Introduction
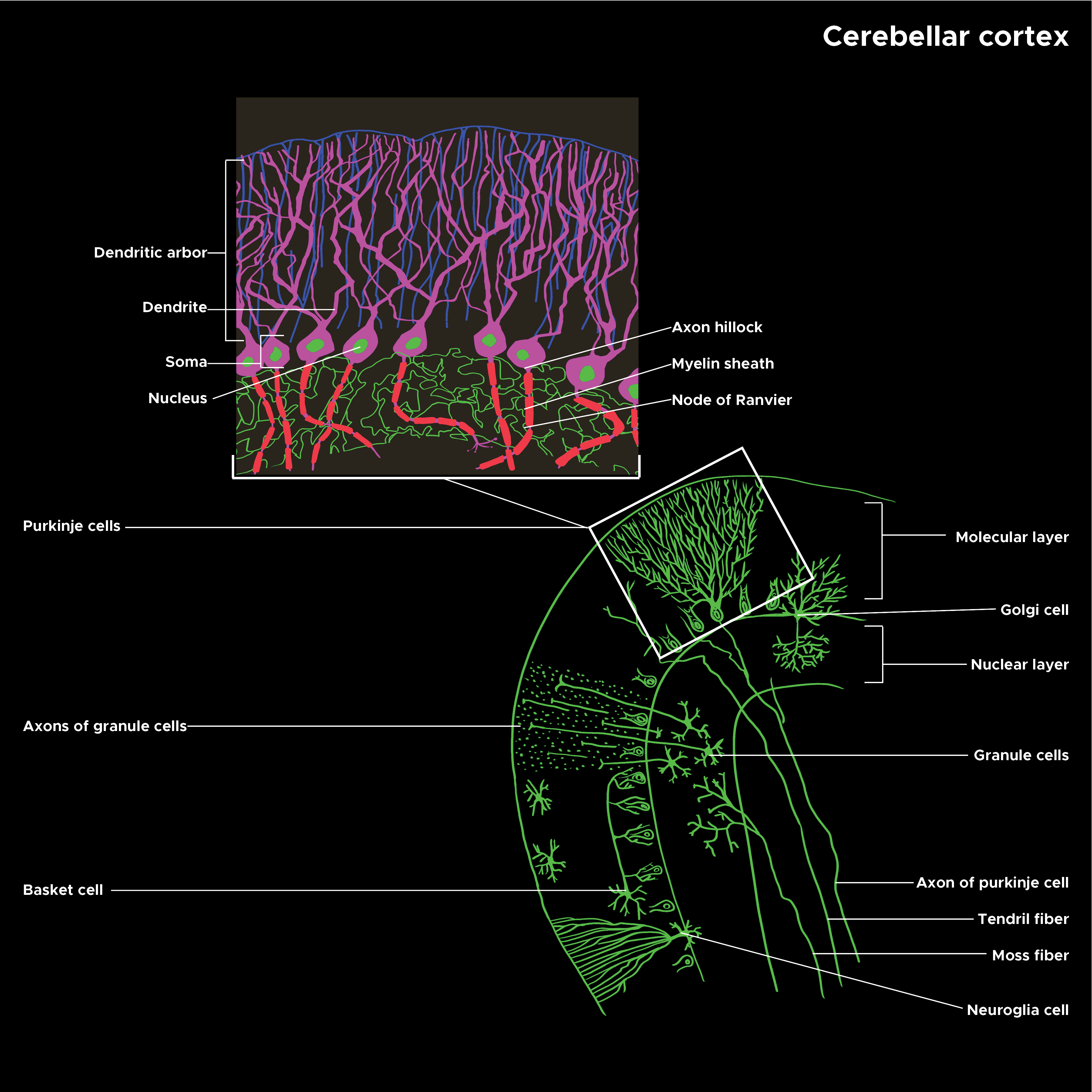
Purkinje cells are a unique type of neuron-specific to the cerebellar cortex. They are remarkable (and instantly recognizable) for their massive, intricately branched, flat dendritic trees, which give them the ability to integrate large amounts of information and learn by remodeling their dendrites. As an important part of the cerebellar circuits, Purkinje cells are necessary for well-coordinated movement and other areas of function, such as cognition and emotion. See Image. Illustration of Cerebellar Cortex Structure.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Purkinje cells start differentiating in early development. Experiments in mice have shown that they begin as a collection of precursor cells in the cerebellar nuclei, which then migrate outward to the cortex. The migrating cells are thought to climb radial glia as directed by chemical signals in the cortex. However, they also may communicate during the migration, as the immature Purkinje cells have been found to synapse with each other while traveling. These synapses then disappear after the migration is complete.[1] The precursor cells start as several hundred “clusters” of cells, which migrate to the same cerebellar regions and serve a single purpose.
After the Purkinje cell layer has formed, the surrounding granule cells, whose axons make up the parallel fiber inputs to the Purkinje cells, also migrate through the Purkinje cell layer to the inner granule cell layer, where they remain in the adult. The parallel fibers and climbing fibers from the inferior olive synapse on the developing Purkinje cells influence their development; obliterating either cell line results in a dendritic tree of lower-than-normal complexity.[2][3][4] Climbing fibers, in particular, develop in association with their Purkinje cell targets. Initially, multiple climbing fibers weakly innervate any given Purkinje cell; however, as development progresses, the climbing fiber that manages to make the most excitatory connections with the Purkinje cell becomes stronger through long-term potentiation of its synapses, while the other fibers become downregulated through long-term depression.[1] At the end of the process, with one climbing fiber remaining, the Purkinje cell’s dendrites will also be restricted to a single plane.[3] Though Purkinje cell dendrite formation may depend on synaptic inputs, the migration can occur independently of these inputs.[3][4][5]
Different neurotransmitters and receptors are essential in Purkinje cell development, although the exact sequence of their use and whether they are excitatory or inhibitory is still under investigation. The N-methyl-D-aspartate glutamate receptor is particularly important as a potential mediator for the long-term potentiation and depression that is so important for dendritic tree remodeling in early development and throughout life; the receptor subunits that express during development have different properties from those expressed in adults. GABA, the neurotransmitter released by Purkinje cells, is also thought to affect the cells during development.[1]
Structure
Microscopically, Purkinje cells have a large, flat, highly branched dendritic tree and a single long axon that forms an inhibitory projection to the cerebellar nuclei. The plane of the dendritic tree is perpendicular to the folds in the cerebellar cortex, with parallel fibers passing through the distal dendritic trees of a large number of Purkinje cells forming weak synapses. A single climbing fiber, as noted above, makes a few hundred synapses to the soma and proximal dendrites. Any given climbing fiber is one of about ten axons from an inferior olivary cell, so the same input is distributed among multiple Purkinje cells.[6] In the 3-layered cerebellar cortex, the Purkinje cells’ bodies make up the middle Purkinje cell layer, while their dendritic trees with the parallel fibers and some of the inhibitory cells, such as basket cells, make up the outer molecular layer.
Function
Though every Purkinje cell is part of the same relatively simple circuit described above, the collective actions of these circuits are many and varied. The most definite evidence is for a major role of the cerebellar circuit in motor coordination, more precisely defined as the ability to fine-tune and course-correct a movement in progress. The large dendritic trees of the Purkinje cell are thought to be critical to this process; they receive complicated inputs from parallel fibers and integrate them into a signal, which represents what the Purkinje cell “thinks” the current motion should be. The climbing fibers are thought to represent an “error signal” that can override or modulate the signal. The entire output then gets routed via the deep cerebellar nuclei to the ventrolateral nucleus of the thalamus, where it feeds into the motor cortex’s ongoing actions and hopefully smooths any error out of the motion. Signals from parallel and climbing fibers route to overlapping areas of the cortex, and Purkinje cell output routes to different areas of the motor cortex.[6][7] As such, the information encoded in this circuit is spread out over large areas of both the cerebellum and cerebrum.
Throughout life, Purkinje cells continue to undergo the processes of long-term potentiation and depression that helped to shape their dendritic trees during embryonic development. Since long-term potentiation and long-term depression are dependent on the simultaneous firing of the synapse and the outgoing axon, those parallel fiber inputs that are most likely to be correlated with their Purkinje cell firing become strengthened over time. This process also contributes to motor coordination, as the correlation between the inputs and the effect of the Purkinje cell on motor activity becomes more refined over time.[6][5] The motor function of the Purkinje cell circuits is the best known and studied because injuries to the cerebellum typically result in motor coordination issues such as ataxia without loss of strength. However, there is some evidence that the cerebellum participates in other important cognitive functions, including language and emotion. The mechanism for its effects on these functions is possibly similar to the error-correction mechanism for motor coordination since they share the same basic circuits.[8]
Histochemistry and Cytochemistry
Numerous molecular markers can be used to label and quantify Purkinje cells. One specific one is L7/Pcp2, a G-protein signaling component only found in Purkinje cell dendrites and proximal axons. Calbindin D28K is also specific to Purkinje cells in the cerebellum. Other reasonably specific markers include pCD6, Pep19/Pcp4, and glutamic acid decarboxylase 67.[9] A particularly interesting marker is zebrin-II, so named because staining a whole cerebellum with it results in a very stereotypical striped pattern with strong conservation between individuals and species. The zebrin stripes appear to correlate with different functional units of the cerebellum, and indeed, the expression of several other genes exactly correlates with zebrin expression. Zebrin-II positive cells also appear to be slightly more resilient to various insults to the Purkinje cells.[10][8]
Clinical Significance
The effects of diseases that affect Purkinje cells are generally the same, although some disease processes have additional findings in other systems. Usually, such patients have ataxia, depending on which areas of the cerebellum are affected.[1] They also show a corresponding cell loss in the inferior olive and the granular layer of the cerebellar cortex, which provide inputs to the Purkinje cells, and losses in the deep cerebellar nuclei, which receive their outputs.[2][10] In the early stages of cell loss, the Purkinje cell somata show a characteristic swelling and darkening; after some time, the dead cells are replaced with reactive Bergman glia, a type of astrocyte found in the cerebellar cortex.[11]
Various insults are specifically harmful to Purkinje cells, often through excessive glutamatergic stimulation and neurotoxicity or changes in calcium metabolism. Global cerebral hypoxia is explicitly known to harm Purkinje cells early in the course. A variety of genetic conditions also affect Purkinje cells, notably the spinocerebellar ataxias, a family of trinucleotide repeat disorders that cause a buildup of proteins in the nucleus. Phenytoin, lithium, and Purkinje cell protein (PCP) increase Purkinje cell death, and seizure disorders also increase Purkinje toxicity by glutamate excitotoxicity. Ethanol specifically causes anterior degeneration of Purkinje cells in the adult and apoptosis in the fetus. Many of these toxins take longer to kill cells positive for zebrin-II, the marker mentioned above, due to some combination of increased defenses against free radicals and expression of other proteins that decrease their metabolic effects.[10]
Media
(Click Image to Enlarge)
References
van Welie I, Smith IT, Watt AJ. The metamorphosis of the developing cerebellar microcircuit. Current opinion in neurobiology. 2011 Apr:21(2):245-53. doi: 10.1016/j.conb.2011.01.009. Epub 2011 Feb 24 [PubMed PMID: 21353528]
Level 3 (low-level) evidenceDesclin JC. Histological evidence supporting the inferior olive as the major source of cerebellar climbing fibers in the rat. Brain research. 1974 Sep 13:77(3):365-84 [PubMed PMID: 4136782]
Level 3 (low-level) evidenceKaneko M, Yamaguchi K, Eiraku M, Sato M, Takata N, Kiyohara Y, Mishina M, Hirase H, Hashikawa T, Kengaku M. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PloS one. 2011:6(5):e20108. doi: 10.1371/journal.pone.0020108. Epub 2011 May 31 [PubMed PMID: 21655286]
Level 3 (low-level) evidenceBradley P, Berry M. The effects of reduced climbing and parallel fibre input on Purkinje cell dendritic growth. Brain research. 1976 Jun 4:109(1):133-51 [PubMed PMID: 1276906]
Level 3 (low-level) evidenceSotelo C, Alvarado-Mallart RM. The reconstruction of cerebellar circuits. Trends in neurosciences. 1991 Aug:14(8):350-5 [PubMed PMID: 1721740]
Level 3 (low-level) evidenceEccles JC. Circuits in the cerebellar control of movement. Proceedings of the National Academy of Sciences of the United States of America. 1967 Jul:58(1):336-43 [PubMed PMID: 5231614]
Palkovits M, Mezey E, Hámori J, Szentágothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Experimental brain research. 1977 May 23:28(1-2):189-209 [PubMed PMID: 881003]
Level 3 (low-level) evidenceBeckinghausen J, Sillitoe RV. Insights into cerebellar development and connectivity. Neuroscience letters. 2019 Jan 1:688():2-13. doi: 10.1016/j.neulet.2018.05.013. Epub 2018 May 7 [PubMed PMID: 29746896]
Level 3 (low-level) evidenceSimons MJ, Pellionisz AJ. Genomics, morphogenesis and biophysics: triangulation of Purkinje cell development. Cerebellum (London, England). 2006:5(1):27-35 [PubMed PMID: 16527761]
Level 3 (low-level) evidenceSarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Progress in neurobiology. 2003 Aug:70(6):473-507 [PubMed PMID: 14568361]
Level 3 (low-level) evidenceGarman RH. Histology of the central nervous system. Toxicologic pathology. 2011 Jan:39(1):22-35. doi: 10.1177/0192623310389621. Epub 2010 Nov 30 [PubMed PMID: 21119051]