Introduction
Erythrocytes, red blood cells (RBC), are the functional components of blood responsible for transporting gases and nutrients throughout the human body. Their unique shape and composition allow for these specialized cells to carry out their essential functions. The role of the erythrocyte is critical in investigating many disease processes in a variety of body systems. Their structure, function, physiology, preparation, microscopy, and clinical importance are the subject of this review article.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
To evaluate the structure, function, and role of the red blood cell in humans and the human disease process.
Structure
The mature erythrocyte has a biconcave, discoid shape and is anucleated.[1] This design allows for the flexibility needed to navigate the cardiovascular system and for an increased surface area which supports sufficient gas exchange and permits the cell to carry out its function. A phospholipid bilayer membrane frames the structure of this unique cell and is maintained by a network of proteins that make up the cytoskeleton. This cytoskeleton is composed of spectrin, actin, band 3, protein 4.1, and ankyrin—which allows for cellular structural integrity as well as malleability. The interactions between these compounds support a structurally sound yet pliable structure.[1][2]
Function
Each red blood cell only lives for about 120 days. In that short time, it must deliver oxygen from the lungs to the peripheral tissues to assist in metabolic processes such as adenosine triphosphate (ATP) synthesis, and it must collect the generated carbon dioxide from the periphery and return it to the lungs for elimination from the body. The deoxygenated blood that arrives at the lungs contains hemoglobin with ferrous heme (Fe) with an affinity for oxygen. Upon arrival to the deoxygenated tissues, the decreased partial pressure of oxygen and low pH cause the heme to lose its affinity for the oxygen, delivering it to the tissue. Carbon dioxide is then taken into the cell and combined with water to form bicarbonate and hydrogen via carbonic anhydrase. Most of the carbon dioxide will travel back to the lungs in the form of bicarbonate and be exhaled.[2]
Tissue Preparation
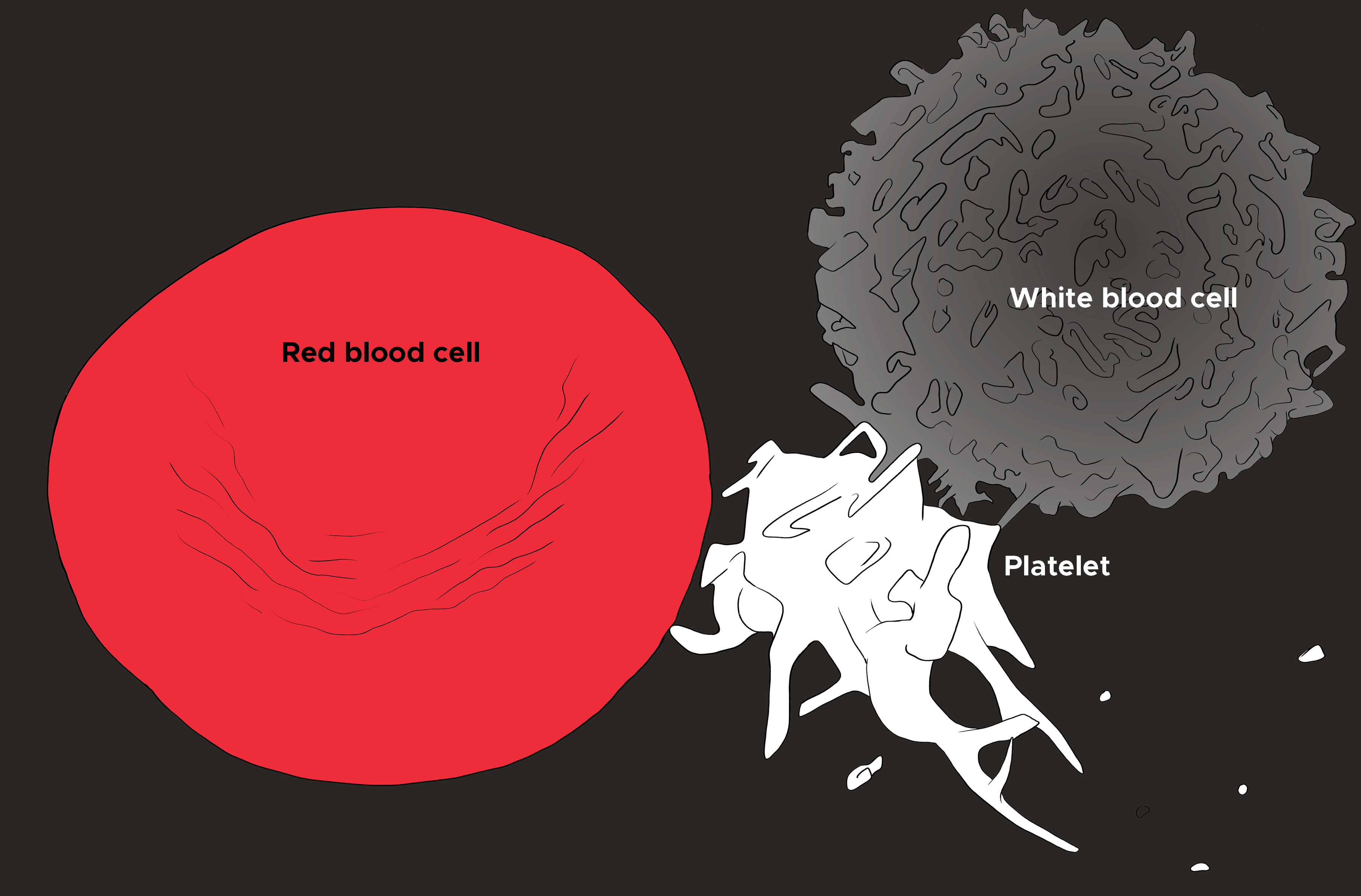
A peripheral blood smear is a standard way to evaluate the red blood cells. Often, it is ordered to confirm the findings on a complete blood count (CBC), evaluate the white blood cells and platelets, and finally analyze the erythrocyte shape, size, color, arrangements, and inclusion bodies (see Image. Red Blood Cell, Platelet, and White Blood Cell). Blood samples are obtained from peripheral veins and stored in a bottle with an anticoagulant solution, usually ethylene diamine tetra-acetic acid (EDTA), and should undergo testing within 2 hours. A sample of blood is deposited on a glass side with a pipette or capillary tube, and a spreader is used to smear the blood. The slide is then dried, labeled, and stained with any additional stains deemed necessary.[3]
Histochemistry and Cytochemistry
Red blood cells can be visualized via light microscopy and electron microscopy, each revealing specific details of the erythrocyte structure. Below are descriptions of how both light microscopy and electron microscopy contribute to the understanding of red blood cells. Histochemical stains have also played a role in identifying key components within a red blood cell, assisting in disease diagnosis. The hallmark stain for histological evaluation of erythrocytes is hematoxylin and eosin. Other stains, though, have allowed for more specific pathologic findings to be identified on light microscopy. For example, Giemsa stains have been utilized in the histology of red blood cells for the identification of the plasmodium of malaria and are useful in the diagnosis of lymphoma.[4] Also, Wright stains can be used to visualize Howell-Jolly bodies, a hallmark in hyposplenism, erythroblastosis, myelodysplasia, megaloblastic anemia, and post-chemotherapy. Heinz bodies can be visualized with supravital or Heinz stains.[5]
Microscopy, Light
Light microscopy of a peripheral blood smear is a mainstay of hematologic evaluation and diagnosis. A typical erythrocyte will be circular in shape with a central area of pallor due to its biconcave shape. When evaluating the erythrocyte size, it can be described as macrocytic (large), microcytic (small), or normocytic. Anisocytosis means a variation in size amongst the red blood cells on a single slide. Color can undergo evaluation as hyperchromic or hypochromic. Anisochromia refers to a variance in the amount of central pallor amongst a group of RBCs. Polychromasia is a variety of colors among the erythrocytes. Red blood cell agglutination means that the cells are clumped together in a cluster, and rouleaux refers to the erythrocytes arranged in a linear formation. The shape can vary drastically with many different forms of red blood cells described in the literature. An ancanthocyte or a spur cell has irregular projections of varying size and distance from either other.
A bite cell has a semi-circular indentation as if someone took a bite from it. Echinocytes are erythrocytes with uniform projections all equidistant apart. Schistocyte is a term that refers to an RBC fragment that has been sheared. A sickle cell is an RBC with a crescentic shape. Spherocytes are erythrocytes that have no central pallor and are uniformly red throughout. Stomatocytes have a linear central clearing rather than a circular one. Target cells have a red center with a central clearing. Teardrop cells taper on one end. One last group of erythrocyte descriptors evaluated with light microscopy is inclusion bodies. These are cytoplasmic or nuclear findings that give clinical clues. For example, Heinz bodies are small round masses seen with supravital or Heinz stains that indicate hemolysis. Howell-Jolly bodies are solid, large, round masses found in the hemoglobin of the cell, are visualized with Wright-stain, and have many clinical significances. Pappenheimer bodies are multiple, small blue inclusion bodies throughout the entire RBC, indicating iron overload, hyposplenism, or myelodysplasia. Finally, basophilic stippling is blue/purple granules seen in thalassemia, lead poisoning, and other pathologies.[5]
Microscopy, Electron
Studies have indicated that electron microscopy of the erythrocyte reveals clear details, such as the cytoskeleton network and pits and vacuoles on the surface of the cells.[6][7] Such findings would be difficult, if not impossible, to view with light microscopy. The observed vacuoles contained ferritin, hemoglobin, membranes, and remnants of mitochondria. Some of these were fused with the cell membrane, possibly indicating a method by which the cell could rid of its wastes.[7] Additionally, the cytoskeleton study has explicitly outlined the general organization of the infrastructure. The article describes several filamentous structures that take convoluted pathways and eventually join end-to-end to form a network, which serves as the basic unit that continuously repeats, ultimately building this complex interwoven and secure shape.[6]
Pathophysiology
Erythrocytes are very sensitive to their surroundings, changing shape and reacting to their environment. In an ideal situation, the erythrocyte exists as a biconcave disc. When exposed to certain chemicals or compounds, the cell morphs in response. It has been proposed that this happens under two conditions: when the environment changes the phospholipid bilayer of the erythrocyte or when exposed to oxidants in the environment. For example, when red blood cells are depleted of their energy source, adenosine triphosphate (ATP), or when there is increased intracellular calcium, the cell develops an echinocyte shape. Also, when an erythrocyte swells with water, it becomes a stomatocyte. These changes occur because of how these situations manipulate the lipid bilayer membrane.
Additionally, the red blood cell has an efficient way to convert hydrogen peroxide to water to prevent its protein degradation and lipid peroxidation abilities. In certain inherited conditions, the red blood cell is void of the enzymes needed to carry out this function and, therefore, suffers from the oxidative stress of its environment. This situation could lead to the formation of Heinz bodies or denatured hemoglobin, such as in patients with glucose-6-phosphate dehydrogenase deficiency. Other oxidative changes to the red blood cell occur in blood bank storage. There are reports that the ability of spectrin to bind actin with protein 4.1 during red blood cell storage decreases due to oxidative changes and loss of phospholipids in the in vitro environment.[1]
Clinical Significance
Erythrocyte size, distribution, and shape variation can all be clues to clinical disease and pathologic processes. For example, acanthocytes may be a byproduct of eryptosis or organized erythrocyte breakdown and death. This process of tabulated cell destruction has been seen in anemia and excess calcium. Agglutination of red blood cells can be an indication of a hypercoagulable state, and microcytosis or small red blood cells can be associated with different forms of microcytic anemia, such as iron deficiency and thalassemia.[8] Additionally, red blood cell morphology has a significant role in the severity of the disease. For example, in sickle cell disease, the concentration of inherited hemoglobin S determines the degree of erythrocyte sickling.
These cells are seen in peripheral blood smears and result in many of the clinical symptoms of the disease, such as vaso-occlusive crisis, leading to pain.[9] Researchers have also noted in the literature that erythrocytes are sensitive to pro-inflammatory states and physiologic changes. Different inflammatory disease states such as (ie, systemic lupus erythematosus) display a large percentage of non-discoid or atypical erythrocyte shapes that were reversible with inflammatory product chelation. This additional finding suggests erythrocyte vulnerability to inflammation and oxidative stress and their significance in chronic inflammatory disease.[8] Additionally, red blood cells and their evaluation can give information about a patient’s general health and what physiologic processes are occurring in their body. Finding nucleated red blood cells in the blood may indicate hemolysis, hemorrhage, or hypoxia and may be involved in leukemias and other cancers.[10]
Media
(Click Image to Enlarge)
References
Smith JE. Erythrocyte membrane: structure, function, and pathophysiology. Veterinary pathology. 1987 Nov:24(6):471-6 [PubMed PMID: 3331858]
Level 3 (low-level) evidenceKuhn V, Diederich L, Keller TCS 4th, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M, Cortese-Krott MM. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxidants & redox signaling. 2017 May 1:26(13):718-742. doi: 10.1089/ars.2016.6954. Epub 2017 Jan 18 [PubMed PMID: 27889956]
Adewoyin AS, Nwogoh B. Peripheral blood film - a review. Annals of Ibadan postgraduate medicine. 2014 Dec:12(2):71-9 [PubMed PMID: 25960697]
Barcia JJ. The Giemsa stain: its history and applications. International journal of surgical pathology. 2007 Jul:15(3):292-6 [PubMed PMID: 17652540]
Ford J. Red blood cell morphology. International journal of laboratory hematology. 2013 Jun:35(3):351-7. doi: 10.1111/ijlh.12082. Epub 2013 Mar 9 [PubMed PMID: 23480230]
Tsukita S, Tsukita S, Ishikawa H, Sato S, Nakao M. Electron microscopic study of reassociation of spectrin and actin with the human erythrocyte membrane. The Journal of cell biology. 1981 Jul:90(1):70-7 [PubMed PMID: 6894761]
Schnitzer B, Rucknagel DL, Spencer HH, Aikawa M. Erythrocytes: pits and vacuoles as seen with transmission and scanning electron microscopy. Science (New York, N.Y.). 1971 Jul 16:173(3993):251-2 [PubMed PMID: 4325866]
Pretorius E, Olumuyiwa-Akeredolu OO, Mbotwe S, Bester J. Erythrocytes and their role as health indicator: Using structure in a patient-orientated precision medicine approach. Blood reviews. 2016 Jul:30(4):263-74. doi: 10.1016/j.blre.2016.01.001. Epub 2016 Feb 2 [PubMed PMID: 26878812]
Azar S, Wong TE. Sickle Cell Disease: A Brief Update. The Medical clinics of North America. 2017 Mar:101(2):375-393. doi: 10.1016/j.mcna.2016.09.009. Epub 2016 Dec 14 [PubMed PMID: 28189177]
Walker HK, Hall WD, Hurst JW, Lynch EC. Peripheral Blood Smear. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250106]