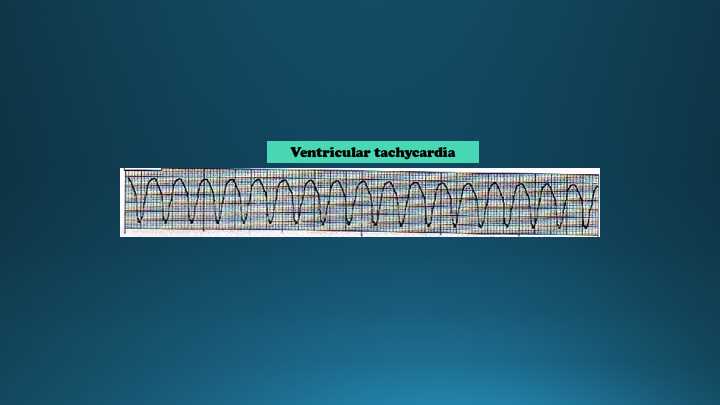
Introduction
Pulseless ventricular tachycardia is a life-threatening cardiac arrhythmia in which coordinated ventricular contractions are replaced by very rapid but ineffective contractions, leading to insufficient organ perfusion and heart failure. Pulseless ventricular tachycardia is a medical emergency.
Due to rapid ventricular contractions, the ventricular filling decreases markedly, leading to a dramatic decrease in cardiac output. As a result, a pulse is absent. Electrophysiology identifying factors for pulseless ventricular tachycardia include; tachycardia (>100 bpm), wide QRS complexes (> 120 milliseconds), atrioventricular (AV) dissociation, presence of fusion or capture beats and an electrical axis between -90 to -180. There are several scoring systems to differentiate ventricular tachycardia from a wide complex supraventricular tachycardia, but 90% of wide complex tachycardia cases will be ventricular tachycardia.
The ventricular arrhythmia can be characterized as either monomorphic (no variation of the QRS from beat to beat) or polymorphic (changing or multiform QRS from beat to beat). The most common cause of pulseless ventricular tachycardia is cardiac ischemia.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pulseless ventricular tachycardia (VT) can result from a multitude of causes and predisposing conditions, including but not limited to, structural heart disease, electrolyte disturbances, drugs/medications, and congenital/inherited channelopathies.[1] The most common cause of ventricular tachycardia (including pulseless VT) is concomitant structural heart disease.
Below are several examples organized into the following categories:
Structural Heart Disease:
- coronary artery disease
- aortic stenosis
- cardiomyopathy
- congestive heart failure
- hypertrophic obstructive cardiomyopathy (HOCM)
- myocardial infarction (MI)
Electrolyte Disturbances:
- hypokalemia
- hyperkalemia
- hypomagnesemia
- hypocalcemia
Channelopathies:
- Lange-Nielsen Syndrome (prolonged QT with deafness)
- Romano-Ward Syndrome (prolonged QT without deafness)
- Brugada Syndrome
Electrophysiologic Phenomena:
- Wolf-Parkinson-White Syndrome (WPW)
- Catecholamine Sensitive Polymorphic Ventricular Tachycardia
Drugs/medications that cause QT prolongation:
- Clarithromycin
- Erythromycin
- Metoclopramide
- Haloperidol
- Methadone
- Droperidol
- Fluoroquinolone antibiotics (i.e., ciprofloxacin)
- Antiemetics (i.e., ondansetron)
- Antiarrhythmics
Other causes:
- Hypovolemia
- Hypoxia
- Acidosis
- Hypothermia
- Tension pneumothorax
- Cardiac tamponade
- Pulmonary embolism
Epidemiology
Ventricular tachycardia (VT) remains a significant contributor to most sudden cardiac deaths in the U.S., at an estimated rate of approximately 300000 deaths per year.[2][3] The majority of these deaths occur in adults greater than 35 years of age, with at least half attributable to ventricular arrhythmias. Estimates of its prevalence vary from 30% to 75% of out-of-hospital cardiac arrests despite recent increases in the incidence of pulseless electrical activity.[2] However, the true incidence is unknown due to inevitable degeneration to asystole if unwitnessed. Despite the decline in coronary artery disease mortality and advancements in resuscitation services, survival from these events remains low, especially if occurring outside the hospital environment, with an associated survival rate of less than 10%.[4]
Pathophysiology
Electrophysiological mechanisms of pulseless ventricular tachycardia are increased automaticity and triggered activity. Electrical impulses originating in ventricular myocardium spread through ischemic myocardium or scar tissue resulting in retrograde atria activation or AV dissociation.
In pulseless ventricular tachycardia, the ventricles contract at a rate too rapid to allow for an adequate filling time during diastole, subsequently resulting in hemodynamic collapse from a diminished cardiac output causing insufficient blood supply to end organs. Abnormal ventricular conduction and asynchrony further reduce the effectiveness of ventricular contractions and aggravate the hemodynamics, leading to sudden collapse. There are two main types of ventricular tachycardia with the morphology of the QRS complexes serving as clues to the cause responsible for the rhythm.
In monomorphic ventricular tachycardia, which is more common, the QRS complexes are identical because there is increased automaticity originating from a single focus in either of the ventricles or a re-entry circuit within the ventricle. The most common cause for monomorphic ventricular tachycardia is scarring of the myocardium from myocardial infarction, which promotes the development of an electrical circuit around the non-conducting fibrotic tissue resulting in the formation of multiple circuits that potentially can evolve into a re-entrant ventricular tachyarrhythmia.[5]
In polymorphic ventricular tachycardia, the QRS complexes vary in morphology and usually result from inherent genetic mutations that disrupt the ion channel function of cardiac myocytes and cause electrophysiological abnormalities during ventricular repolarization.[6] Congenital conditions predisposing to polymorphic ventricular tachycardia usually exhibit prolongation of the QT interval on the EKG. Acquired etiologies that may lead to the development of polymorphic ventricular tachycardia also include drug toxicity of antiarrhythmic drugs, antibiotics, and antipsychotic medications that prolong the QT interval.
History and Physical
Before the event, patients with pulseless ventricular tachycardia may complain of chest pain, palpitations, dyspnea, light-headedness, and syncope. On physical examination, there will be signs of impaired perfusion, hypotension, tachypnea, elevated jugular venous pressure, and S1 heart sound. Sudden collapse results from ineffective ventricular contractions and low cardiac output.
At the time of the event, patients with pulseless ventricular tachycardia are unconscious and unresponsive without a palpable pulse. Any delay in starting defibrillation dramatically reduces survival, and death may occur in a few minutes.
Evaluation
The diagnosis of pulseless VT is based on physical examination and ECG. Cardiopulmonary resuscitation, according to Advanced Cardiac Life Support guidelines, should be started immediately as far as the diagnosis is confirmed. ECG findings usually include regular R-R intervals, rapid ventricular rate with an undistinguishable atrial rate (absence of p-waves), AV dissociation, and a wide QRS complex (more than 0.12 sec).[7]
Several ECG criteria have been created to help diagnose VT.[8] These criteria include:
The Classical Criteria:
- AV dissociation
- Capture or fusion beats
- Negative or positive concordance
- Right bundle branch configuration
- QRS width >140 ms and left axis
- QR, R, or RSr' complex in V1
- RS >1 in V6 or QS in V6
- Left bundle branch configuration
- QRS width >160 ms and right axis
- Initial R in V1 >30 ms
- Slurring or notching of the downstroke of the S-wave in V1-2
- Begin QRS-nadir S-wave in V1-2
- Any Q in V6
Brugada Criteria:
- Absence of an RS complex in all precordial leads
- The longest R to S interval >100 ms in any precordial lead
- AV dissociation
- Classical criteria for both VT present in both lead V1-2 and V6
If any single one of the above Brugada criteria is present, then VT is diagnosed.
The Vereckei Criteria:
- The presence of AV dissociation
- In lead aVR (any one of the following):
- The presence of an initial R-wave
- Initial R or Q > 40ms
- Notch in the descending limb of a negative onset and predominantly negative QRS
- Vi/Vt ≤ 1
In general, per the AHA, basic criteria to support the diagnosis of VT includes [9]:
- AV dissociation
- QRS complex >0.14 seconds
- Monophasic R wave in aVR
- Specific QRS morphologies (i.e., positively or negatively concordant QRS complex in precordial leads)
- The absence of an RS complex in all precordial leads
- An RS interval >100 ms in at least 1 precordial lead
Treatment / Management
Pulseless ventricular tachycardia is a medical emergency, and its management should follow advanced life support guidelines.[7] Pulseless VT requires immediate electrical cardioversion with high-energy defibrillator (150-200 J on biphasic and 360 J on monophasic).[10] Delaying defibrillation for 2 minutes or more decrease survival rate compared with patients received immediate defibrillation (39,3% vs. 22,2%).[11](B2)
In a series of shocks, each following shock should be higher in energy than the previous shock. After the first shock delivered, 5 cycles of CPR should be performed. Each CPR cycle should contain 30 chest compressions followed by 2 breaths.[11][7] Cardiopulmonary resuscitation is performed only until the defibrillator is ready. Defibrillation requires fewer joules if it is done early. After every shock, chest compressions should be performed, along with oxygen delivery and intravenous injection of vasopressors and antiarrhythmic drugs.[7][12](B2)
After the conversion to sinus rhythm, the patient should be monitored continuously. Metabolic acidosis quickly follows cardiovascular collapse. If the arrhythmia is terminated within 30 to 60 seconds, significant acidosis does not occur. If a patient is unresponsive after return to a normal rhythm or the event happened out-of-hospital, hypothermia cooling to 32-34 degrees Celcius for 24 hours should be initiated for possible neurologic recovery.[7]
In every case of pulseless VT acute myocardial infarction should be suspected and urgent coronary angiography and PCI should be considered if feasible.[10][13] Fibrinolytic therapy during CPR for pulseless VT is not recommended.(A1)
Medical treatment of pulseless VT usually is carried out along with defibrillation and includes intravenous vasopressors and antiarrhythmic drugs. 1 mg of epinephrine IV should be given every 3 to 5 minutes. Epinephrine can be replaced by vasopressin given 40 units IV once.[14] Amiodarone is the most studied drug and is used for the prevention of sudden cardiac death (SCD). Sotalol is the most widely used alternative but is associated with an increased risk of SCD due to decreasing defibrillation threshold. Patients on beta-blockers reported having lower mortality rates.(A1)
For primary prevention of SCD in patients from the high-risk group, as well as for secondary prevention in patients who already have a prior history of sustained VT or VF, ICD implantation is recommended.[15][16] Implantable cardiac defibrillators (ICDs) have benefits in terms of mortality and survival rates compared to medical treatment.(A1)
Differential Diagnosis
The following signs speak in favor of ventricular tachycardia: QRS complex greater than 0.14 seconds with right bundle branch morphology or greater than 0.16 seconds with left bundle branch morphology, RS interval > 100 ms in a precordial lead, AV dissociation, negative QRS concordance in the precordial leads and ventricular fusion beats.[12]
If the patient’s condition allows, differential diagnosis of a wide-complex tachycardia should be made between ventricular tachycardia (80% of wide-QRS tachycardia cases), supraventricular tachycardia (SVT) with aberrant conduction (15% to 20%) and SVT with pre-excitation and antidromic atrioventricular reentrant tachycardia (AVRT) (1% to 5%).[16] Every wide complex tachycardia should be considered as VT until proven otherwise.[17][18]
Prognosis
Prognosis of pulseless ventricular tachycardia heavily depends on the time from the onset of tachycardia to defibrillation and restoration of sinus rhythm and efficient perfusion. Shorter delays in defibrillation associated with higher survival rates, up to 50%. Delays in treatment up to 15 minutes decrease the survival rates to as low as 5%.[18] The prognosis also depends on a patient's cardiac status, including underlying ischemic or structural heart disease, as well as other comorbidities.
Complications
Pusless ventricular tachycardia can lead to cardiac arrest and insufficient organ perfusion, which is the main cause of most complications. Anoxic brain injury and lifelong neurological complications can result from tachycardia that lasts for greater than 3 minutes.[19] Anoxic brain injury is one of the multiple pathophysiological conditions that can occur in post-cardiac arrest syndrome.
Other complications of the post-cardiac arrest syndrome include post-cardiac arrest myocardial dysfunction and systemic ischemia/reperfusion response. Myocardial dysfunction from ischemia can lead to transient global dysfunction and is a common cause of early mortality after cardiac arrest.[20] Reperfusion after ischemia causes activation of immunologic and coagulation pathways, which increases the risk of multiple organ failure and infections. The ischemic-reperfusion injury also depletes intravascular volume and impairs vasoregulation and oxygen delivery to organ tissues.[20] Treatment for post-cardiac arrest syndrome includes multiple areas of treatment measures, including hemodynamic monitoring, airway management, and neurological assessments.
Deterrence and Patient Education
Pulseless ventricular tachycardia is most commonly due to cardiac ischemia. Patients with ischemic heart disease should be educated on lifestyle changes such as low cholesterol and low salt diet and regular exercise. Patients who smoke should be encouraged to quit smoking. Patients should be sure to establish with a cardiologist for follow up and for proper medical management to ensure the underlying cause is being properly treated and to prevent reoccurrence. In patients where the cause of ventricular tachycardia was due to medication side effects, proper medication education for all patients is essential.
Pearls and Other Issues
Pearls and Key Issues
- Pulseless VT is a medical emergency that requires immediate defibrillation.
- The energy of 150-200 J on biphasic and 360 J on monophasic defibrillator should be used.
- Delaying defibrillation of pulseless VT dramatically decreases the survival rate.
- Critical interventions in the post-arrest phase are coronary angiography and PCI.
- Implantation of ICD is indicated both for primary and secondary prevention of VT/V-fib.
Enhancing Healthcare Team Outcomes
Pulseless ventricular tachycardia is a life-threatening arrhythmia. Early recognition and prompt cardiopulmonary resuscitation with defibrillation can be life-saving and may also prevent lifelong complications. Interprofessional healthcare teams usually include nurses, physicians, mid-level providers, anesthesia, respiratory therapists and pharmacists. Effective communication between all members of the healthcare team is essential for early initiation of life-saving measures in patients who develop pulseless ventricular tachycardia. Each minute in treatment delay decreases the chance of survival by approximately 10%.[21] Continuing medical education keeps providers and medical staff up to date on The American Heart Association ACLS protocol, and all healthcare team members need to be certified and remain current. In persons who recover, prompt evaluation by a cardiologist is essential for the prevention of recurrent arrhythmias and complications. [Level 1]
A board-certified cardiology pharmacist will play a vital role in these cases, given the nature of the drugs involved. They will consult with the cardiology team on agent selection, appropriate dosing, check for interactions, and provide direction to nursing staff on adverse events. Nursing will monitor the patient after assisting in the emergency procedures upon arrival at the healthcare facility. These interprofessional actions will increase survival in cases of pulseless VT. [Level 5]
Bystander CPR and public access to a defibrillator have helped increase the survival rate of out of hospital cardiac arrest, although survival remains under 10%.[22] Once the patient has arrived at the hospital, temperature control, and cardio-cerebral resuscitation measure further improve overall survival.[22] [Level 1]
Regardless of the location, early recognition and prompt initiation of advanced cardiac life support protocol are crucial elements to survival.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
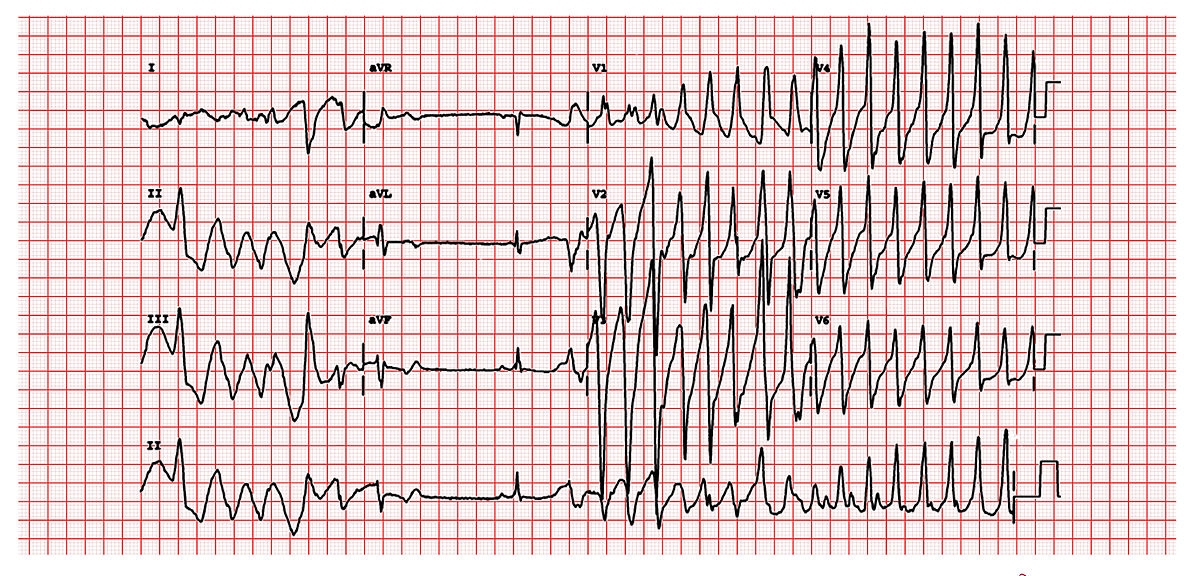
This is a classic 12 lead EKG of a patient with Torsade de Pointes. It shows the polymorphic nature of the tachycardia, the long QT interval and the initiation of the tachycardia with a late coupled P.V.C. Contributed by Michael Rosengarten BEng, MD.McGill (CC BY-SA 3.0 https://creativecommons.org/licenses/by-sa/3.0/deed.en)
References
Baldzizhar A, Manuylova E, Marchenko R, Kryvalap Y, Carey MG. Ventricular Tachycardias: Characteristics and Management. Critical care nursing clinics of North America. 2016 Sep:28(3):317-29. doi: 10.1016/j.cnc.2016.04.004. Epub 2016 Jun 22 [PubMed PMID: 27484660]
Tang PT, Shenasa M, Boyle NG. Ventricular Arrhythmias and Sudden Cardiac Death. Cardiac electrophysiology clinics. 2017 Dec:9(4):693-708. doi: 10.1016/j.ccep.2017.08.004. Epub [PubMed PMID: 29173411]
McNally B, Robb R, Mehta M, Vellano K, Valderrama AL, Yoon PW, Sasson C, Crouch A, Perez AB, Merritt R, Kellermann A, Centers for Disease Control and Prevention. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002). 2011 Jul 29:60(8):1-19 [PubMed PMID: 21796098]
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association and the Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. European heart journal. 2006 Sep:27(17):2099-140 [PubMed PMID: 16923744]
Level 1 (high-level) evidenceJohn RM, Tedrow UB, Koplan BA, Albert CM, Epstein LM, Sweeney MO, Miller AL, Michaud GF, Stevenson WG. Ventricular arrhythmias and sudden cardiac death. Lancet (London, England). 2012 Oct 27:380(9852):1520-9. doi: 10.1016/S0140-6736(12)61413-5. Epub [PubMed PMID: 23101719]
Staikou C, Chondrogiannis K, Mani A. Perioperative management of hereditary arrhythmogenic syndromes. British journal of anaesthesia. 2012 May:108(5):730-44. doi: 10.1093/bja/aes105. Epub [PubMed PMID: 22499746]
Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer JM, Kleinman ME, Kronick SL, Lavonas EJ, Link MS, Mancini ME, Morrison LJ, O'Connor RE, Samson RA, Schexnayder SM, Singletary EM, Sinz EH, Travers AH, Wyckoff MH, Hazinski MF. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015 Nov 3:132(18 Suppl 2):S315-67. doi: 10.1161/CIR.0000000000000252. Epub [PubMed PMID: 26472989]
Alzand BS, Crijns HJ. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011 Apr:13(4):465-72. doi: 10.1093/europace/euq430. Epub 2010 Dec 3 [PubMed PMID: 21131372]
Dalia AA, Essandoh M, Cronin B, Hussain N, Gerstein NS, Schulman P. A Narrative Review for Anesthesiologists of the 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Journal of cardiothoracic and vascular anesthesia. 2019 Jun:33(6):1722-1730. doi: 10.1053/j.jvca.2019.01.004. Epub 2019 Jan 4 [PubMed PMID: 30685157]
Level 3 (low-level) evidenceInternational Liaison Committee on Resuscitation. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 8: Interdisciplinary topics. Resuscitation. 2005 Nov-Dec:67(2-3):305-14 [PubMed PMID: 16324994]
Level 3 (low-level) evidenceChan PS, Krumholz HM, Nichol G, Nallamothu BK, American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. The New England journal of medicine. 2008 Jan 3:358(1):9-17. doi: 10.1056/NEJMoa0706467. Epub [PubMed PMID: 18172170]
Level 2 (mid-level) evidencePerkins GD, Handley AJ, Koster RW, Castrén M, Smyth MA, Olasveengen T, Monsieurs KG, Raffay V, Gräsner JT, Wenzel V, Ristagno G, Soar J, Adult basic life support and automated external defibrillation section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015 Oct:95():81-99. doi: 10.1016/j.resuscitation.2015.07.015. Epub 2015 Oct 15 [PubMed PMID: 26477420]
Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, European Society of Cardiology Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, EACTS Clinical Guidelines Committee, Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Zamorano JL, Task Force on Myocardial Revascularization of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery, European Association of Percutaneous Cardiovascular Interventions. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014 Oct:46(4):517-92. doi: 10.1093/ejcts/ezu366. Epub 2014 Aug 29 [PubMed PMID: 25173601]
Level 1 (high-level) evidenceEuropean Heart Rhythm Association, Heart Rhythm Society, Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL, American College of Cardiology, American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). Journal of the American College of Cardiology. 2006 Aug 15:48(4):854-906 [PubMed PMID: 16904574]
Level 1 (high-level) evidenceCallaway CW. Epinephrine for cardiac arrest. Current opinion in cardiology. 2013 Jan:28(1):36-42. doi: 10.1097/HCO.0b013e32835b0979. Epub [PubMed PMID: 23196774]
Level 3 (low-level) evidenceKusumoto FM, Bailey KR, Chaouki AS, Deshmukh AJ, Gautam S, Kim RJ, Kramer DB, Lambrakos LK, Nasser NH, Sorajja D. Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart rhythm. 2018 Oct:15(10):e253-e274. doi: 10.1016/j.hrthm.2017.10.037. Epub 2017 Nov 4 [PubMed PMID: 29097318]
Level 1 (high-level) evidenceBrugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991 May:83(5):1649-59 [PubMed PMID: 2022022]
Holmberg M, Holmberg S, Herlitz J. Incidence, duration and survival of ventricular fibrillation in out-of-hospital cardiac arrest patients in sweden. Resuscitation. 2000 Mar:44(1):7-17 [PubMed PMID: 10699695]
Kang JY, Kim YJ, Shin YJ, Huh JW, Hong SB, Kim WY. Association Between Time to Defibrillation and Neurologic Outcome in Patients With In-Hospital Cardiac Arrest. The American journal of the medical sciences. 2019 Aug:358(2):143-148. doi: 10.1016/j.amjms.2019.05.003. Epub 2019 May 21 [PubMed PMID: 31200920]
Kang Y. Management of post-cardiac arrest syndrome. Acute and critical care. 2019 Aug:34(3):173-178. doi: 10.4266/acc.2019.00654. Epub 2019 Aug 31 [PubMed PMID: 31723926]
Ali B, Zafari AM. Narrative review: cardiopulmonary resuscitation and emergency cardiovascular care: review of the current guidelines. Annals of internal medicine. 2007 Aug 7:147(3):171-9 [PubMed PMID: 17679705]
Level 3 (low-level) evidencevan Diepen S, Girotra S, Abella BS, Becker LB, Bobrow BJ, Chan PS, Fahrenbruch C, Granger CB, Jollis JG, McNally B, White L, Yannopoulos D, Rea TD. Multistate 5-Year Initiative to Improve Care for Out-of-Hospital Cardiac Arrest: Primary Results From the HeartRescue Project. Journal of the American Heart Association. 2017 Sep 22:6(9):. doi: 10.1161/JAHA.117.005716. Epub 2017 Sep 22 [PubMed PMID: 28939711]