing kidney lesion primarily is renal cell carcinoma and oncocytoma. The Bosniak classification system assesses imaging factors that influence the probability of a partially cystic renal lesion being renal cell carcinoma. A common approach is to only biopsy Bosniak category 3 or growing Bosniak category 2F lesions, which are the types most indeterminate for malignancy.[12]
Imaging characteristics that encourage proceeding to renal mass biopsy are discussed in detail by Caoili [13] and are summarized below. If these features are present, a diagnosis of cancer should be considered.
- An infiltrative mass that preserves a reniform shape in the absence of risk factors for urinary tract infection should raise suspicion for lymphoma or urothelial carcinoma over standard renal cell carcinoma.
- Multiple enhancing masses without macroscopic fat should raise suspicion for lymphoma or a hereditary cancer syndrome over standard renal cell carcinoma.
Non-Targeted Biopsies for Differentiating Causes of Acute Kidney Injury or Chronic Kidney Disease
Non-targeted biopsies are performed to differentiate etiologies of acute kidney injury or chronic kidney disease in patients with the potential for recoverable renal function, as the treatment approach may vary depending on the underlying etiology.
- The 2024 American College of Rheumatology (ACR) guidelines (available on the society's website) recommend performing kidney biopsy in patients with systemic lupus erythematosus who have high protein levels in the urine or impaired kidney function that is not otherwise explained.
- The 2023 joint ACR-SIR guidelines (available on the ACR website) state only that non-targeted biopsies are appropriate for determining the nature and extent of diffuse parenchymal diseases, such as renal transplant rejection and glomerulonephritis.
- The 2013 ACR guidelines indicate that, for acute kidney injury, a biopsy is the next most appropriate radiologic test after performing an ultrasound of both the kidneys and bladder. For chronic kidney disease, the ultrasound of the kidneys and bladder should be performed first, but a biopsy is of higher or equivalent utility compared to any other radiologic test.[14] The 2020 updated version of these guidelines omitted this commentary.
For non-targeted biopsies, neither the American Society of Nephrology nor the National Kidney Foundation provide guidelines in any general or specific setting, such as acute kidney injury, chronic kidney disease, or post-transplant care.[15] These guidelines do not address many of the specific questions or issues encountered in everyday medical practice pertaining to the use of specific imaging or clinical parameters to differentiate types of diffuse parenchymal renal diseases with high positive predictive value.
Fiorentino [16] created an evidence-based list of indications and conclusions, and Fuiano [17] conducted a worldwide survey to obtain opinions on indications for non-targeted biopsies. The specific relative indications for non-targeted biopsies described by Fiorentino and Fuiano—including various types of nephrotic syndrome and glomerulonephritis—are extensive. Readers are encouraged to refer to their papers, the National Kidney Foundation guidelines,[15] or numerous other available non-consensus guideline-based sources with suggested indications for non-targeted biopsies.
Other Clinical Scenarios Where a Biopsy May Be Appropriate
- High surgical risk due to comorbidities: A biopsy may be considered for a tumor that is likely to be oncocytoma or renal cell carcinoma in a patient who has comorbidities associated with high surgical risks. These risks can be estimated using the American College of Surgeons calculator (riskcalculator.facs.org/RiskCalculator). A patient with high perioperative mortality risk and with a predicted life expectancy shorter than the anticipated progression of renal cell carcinoma to a terminal state may expect to have a confirmed tissue diagnosis of malignancy. This confirmation can help them decide whether to proceed with a major surgery that may not add longevity or quality of life. In addition, it may influence their choice of alternative treatment strategies with less chance of cure in managing known malignancy, such as percutaneous ablation or active surveillance.
- Unresectable renal tumors: When a renal tumor cannot be surgically removed (unresectable), a tissue sample is not available, preventing a definitive histological diagnosis. In such cases, medical or radiation oncologists often prefer to have a confirmed tissue diagnosis before offering treatment, as it helps guide appropriate therapy. However, professional guidelines, including those from the NCCN, do not universally require a tissue diagnosis for all patients with unresectable tumors.[18]
- Multiple tumors in the kidney or elsewhere: Multiple metastases to the kidney are much less common than metastases from the kidney or synchronous renal primary tumors. When two or more renal lesions have imaging features consistent with renal cell carcinoma, the staging of cancer should be performed by biopsying the safest lesion outside the kidney. This approach aims to establish a World Health Organization stage 4 renal cell carcinoma diagnosis, thus providing histologic confirmation of the extrarenal spread of the disease. If the results of the extrarenal biopsy indicate a cancer diagnosis that is not of renal origin, the renal tumor should be assessed on its own merit. If the tumor's imaging features are compatible with renal cell carcinoma, a second biopsy of the renal lesion is not needed just to prove that the lesion is truly a renal cell carcinoma and not a metastasis from other cancer sites.[19][20]
- Active surveillance instead of urgent treatment: Some institutions may require patients to undergo a renal biopsy as part of a non-operative surveillance program, using histological grading of renal cell carcinoma to better advise patients on the chances for potential tumor growth and metastasis. This strategy is endorsed by the EAU [11] but is not recommended by the NCCN [18] or AUA.[21] Management of renal cell carcinoma based on biopsy tissue grading has not yet been shown to be more clinically appropriate than management based on imaging surveillance alone, and the AUA currently only endorses the latter.[21] The NCCN states that a biopsy can be considered in these situations.[18]
- Renal tumors underdoing tumor ablation therapy: The AUA recommends a tissue biopsy before ablation therapy.[21] The NCCN recommends biopsy after ablation therapy for renal cell carcinoma if surveillance imaging shows new enhancement, a progressive increase in the size of an ablated neoplasm, new nodularity in or around the treated zone, failure of the treated lesion to regress over time, or satellite or port site lesions.[18]
Contraindications
The 2016 AUA guidelines stated that biopsy is typically not indicated for a solid tumor in the following scenarios:
- Young or healthy patients who are unwilling to accept the uncertainties associated with biopsy or
- Older or frail patients who are treated conservatively independent of renal mass biopsy findings [22]
Imaging characteristics that discourage proceeding to renal mass biopsy are discussed in detail by Caoili [13] and are briefly summarized below.
- Hemorrhage and protein within a complicated cyst: Dense fluid can simulate solid tissue on either pre- or post-contrast ultrasound, CT, or MRI. Comparing pre- and post-contrast images and using contrast subtraction imaging technology can help clarify this finding.
- Pseudoenhancement: This is an artifactual increase in attenuation (typically 1 to 25 HU) or intensity within a lesion on post-contrast imaging. This artifact is more likely to be observed in small (<2 cm) hypodense or hypointense lesions adjacent to the dense renal parenchyma. Given the small lesion size in these situations, active surveillance is sometimes a reasonable decision depending on other factors.
- Lesion enhancement equivalent to blood pool: A lesion that enhances to the same degree as the blood pool, that is, with similar intensity or density to the aorta, may initially appear solid but could represent a vascular anomaly, such as a pseudoaneurysm. In this situation, further evaluation with ultrasound or MRI sequences optimized for detecting blood vessels is recommended as the next step.
Biopsy of isolated hemorrhage/protein or pseudo-enhancement lesions typically results in a nondiagnostic specimen. In contrast, a biopsy of a vascular anomaly has a high pre-test probability of resulting in a hemorrhagic complication.
The following CT/MRI findings favor a benign diagnosis.
- Macroscopic fat: This finding is diagnostic of a benign angiomyolipoma without calcifications or other aggressive features.
- Microscopic fat (intracellular/minimal fat): Minimal fat angiomyolipomas are hypointense to normal parenchyma on T2 MRI, similar to papillary renal cell carcinoma, and demonstrate avid arterial enhancement, similar to clear renal cell carcinoma. The key to the diagnosis is that the two cancers only have one of these features, whereas minimal fat angiomyolipomas have both.
- Rapidly expanding ill-defined mass in a patient with urinary tract infection risk factors or clinical findings: This finding suggests focal bacterial pyelonephritis. If the finding persists on repeat imaging after clinical improvement of the infection, a biopsy may help determine whether the lesion is infectious or neoplastic.
Risks of the biopsy procedure, such as hemorrhage, may serve as relative contraindications if the patient is not able to tolerate the possible adverse outcome physically or psychologically. The 2013 SIR guidelines placed renal biopsy—even when performed through the transjugular approach, which is typically considered safer as it somewhat lowers the risk of significant hemorrhage—in the highest of its 3 risk categories for hemorrhagic complications.[23] The 2019 updated SIR guidelines reduced its risk categories to 2, with renal biopsy remaining in the higher risk category.[9]
Biopsies should be delayed until coagulation parameters are corrected to ensure that the risks of clinically significant hemorrhage do not outweigh the benefits of a tissue diagnosis. The SIR recommends a minimum platelet count of 50,000/µL and a maximum International Normalized Ratio of 1.5 to 1.8. The SIR no longer recommends maximum activated partial thromboplastin time, although it was previously 1.5 times the upper limit of normal for the hospital's control value. The SIR also recommends withholding specific antiplatelet drugs and anticoagulants for precise times before the procedure.[24] These times range from 2 hours to 10 days.
Extreme hypertension worsens hemorrhagic risks. In a randomized trial, desmopressin administration was shown to reduce blood loss before kidney biopsy, although the study did not evaluate whether the results changed clinical outcomes.[25] Patient allergies should be assessed before the procedure to mitigate the risk of anaphylaxis from any planned medications.[26]
Equipment
Transvenous renal biopsy is performed by placing a catheter, typically through jugular vein access, into the renal vein in the setting of non-targeted biopsies. A sheath is then placed into the renal vein that allows the insertion of a spring-loaded needle attached to a long extension support device. A detailed discussion of the equipment and technique is beyond the scope of this activity and is discussed elsewhere.[27][28]
Percutaneous targeted and non-targeted biopsies can be performed using various image guidance techniques, including ultrasound alone, ultrasound with needle guide technology, intermittent CT, fluoroscopic CT, or MRI, in some specialized centers. A retrospective case review found that an ultrasound-guided biopsy was associated with a lower risk of bleeding complications compared to a blind biopsy.[29]
Agarwal provided an in-depth review of needles for percutaneous access and the types of equipment used to evaluate the specimens.[30] In most published trials, both targeted and non-targeted biopsies have been performed using an 18- or 20-gauge mechanized spring-loaded needle, sometimes referred to as a gun, inserted coaxially through a 17- or 19-gauge needle, respectively. Head-to-head comparisons of different types of needle guns have been conducted and are available elsewhere in the literature. Some clinicians prefer larger gauge needles; however, no large prospective randomized study has demonstrated that they reduce the number of passes required. A meta-analysis comparing 16-gauge needles with smaller needles found an improved number of glomeruli retrieved and fewer passes with the 16-gauge needle, with no increase in major complications; however, the overall complication rate was higher.[31] Laboratories evaluating non-targeted biopsy specimens do not accept fine-needle aspiration samples. Needles with removable hubs, such as Van Sonnenberg needles, can be used to aid in the setting of a potentially difficult target to reduce the chance of multiple errant punctures with a larger gauge needle.
Personnel
In addition to the proceduralist performing the biopsy, several other healthcare practitioners perform critical functions during the procedure, including:
- A nurse trained in conscious sedation, nurse anesthetist, or anesthesiologist to administer conscious sedation and monitor vital signs.
- A technologist to assist with the equipment.
- A cytotechnologist or pathologist to assess the adequacy of specimens (rapid on-site evaluation) and to ensure that specimens are deposited in the most appropriate preservatives for the laboratory tests that best address the clinical scenario.
Preparation
The AUA stated that patients should be counseled regarding rationale, positive and negative predictive values, potential risks, and non-diagnostic rates of the procedure.[21] The referring clinician, such as a urologist or nephrologist, who determines the need for a biopsy should address these considerations before scheduling the procedure. Otherwise, the referring clinician should be available during the consent in conjunction with the performing clinician to address the rationale, benefits, and alternatives. If neither of these situations has occurred and the patient wishes to reconsider the procedure after hearing about its risks from the performing clinician, clinicians should accept that the patient may wish to delay the procedure until the referring clinician addresses such issues. The patient should be informed about positioning, typically prone oblique or prone, and the sedation/analgesia to be used.[32] The patient should be prepared for post-procedure monitoring, including arranging for a responsible family member or friend to provide transportation and assistance if any concerning symptoms arise after discharge.
The depth of the target and route of the needle should be pre-planned before the procedure date to avoid finding that the intended target is not accessible using the intended image guidance technique. Extra-long needles may need to be specially ordered if the patient is obese.
Patients with difficult-to-reach targets are served best by coordinating the biopsy so that both ultrasound and CT can be used as necessary to afford the advantages of both modalities, such as the speed of ultrasound, tissue contrast, and resolution of CT. CT-guided biopsies of upper pole lesions protected by ribs may be made feasible only through CT gantry angling or trigonometric analysis to estimate the necessary insertion angle. The angle at which to direct the needle concerning the level of the table can be calculated by first dividing the depth of the lesion by the distance of the lesion from the skin entry point and then by calculating the inverse tangent of that number.
Technique or Treatment
The 2014 EAU guidelines and the 2016 AUA guidelines stated that the coaxial technique is the preferred approach for percutaneous biopsy and has not been associated with tract tumor seeding based on data from a large series of patients. The alternative to the coaxial technique is removing and reinserting a needle for each pass. A small retrospective study of renal biopsies found no difference in complications or outcomes between the two techniques, but a randomized controlled trial comparison has not been made.[33]
For both targeted and non-targeted biopsies, core biopsy specimens have greater sensitivity and specificity compared to fine-needle aspiration specimens.[34] Consequently, the AUA guidelines recommend multiple core biopsies over fine needle aspiration for targeted biopsy, a recommendation also supported by the EAU guidelines. There is no standard minimum amount of tissue requirement for a targeted biopsy, as some pathologists can diagnose with the yield from a single fine-needle aspiration pass. Clinicians recommending the biopsy should consult with the pathologist providing the diagnostic interpretation to discuss the desirable molecular testing, which may affect the amount of tissue needed. The pathologist, in turn, should consult with the clinician performing the procedure (if different) to specify the amount of tissue required to conduct tests for genetic markers, flow cytometry, culture, and sensitivity. The necessary sample size can vary from one pathologist/institution to another; there are no standardized guidelines for the minimum tissue volume needed in advance.
For non-targeted biopsies, a minimum of about 5 to 10 intact glomeruli is required for diagnosis.[35] Yoshinari demonstrated that each 1 cm length of an 18-gauge specimen of the renal cortex averaged over 11 glomeruli.[36] A minimum of 2 to 3 specimens is needed to enable evaluation under light microscopy, electron microscopy, and immunofluorescence.[37]
Some clinicians plug the needle track with thrombin, fresh frozen plasma, or gelatin sponge; however, no randomized controlled trials have compared these products against no intervention or placebo for percutaneous renal biopsies in individuals. There are no national guidelines for post-biopsy monitoring. Some institutions allow discharge after only 1 hour, whereas others require much longer.[38]
Complications
The SIR 2013 practice guidelines (citing primary sources) state that bleeding complications requiring transfusion when using an 18-gauge or smaller needle are expected to be less than 3%, and that a 5% rate should result in a change in practice habits.[23][39][23] The guidelines also state that using a larger needle is expected to result in a slightly higher complication rate but do not address these other complications more specifically.
Patel stated the following risk percentages:
- Clinically significant renal hematoma (4.9%)
- Clinically significant pain (1.2%)
- Pneumothorax (0.6%)
- Hemorrhage requiring transfusion (0.4%) [40]
Anecdotally, vascular injuries that do not require treatment are much more common than those reported in such studies, which do not screen all patients post-biopsy with ultrasound to assess for their presence. Although neither of the previously cited sources mentions arteriovenous fistula, a study conducted by an experienced clinician found that arteriovenous fistula requiring clinical attention occurred after about 1% of biopsies. In the author's experience, arteriovenous fistulas are detected at a higher rate when physicians specifically look for them using post-biopsy ultrasound.[41]
Nearly every patient develops at least some degree of perirenal hematoma, which is detected more easily on CT or MRI than on ultrasound. One in several thousand patients may lose a kidney due to complications, and fatalities have occurred following severe complications from renal biopsies.
Clinical Significance
Kidney biopsy should not be performed if, based on a comprehensive noninvasive radiological workup, it has a low to zero chance of changing treatment.
Multiple studies report that core targeted biopsies have high sensitivity, specificity, and positive predictive value with a comparatively low (~50%) negative predictive value.[42][43][44]. A meta-analysis of nearly 3000 patients found the following:
- Mean sensitivity more than 95% (95% CI 78-100)
- Mean specificity more than 95% (95% CI 75-100)
- Mean positive predictive value more than 99% (95% CI 97-100) [40]
Histologic subtyping of renal cell carcinoma, such as clear cell, papillary, or chromophobic subtypes, is about 95% accurate. However, as of now, subtyping does change management according to AUA or NCCN guidelines.[40][45]
Richard concluded that in patients with questionable renal cell carcinoma versus oncocytoma, surgical treatment could have been avoided in at least 26% of cases because the biopsied lesion was benign.[45] However, the converse point is that about 75% of patients in the study population underwent unnecessary biopsies and could have proceeded straight to surgical resection.
Targeted biopsy limitations include the following:
- The non-diagnostic rate of the first targeted biopsy is approximately 10% or higher.[45][40]
- A small (<5%) subset of patients with benign oncocytoma have concurrent renal cell carcinoma that could be missed with targeted biopsy.[46]
- Targeted biopsy has a low negative predictive value for malignant tumors, with a percentage likely no higher than in the low 60s, indicating a false-negative rate of approximately 30%.[40]
- Fuhrman histologic grading based on core biopsy is only 50% to 75% accurate.[40] The AUA guidelines commented on this fact, indicating that the accuracy of histologic grading is variable.[21]
A prospective study of 80 patients found that non-targeted biopsy changed the pre-biopsy clinical diagnosis in 44% of cases and altered therapy in 31% of cases, indicating that non-targeted biopsy was unnecessary in about 70% of cases.[47] A larger or more recent prospective study was not identified in the literature.
Therefore, targeted and non-targeted biopsies play a role in confirming or changing management decisions, but many patients may not derive any benefit.
Enhancing Healthcare Team Outcomes
Renal biopsies can lead to complications. Clinicians and nurses can collaborate to educate patients so that patients feel confident that the benefit outweighs the risks and are empowered when addressing different scenarios before and after the biopsy. The clinical nurse and the technologist aim to minimize procedure complications and increase the likelihood of retrieving an adequate biopsy specimen on the first attempt. Consulting a surgeon is essential when a laparoscopic biopsy may be preferable to a percutaneous biopsy.
The research on renal biopsies mentioned earlier (primarily Oxford Centre for Evidence-based Medicine version 2.1 level 3) indicates that renal biopsies usually succeed in diagnosing without complications. However, they can influence patient outcomes in a minority of cases if patients are not selected carefully.
Ethical principles play a central role in renal biopsy decision-making. Informed consent is essential, respecting patient autonomy and ensuring beneficence and non-maleficence. Patient preferences are central to decisions, promoting shared decision-making. Education and training keep the team updated on best practices. Ongoing professional development ensures that healthcare practitioners are equipped to respond effectively to complications of renal biopsy. A patient-centered approach places the patient's well-being and preferences at the forefront of all decisions. When approaching a renal biopsy, an interprofessional healthcare team ensures a comprehensive response, minimizes complications, and prioritizes patient safety and care quality.
Media
References
Müller RU, Benzing T. Cystic Kidney Diseases From the Adult Nephrologist's Point of View. Frontiers in pediatrics. 2018:6():65. doi: 10.3389/fped.2018.00065. Epub 2018 Mar 22 [PubMed PMID: 29623269]
Avni FE, Garel C, Cassart M, D'Haene N, Hall M, Riccabona M. Imaging and classification of congenital cystic renal diseases. AJR. American journal of roentgenology. 2012 May:198(5):1004-13. doi: 10.2214/AJR.11.8083. Epub [PubMed PMID: 22528889]
Bordoni B, Sugumar K, Leslie SW. Anatomy, Abdomen and Pelvis, Pelvic Floor. StatPearls. 2025 Jan:(): [PubMed PMID: 29489277]
Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. The Journal of urology. 2021 Aug:206(2):199-208. doi: 10.1097/JU.0000000000001911. Epub 2021 Jul 11 [PubMed PMID: 34115547]
Heilbrun ME, Remer EM, Casalino DD, Beland MD, Bishoff JT, Blaufox MD, Coursey CA, Goldfarb S, Harvin HJ, Nikolaidis P, Preminger GM, Raman SS, Sahni A, Vikram R, Weinfeld RM. ACR Appropriateness Criteria indeterminate renal mass. Journal of the American College of Radiology : JACR. 2015 Apr:12(4):333-41. doi: 10.1016/j.jacr.2014.12.012. Epub [PubMed PMID: 25842014]
Vikram R, Beland MD, Blaufox MD, Moreno CC, Gore JL, Harvin HJ, Heilbrun ME, Liauw SL, Nguyen PL, Nikolaidis P, Preminger GM, Purysko AS, Raman SS, Taffel MT, Wang ZJ, Weinfeld RM, Remer EM, Lockhart ME. ACR Appropriateness Criteria Renal Cell Carcinoma Staging. Journal of the American College of Radiology : JACR. 2016 May:13(5):518-25. doi: 10.1016/j.jacr.2016.01.021. Epub 2016 Mar 23 [PubMed PMID: 27016804]
Jacobs BL, Tan HJ, Montgomery JS, Weizer AZ, Wood DP, Miller DC, Wolf JS Jr, Hafez KS. Understanding criteria for surveillance of patients with a small renal mass. Urology. 2012 May:79(5):1027-32. doi: 10.1016/j.urology.2011.12.052. Epub [PubMed PMID: 22546379]
Level 2 (mid-level) evidenceSheth RA, Baerlocher MO, Connolly BL, Dariushnia SR, Shyn PB, Vatsky S, Tam AL, Gupta S. Society of Interventional Radiology Quality Improvement Standards on Percutaneous Needle Biopsy in Adult and Pediatric Patients. Journal of vascular and interventional radiology : JVIR. 2020 Nov:31(11):1840-1848. doi: 10.1016/j.jvir.2020.07.012. Epub 2020 Oct 1 [PubMed PMID: 33011015]
Level 2 (mid-level) evidencePatel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, Wilkins LR, Sarode R, Weinberg I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. Journal of vascular and interventional radiology : JVIR. 2019 Aug:30(8):1168-1184.e1. doi: 10.1016/j.jvir.2019.04.017. Epub 2019 Jun 20 [PubMed PMID: 31229333]
Level 3 (low-level) evidenceVeltri A, Bargellini I, Giorgi L, Almeida PAMS, Akhan O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovascular and interventional radiology. 2017 Oct:40(10):1501-1513. doi: 10.1007/s00270-017-1658-5. Epub 2017 May 18 [PubMed PMID: 28523447]
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A. EAU guidelines on renal cell carcinoma: 2014 update. European urology. 2015 May:67(5):913-24. doi: 10.1016/j.eururo.2015.01.005. Epub 2015 Jan 21 [PubMed PMID: 25616710]
Level 1 (high-level) evidenceLang EK, Macchia RJ, Gayle B, Richter F, Watson RA, Thomas R, Myers L. CT-guided biopsy of indeterminate renal cystic masses (Bosniak 3 and 2F): accuracy and impact on clinical management. European radiology. 2002 Oct:12(10):2518-24 [PubMed PMID: 12271393]
Level 2 (mid-level) evidenceCaoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Seminars in interventional radiology. 2014 Mar:31(1):20-6. doi: 10.1055/s-0033-1363839. Epub [PubMed PMID: 24596436]
Remer EM, Papanicolaou N, Casalino DD, Bishoff JT, Blaufox MD, Coursey CA, Dighe M, Eberhardt SC, Goldfarb S, Harvin HJ, Heilbrun ME, Leyendecker JR, Nikolaidis P, Oto A, Preminger GM, Raman SS, Sheth S, Vikram R, Weinfeld RM. ACR Appropriateness Criteria(®) on renal failure. The American journal of medicine. 2014 Nov:127(11):1041-1048.e1. doi: 10.1016/j.amjmed.2014.05.014. Epub 2014 May 24 [PubMed PMID: 24865874]
National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Nov:60(5):850-86. doi: 10.1053/j.ajkd.2012.07.005. Epub [PubMed PMID: 23067652]
Level 2 (mid-level) evidenceFiorentino M, Bolignano D, Tesar V, Pisano A, Van Biesen W, D'Arrigo G, Tripepi G, Gesualdo L, ERA-EDTA Immunonephrology Working Group. Renal Biopsy in 2015--From Epidemiology to Evidence-Based Indications. American journal of nephrology. 2016:43(1):1-19. doi: 10.1159/000444026. Epub 2016 Feb 5 [PubMed PMID: 26844777]
Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C, Andreucci M, Andreucci VE. Current indications for renal biopsy: a questionnaire-based survey. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000 Mar:35(3):448-57 [PubMed PMID: 10692270]
Level 3 (low-level) evidenceMotzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman M, Gallagher TH, Gore JL, Hancock SL, Harrison MR, Kim W, Kyriakopoulos C, LaGrange C, Lam ET, Lau C, Michaelson MD, Olencki T, Pierorazio PM, Plimack ER, Redman BG, Shuch B, Somer B, Sonpavde G, Sosman J, Dwyer M, Kumar R. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2017 Jun:15(6):804-834. doi: 10.6004/jnccn.2017.0100. Epub [PubMed PMID: 28596261]
Level 1 (high-level) evidenceUppot RN, Harisinghani MG, Gervais DA. Imaging-guided percutaneous renal biopsy: rationale and approach. AJR. American journal of roentgenology. 2010 Jun:194(6):1443-9. doi: 10.2214/AJR.10.4427. Epub [PubMed PMID: 20489082]
Tan HJ, Jacobs BL, Hafez KS, Montgomery JS, Weizer AZ, Wood DP Jr, Miller DC, Wolf JS Jr. Understanding the role of percutaneous biopsy in the management of patients with a small renal mass. Urology. 2012 Feb:79(2):372-7. doi: 10.1016/j.urology.2011.09.050. Epub [PubMed PMID: 22310755]
Level 2 (mid-level) evidenceCampbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, Clark PE, Davis BJ, Derweesh IH, Giambarresi L, Gervais DA, Hu SL, Lane BR, Leibovich BC, Pierorazio PM. Renal Mass and Localized Renal Cancer: AUA Guideline. The Journal of urology. 2017 Sep:198(3):520-529. doi: 10.1016/j.juro.2017.04.100. Epub 2017 May 4 [PubMed PMID: 28479239]
Campbell SC, Uzzo RG, Karam JA, Chang SS, Clark PE, Souter L. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-up: AUA Guideline: Part II. The Journal of urology. 2021 Aug:206(2):209-218. doi: 10.1097/JU.0000000000001912. Epub 2021 Jul 11 [PubMed PMID: 34115531]
Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WE, Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement, Standards of Practice Committee of the Society of Interventional Radiology. Addendum of newer anticoagulants to the SIR consensus guideline. Journal of vascular and interventional radiology : JVIR. 2013 May:24(5):641-5. doi: 10.1016/j.jvir.2012.12.007. Epub [PubMed PMID: 23622037]
Level 3 (low-level) evidenceDavidson JC, Rahim S, Hanks SE, Patel IJ, Tam AL, Walker TG, Weinberg I, Wilkins LR, Sarode R. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part I: Review of Anticoagulation Agents and Clinical Considerations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. Journal of vascular and interventional radiology : JVIR. 2019 Aug:30(8):1155-1167. doi: 10.1016/j.jvir.2019.04.016. Epub 2019 Jun 20 [PubMed PMID: 31229332]
Level 3 (low-level) evidenceSattari SA, Shahoori A, Shahbazian H, Sabetnia L, Aref A, Sattari AR, Ghorbani A. Desmopressin Acetate in Percutaneous Ultrasound-Guided Native Kidney Biopsy in Patients with Reduced Kidney Function: A Double-Blind Randomized Controlled Trial. Iranian journal of kidney diseases. 2022 Jul:16(4):238-245 [PubMed PMID: 35962638]
Level 1 (high-level) evidenceRafiei P, Walser EM, Duncan JR, Rana H, Ross JR, Kerlan RK Jr, Gross KA, Balter S, Bartal G, Abi-Jaoudeh N, Stecker MS, Cohen AM, Dixon RG, Thornton RH, Nikolic B, Society of Interventional Radiology Health and Safety Committee. Society of Interventional Radiology IR Pre-Procedure Patient Safety Checklist by the Safety and Health Committee. Journal of vascular and interventional radiology : JVIR. 2016 May:27(5):695-9. doi: 10.1016/j.jvir.2016.03.002. Epub 2016 Mar 31 [PubMed PMID: 27038685]
Mal F, Meyrier A, Callard P, Altman JJ, Kleinknecht D, Beaugrand M, Ferrier JP. Transjugular renal biopsy. Lancet (London, England). 1990 Jun 23:335(8704):1512-3 [PubMed PMID: 1972443]
Levi IM, Ben-Dov IZ, Klimov A, Pizov G, Bloom AI. Transjugular kidney biopsy: enabling safe tissue diagnosis in high risk patients. The Israel Medical Association journal : IMAJ. 2011 Jul:13(7):425-7 [PubMed PMID: 21838185]
Level 2 (mid-level) evidenceMaya ID, Maddela P, Barker J, Allon M. Percutaneous renal biopsy: comparison of blind and real-time ultrasound-guided technique. Seminars in dialysis. 2007 Jul-Aug:20(4):355-8 [PubMed PMID: 17635829]
Level 2 (mid-level) evidenceAgarwal SK, Sethi S, Dinda AK. Basics of kidney biopsy: A nephrologist's perspective. Indian journal of nephrology. 2013 Jul:23(4):243-52. doi: 10.4103/0971-4065.114462. Epub [PubMed PMID: 23960337]
Level 3 (low-level) evidenceZhan T, Lou A. Comparison of outcomes of an 18-gauge vs 16-gauge ultrasound-guided percutaneous renal biopsy: a systematic review and meta-analysis. Renal failure. 2023:45(2):2257806. doi: 10.1080/0886022X.2023.2257806. Epub 2023 Sep 19 [PubMed PMID: 37724553]
Level 1 (high-level) evidenceGesualdo L, Cormio L, Stallone G, Infante B, Di Palma AM, Delli Carri P, Cignarelli M, Lamacchia O, Iannaccone S, Di Paolo S, Morrone L, Aucella F, Carrieri G. Percutaneous ultrasound-guided renal biopsy in supine antero-lateral position: a new approach for obese and non-obese patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008 Mar:23(3):971-6 [PubMed PMID: 17977877]
Level 1 (high-level) evidenceHatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. AJR. American journal of roentgenology. 2008 Feb:190(2):413-7. doi: 10.2214/AJR.07.2676. Epub [PubMed PMID: 18212227]
Level 2 (mid-level) evidenceMarconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, Bex A, Bensalah K, Canfield SE, Hora M, Kuczyk MA, Merseburger AS, Mulders PFA, Powles T, Staehler M, Ljungberg B, Volpe A. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. European urology. 2016 Apr:69(4):660-673. doi: 10.1016/j.eururo.2015.07.072. Epub 2015 Aug 29 [PubMed PMID: 26323946]
Level 1 (high-level) evidenceSakai H, Abe K, Kobayashi Y, Koyama A, Shigematsu H, Harada T, Yoshikawa N, Arakawa M, Itoh H, Osawa G. Clinical guidelines of IgA nephropathy. Nihon Jinzo Gakkai shi. 1995 Aug:37(8):417-21 [PubMed PMID: 7563948]
Yoshinari M, Suzuki R, Watanabe K, Katoh T, Watanabe T. How long is enough: length of renal needle biopsy specimen for histological diagnosis. American journal of nephrology. 2002 Jul-Aug:22(4):402 [PubMed PMID: 12169878]
Level 3 (low-level) evidenceWalker PD, Cavallo T, Bonsib SM, Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004 Dec:17(12):1555-63 [PubMed PMID: 15272280]
Level 1 (high-level) evidencede Fátima Lucena A, Oliveira MC, Manfro RC. Reduction of patients' bed rest time after percutaneous renal biopsy evaluated by the Nursing Outcomes Classification: Randomized clinical trial. International journal of nursing knowledge. 2024 Jul:35(3):308-316. doi: 10.1111/2047-3095.12447. Epub 2023 Sep 7 [PubMed PMID: 37676727]
Level 1 (high-level) evidenceManno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, Gesualdo L, Schena FP. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney international. 2004 Oct:66(4):1570-7 [PubMed PMID: 15458453]
Patel HD, Johnson MH, Pierorazio PM, Sozio SM, Sharma R, Iyoha E, Bass EB, Allaf ME. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. The Journal of urology. 2016 May:195(5):1340-1347. doi: 10.1016/j.juro.2015.11.029. Epub 2016 Feb 18 [PubMed PMID: 26901507]
Level 1 (high-level) evidenceRoccatello D, Sciascia S, Rossi D, Naretto C, Bazzan M, Solfietti L, Baldovino S, Menegatti E. Outpatient percutaneous native renal biopsy: safety profile in a large monocentric cohort. BMJ open. 2017 Jun 21:7(6):e015243. doi: 10.1136/bmjopen-2016-015243. Epub 2017 Jun 21 [PubMed PMID: 28637732]
von Rundstedt FC, Mata DA, Kryvenko ON, Roth S, Degener S, Dreger NM, Goedde D, Assaid A, Kamper L, Haage P, Stoerkel S, Lazica DA. Diagnostic Accuracy of Renal Mass Biopsy: An Ex Vivo Study of 100 Nephrectomy Specimens. International journal of surgical pathology. 2016 May:24(3):213-8. doi: 10.1177/1066896915625178. Epub 2016 Jan 25 [PubMed PMID: 26811388]
Lebret T, Poulain JE, Molinie V, Herve JM, Denoux Y, Guth A, Scherrer A, Botto H. Percutaneous core biopsy for renal masses: indications, accuracy and results. The Journal of urology. 2007 Oct:178(4 Pt 1):1184-8; discussion 1188 [PubMed PMID: 17698122]
Level 2 (mid-level) evidenceLeveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shiff DA, Fernandes K, Jewett MA. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. European urology. 2011 Sep:60(3):578-84. doi: 10.1016/j.eururo.2011.06.021. Epub 2011 Jun 24 [PubMed PMID: 21704449]
Level 2 (mid-level) evidenceRichard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, Hermanns T, Juvet T, Finelli A. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. European urology. 2015 Dec:68(6):1007-13. doi: 10.1016/j.eururo.2015.04.004. Epub 2015 Apr 18 [PubMed PMID: 25900781]
Ginzburg S, Uzzo R, Al-Saleem T, Dulaimi E, Walton J, Corcoran A, Plimack E, Mehrazin R, Tomaszewski J, Viterbo R, Chen DY, Greenberg R, Smaldone M, Kutikov A. Coexisting hybrid malignancy in a solitary sporadic solid benign renal mass: implications for treating patients following renal biopsy. The Journal of urology. 2014 Feb:191(2):296-300. doi: 10.1016/j.juro.2013.07.059. Epub 2013 Jul 27 [PubMed PMID: 23899990]
Level 2 (mid-level) evidenceTurner MW, Hutchinson TA, Barré PE, Prichard S, Jothy S. A prospective study on the impact of the renal biopsy in clinical management. Clinical nephrology. 1986 Nov:26(5):217-21 [PubMed PMID: 3802585]