Introduction
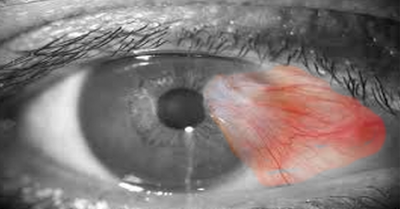
Pterygium is one of the common ocular surface disorders. From two Greek words, the word "pterygium" has been derived: (pteryx) meaning wing and (pterygion) meaning fin. Sushruta was the first to describe it in 1000 BC, the first recorded ophthalmic surgeon.[1] Pterygium is basically a fibrovascular overgrowth of the subconjunctival tissue, triangular in shape, and encroaching on to the cornea in the medial and lateral palpebral fissure.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The various known risk factors are immune mechanism, genetic predisposition, and chronic environmental irritation, which include UV (ultraviolet) rays, hot and dry weather, wind, dusty atmosphere, and the period of exposure to such conditions. However, the most common is the increased time of exposure to UV rays of the sunlight, followed by chronic eye irritation from dry and dusty conditions.[2]
Epidemiology
The prevalence rates are different in various parts of the world. It is highest in the “pterygium belt” described by Cameron, which lies between 37° north and south of the equator. The prevalence of pterygium was stated to vary widely from 0.3 to 29 percent in the world. In India, the prevalence ranges from 9.5 to 13%.[3] It is more commonly found in rural parts of the country.[4] The prevalence of pterygium in Barbados was 23.4% of the black population, 23.7% of the mixed Black and White population, and 10.2% of the White population.[5] Other countries have different prevalence, like 10.1% in Singapore, 30.8% in Japan, 14.49% in a Tibetan population in China, and 17.9% in the elderly Mongolian population at Henan county, China.[6][7][8][7][6][9]
The predominance of pterygia on the nasal side in the interpalpebral zone is speculated to result from the following mechanisms:
- Light passes through the cornea medially, concentrating on the nasal limbus region, while the nose shadow decreases the strength of light transmitted to the temporal limbus.
- Longer temporal upper lid eyelashes and outer two-third bowing of these lashes filtering light falling on temporal conjunctiva and cornea.
- The natural flow of tears is from the temporal to the nasal side towards the punctum, and any amount of dust that reaches the conjunctival sac irritates the nasal conjunctive further.
- Since there are two anterior ciliary arteries on the nasal side while there is only one on the temporal side, any irritant may result in increased nasal hyperemia.
Males work outdoors much longer than females, so it has been shown that pterygium is found more often in males compared to females.[10]
Pathophysiology
UV rays cause the insufficiency of the limbal stem cells of the cornea. It causes activation of the tissue growth factors, which further lead to angiogenesis and cell proliferation. The limbal stem cells are damaged by the UV rays that cause conjunctivalization of the cornea, and the cornea is invaded by aggressive fibroblasts. UV radiation may cause mutations in the p53 tumor suppressor gene, resulting in the abnormal pterygial epithelium.[11][12]
Recent studies have indicated that human papillomavirus could also be involved in the pathogenesis of the pterygium.[13] The latest studies have shown that human papillomavirus can also engage in the pathogenesis of pterygium. Such findings indicate that pterygium is not simply a degenerative lesion but can arise from unregulated cell proliferation. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) at the pterygium's advancing edge may be responsible for inflammation, tissue remodeling, Bowman's layer degradation, and corneal pterygium invasion.[14]
It was also hypothesized that a pterygium might represent a region with a localized limbal stem cell deficiency, resulting in a conjunctive invasion of the adjacent cornea.[15]
Recurrent Pterygium Pathophysiology
Pathophysiology of recurrence is the reactivation of the inflammatory process, which is present in the primary form. Sometimes the surgical trauma serves as an enhancer of the inflammatory response. Proliferative cytokines and growth factors (including vascular endothelial growth factor or VEGF) can increase after surgery if the limbal stem cells remain activated, and fibroelastic tissue is also involved.[16] Because of this, there is an acceleration of fibrovascular proliferation and an increase in metalloproteinase synthesis that destroys the Bowman membrane and the stromal collagen that may increase the progress of pterygium.
Histopathology
Microscopically, pterygium is considered to be composed of elastotic fibro-vascular tissue. Leaving the top, mostly, the pterygium is covered by conjunctival epithelium. Extensions of fibrous tissue are seen on the top of pterygium, and as the head encroaches on to the cornea, the Bowman’s layer gets involved and is fragmented. The fibrous connective tissue fills these cavities. Histopathological hallmarks of chronic inflammation can also be seen. There is the presence of lymphocytic infiltration consisting of plasma cells, mastocytes, and T-lymphocytes.[17] There may be an increase of newly formed blood vessels, degenerative collagen fibers, and abnormal elastic fibers. The pathognomonic feature in histopathology is elastotic degeneration of conjunctival stroma, and also, there is an excessive fibro-proliferative reaction.
History and Physical
Patients often present with the symptoms of irritation in the eye, lacrimation, foreign body sensation, cosmetic blemish, and various functional problems, which include diminution of vision and difficulty in the fitting of contact lens.
Detailed history including age, sex, occupation, exposure to irritants like sun, smoke, and dust should be taken. Any previous treatment history and family history of pterygium are to be asked. General physical examination and systemic examination to exclude the presence of collagen-vascular or mucocutaneous disorders should be carried out.
History should be taken to classify the pterygium into (a) primary pterygium (b) recurrent pterygium.
Old textbooks define malignant pterygium as a recurrent pterygium with symblepharon formation and restrictions of ocular movements. However, pterygium is a non-malignant condition and must be differentiated from malignant conditions like ocular surface squamous neoplasia (OSSN) by clinical features, impression cytology, and/or histopathology.
Evaluation
Comprehensive ocular examination, including visual acuity, extraocular movements (EOM), and anterior segment evaluation is to be done. Detailed refraction should be done to note down the amount and type of astigmatism.
Dry eye should be ruled out by conducting Schirmer’s test or tear film break-up time.
Pterygium should be evaluated under the following criteria: location, size, vascularity, extent, and area of corneal involvement [chord at limbus and extent of corneal encroachment from limbus is measured using Castroviejo’s calipers] can be carried out by oblique illumination by torchlight and further substantiated by slit-lamp examination. The presence or absence of Stocker’s line is noted.
A pterygium has three parts:
- Cap - At the leading edge, which is distinguished by a halo-like avascular zone
- Head - peripheral to the cap
- Body - the main part of the pterygium communicating with the bulbar conjunctiva
Pterygium may be classified into:
- Progressive: thick, fleshy, vascular, progressively encroaching towards the center of the cornea.
- Atrophic: Thin, attenuated, poor vascularity, stationary
Treatment / Management
There is a lack of agreement among ophthalmologists on optimal pterygia management in both medical and surgical terms. Medical care is given to relieve the patient from the inflammatory symptoms and reduce the persistent discomfort that persists. Throughout the years, medical treatment for pterygium made use of relatively benign agents such as bile and urine and more toxic agents such as lead-acid, mercuric lanolin, radiotherapy, thiotepa, 5-fluorouracil, and more recently, mitomycin C in a search for a safe and effective therapy.
Medical treatment in modern times includes lubrication with artificial tear drops or decongestants to provide short-term comfort and a slight improvement in cosmetics. Topical NSAIDs, eye drop loteprednol, brings added comfort. Vasoconstrictive agents minimize redness and enhance the appearance and add antihistamines to the decongestant drops to help prevent the effect of histamine associated edema and itching.[18](A1)
However, surgical treatment remains the preferred option. Operation with pterygium was mentioned as far back as 1000 B.C. In patients with pterygium, the reasons for surgery are decreased vision due to visual axis encroachment, chronic pain, persistent inflammation, abnormal astigmatism, restrictive ocular motility, and cosmesis.
The patient was prepared to lie recumbent on an operating table during Susruta's surgical operation, and the pterygium was loosened and irritated by sprinkling powdered salt into the eye.[19] Then, the pterygium was fomented with the hand, heated by the finger rubbing. The patient was asked to look laterally, a sharp hook was used to hold the growth at its loosened upturned section and was held up with a toothed forceps, or a threaded needle was passed from below the thread-holding section. Then the pterygium was removed by scratching it with a sharp round-top tool. The pterygium root was pushed to the medial canthus from the black outline (cornea) of the eye, then excised and removed.
Few examples of past surgeries include Celsus's procedure (Rome—50 A.D.), which used a thread with a sawing motion to separate the pterygium from the ocular tissues toward the pupil. Paul's operation (Paulus Aegineta, Eye Surgeon, Greece, 7th century) used a small hook to seize the pterygium.
Many surgical techniques have been used since past to present, though none is universally accepted because of variable recurrence rates. In most of the studies, it has been seen that 90% recurrences occur between the first and the third month.[19] The reason for recurrence may be surgery related or due to the patient and environmental conditions. Surgery related causes may be due to incomplete primary surgery, which includes incomplete removal of the affected tissue, presence of fibrotic tissue debris left in the cornea and limbus, presence of sclera-corneal rough surface with irregularities, the tension in conjunctival suture edges, conjunctival edge dehiscence or poorly controlled inflammatory reaction. The patient and environment-related causes include male patients, age less than 40 years, patients of Asian, African, American and Hispanic origin, continuous exposure to dry and dusty environment, and presence of a dry eye syndrome.
Pterygium surgery has always remained a concern for the operating surgeon because of its recurrence and cosmesis post-surgery.
Grading of the cosmetic results after pterygium surgery was done in the study conducted by Prabhasawat et al. which is as follows:[20](B3)
- Grade 1: Appearance of the operated site is not different from the normal appearance
- Grade 2: Some fine episcleral vessels are seen in the excised area extending up to but not beyond the limbus and without any fibrous tissue
- Grade 3: Additional fibrous tissues are seen in the excised area that does not invade the cornea
- Grade 4: A true recurrence with fibrovascular tissue invading the cornea is seen
Hirst, in his study, graded the cosmetic results post-surgery into 6 grades, which is as follows:[21](A1)
Normal: Eye, which is quiet and white with no abnormal vasculature, conjunctival changes, or corneal opacities.
Excellent: Eye in which a white opacity in the cornea is visible.
Good: Eye in which there may or may not be a white corneal opacity, but where the eye exhibits some conjunctival vascular injection, not only nasally at the possible pterygium site but also temporally, suggesting nonsurgical attribution.
Fair: Eye in which conjunctival vascular changes i.e., injection or plexus, is present only at the nasal surgical site.
Poor: Eye in which there are not only nasal conjunctival vascular abnormalities but also a spectrum of other conjunctival changes, from simple scarring to puckering nasally.
Upgradeable: Eye, where the nasal area is out of focus, making a true grading difficult.
Regardless of the problem of recurrence of pterygium, various surgical methods have been described for the removal.
Avulsion Technique[22]
The bulbar conjunctiva at the edge of the scleral portion of pterygium is incised with Westcott scissors, and this portion is freed from the underlying sclera by blunt dissection. The freed portion of the pterygium is then grasped with toothed forceps and torn from the cornea, and a second forceps grasps the perilimbal tissue away to give counter traction. Residual tissue is scraped from the - corneal surface with a beaver blade, and the surface is then polished with a diamond burr. The recurrence rate (23% to 75%) is similar to other techniques for primary removal.[23](B3)
Simple excision technique has a recurrence rate of 30 percent to 100 percent.[24][25]
The Bare Sclera Technique
This method was described by D'Ombrain in 1948.[26] It involves excising the head and body of the pterygium while allowing the bare scleral bed to re-epithelialize. Dissection from the corneal side and the uninvolved perimeter of normal conjunctiva is done. The cornea is left as smooth as possible. All of Tenon's capsule from beneath and pterygium is excised. Bare scleral excision can be started by an incision around the conjunctival body of the pterygium or from the corneal apex.[27] High recurrence rates, between 24 percent and 89 percent, have been documented in various reports. Additional methods were used to overcome the problem of recurrence, which included 5-FU, thiotepa, intraoperative mitomycin C, and postoperative mitomycin C. They yield more successful results. In the study conducted by Kareem et al., the recurrence rate was 32% when applied with bare sclera technique, 8% when applied along with intraoperative mitomycin C and 18% when applied along with intraoperative 5-FU.[28]
Verma et al. reported the recurrence rate as 48% when applied together with the bare sclera technique on the cases with recurrent pterygium and around 3% with the use of intraoperative mitomycin C.[17] The study of Yanyaliet al.[29] showed that the recurrence rate was 57.8% when applied with bare sclera technique and 21% when applied with intraoperative mitomycin C; whereas, Demirok et al. found this rate to be 40% along with the bare sclera technique and 5.9% when applied with intraoperative mitomycin C.[30](A1)
In the 1960s, radiotherapy with strontium 90 application was used commonly and did reduce the recurrence rate to a range of 6.4% to approximately 12%. However, it was not realized until decades later that there was an unacceptable rate of scleromalacia of 13%, pain, and even secondary endophthalmitis. Although these published series do not seem to favor bare sclera resection alone, this technique is still in use both for therapeutic and research purposes. It is difficult to rationalize the use of these agents for a disease that can be successfully treated with extremely low complication rates with a conjunctival autograft when the reason for their use is speed and simplicity only.
Primary Closure[31]
Head and mid-body of the pterygium are excised. It is followed by a tenonectomy. Tenonectomy is extended beneath the conjunctiva to the adjacent rectus muscle. Relaxing conjunctival incisions are created around the limbus, both superiorly and inferiorly. The conjunctiva is then primarily and carefully closed. The uncommon recurrences (2.1 percent) happened only in 2 cases of wound contamination and in 15 cases of wound dehiscence.[31]
Transplantation of the Head of the Pterygium
The hypothesis is that the pterygium's head is the source of its development. In the procedure defined by Mc Reynolds,[32] the conjunctiva along the lower margin of the body is incised after separating the head of the pterygium from the cornea, and it is weakened above and below the incision, forming a cul-de-sac towards the lower fornix. The pterygium's body is made to run more vertically, rather than horizontally. The blood vessels of the body are tightly extended over the sclera to obliterate with time and cover the pterygium with the lower cul-de-sac conjunctive. The pterygium's new course is structured such that the raw region at the limbus, created by removal of the head and back, is covered by conjunctiva. However, high recurrence rates were found with this procedure.
Stocker described a method of conjunctival z-plasty for primary closure.[33] Wilson and Bourne[33] described an alternate type of z-plasty consists of placing a flap of normal tissue between the body of the pterygium and the corneal limbus. According to the authors, normal tissue acts as a barrier to the regrowth of the pterygium and preserves the superior bulbar conjunctiva for use in a conjunctival autograft procedure in the event of a recurrence.
Conjunctival Autograft
It was first done by Kenyon et al. in 1985.[34] In this method, a conjunctival autograft is taken from the upper temporal part of the bulbar conjunctiva within the same eye or in the other eye. This autograft is sutured on the location of the pterygium tissue excised. In the study conducted by Kenyon et al., the recurrence rate was reported to be at a lower rate, like 5.3%. Lewallen et al. reported the recurrence rate, as 21% in the tropical region.[34] The study conducted by Ti et al., showed that the recurrence rate in the cases with primary pterygium was 20.8% and 31.2% in the cases with recurrent pterygium.[35] Conjunctival autograft technique requires experience, and the time of surgery is a bit more. Various studies showed that the duration of the operation could be shortened by using fibrin glue, and patient comfort during the postoperative period is better when compared with the surgery performed using the suture.[36](A1)
The use of autologous blood fibrin can be used as an alternative as fibrin glue is costly, and it has the risk of causing infection. Autologous blood fibrin is a synthetic tissue adhesive prepared from a donor plasma, and it was first described for pterygia surgery by Cohen in 1993.[37] Successful results have also been reported concerning the use of autologous blood fibrin in conjunctival grafting procedures.[37](B3)
Complications seen with this technique are corneoscleral dellen, graft edema, epithelial cysts, suture granuloma, flap retraction, and necrosis.
Conjunctival Rotational Autograft (CRA)
Spaeth first described the advantage of CRA over other graft techniques, namely the preservation of normal conjunctiva in other non-affected areas.[38] In this 90-degree rotation, autograft of excised pterygial tissue was done and re-sutured into its bed. Later, various modifications of the technique were done. Haider et al. evaluated the safety, efficacy, and recurrence following 90 degrees versus zero degrees and 180 degrees rotation autograft for treating primary pterygium and reported that all techniques were effective and 90 degrees rotation autograft was safe and an easy procedure to perform for the management of pterygium with a lower recurrence rate compared with zero and 180 degrees rotation autograft.[39] Alp et al. evaluated the safety and efficacy of CRA and reported a recurrence rate of 16.6% in a mean follow-up period of 15.9 months. There were no intraoperative and postoperative complications and concluded that the 180-degree CRA technique was effective and safe in preventing the recurrence of primary pterygium.[40](B2)
Limbal Conjunctival Autotransplant (LCAT)
It is probably the most common procedure to be combined with bare scleral pterygium removal, where the bare area is covered with conjunctiva removed from another area of the bulbar surface.
Limbal conjunctival autograft contains approximately 0.5 mm of limbal and peripheral corneal tissue. A better anatomic and functional result will be achieved by including limbus epithelium in the conjunctival graft, and thus, the barrier function in the limbus will have been ensured once again, and the pterygium recurrence will be minimized. [41] The recurrence rate of limbal autograft varies between 0 and 15%. Malik et al. reported the recurrence rate after limbal conjunctival autograft as 2.5%,[42], whereas Al Fayez et al. reported it as 1% [43] and Masters et al. reported it as 2.14%.[44] (A1)
- The superior conjunctival autotransplant has been used widely since the early 1980s and has been associated with a recurrence rate of approximately 2% to 12%, very few complications, and none associated with loss of all vision but is a lengthier and more complex procedure.[45]
- The inferior conjunctival autotransplants result in 3.3% to 5.25% recurrence rates with either no recurrence or regrowth of more than 1.5 mm onto the cornea. [46] (B2)
The important causes for recurrence after LCAT are cross-graft recurrence, outflanking, reproliferation of previously unexcised pterygial tissue, and transformation of the epithelial graft into the pterygial tissue.
Limbal Stem Cells Transplantation[21] (A1)
This procedure seems likely to enable the epithelialization of the peripheral cornea, the limbus, and also the neighboring denuded sclera by the corneal epithelium. Maybe the limbal region can then benefit from circumferential migration of limbal cells. Limbal stem cells are not needed to preserve a phenotypically stable limbus until the underlying tissue threatens it.[41]
Amniotic Membrane Grafting
It is also used to avoid the recurrence of pterygium. However, the exact mechanism which confers its beneficial effect on the amniotic membrane has not yet been established. Due to their anti-inflammatory properties, their encouragement of epithelial development, and their suppression of the transforming growth factor-beta (TGF-beta), these grafts are believed to facilitate healing and minimize recurrence levels and fibroblast proliferation. The amniotic membrane controls the cytokine rates and growth factors. It presents benefits in wide-area lesions or in situations such as wounds in which conjunctival grafts can not be done, and even in patients for which no transplant can be conducted afterward in terms of glaucoma surgery. Unfortunately, levels of recurrence differ greatly between current research, somewhere between 2.6 percent and 10.7 percent for primary pterygia and as high as 37.5 percent for recurrent pterygia.[47] Researchers typically pair the amniotic membrane graft with mitomycin. A comprehensive tenonectomy is done. A variable amount of postoperative steroid injections is given. This helps in obtaining the low recurrence risk.
Postulating that the reduced risk of recurrence could be attributed to mitomycin or tenonectomy, and not the amniotic membrane, would appear reasonable. A distinct value of this procedure, though, is the protection of the bulbar conjunctiva.[48](B2)
Lamellar or Penetrating Keratoplasty
In cases of recurrent pterygium, there is significant residual scarring and thinning of the cornea. In these scenarios, tissue from the lamellar corneal graft can be used. It helps remove thin, or scarred corneal parts. Corneal trephine (normally 8 mm) is used after superficial excision to incise the limbus to a depth of 0.3 mm. The host bed is then dissected, and then a smooth surface is created. The defect is filled with a 0.4 mm thick fresh donor button, sutured in place with interrupted 10-0 nylon. In the study carried out by Laughrea PA and Arentsen JJ, recurrence occurred in three of ten cases.[49](B2)
Adjuvant Treatments
To prevent a recurrence, these treatments are performed along with surgical treatments to prevent the recurrence. They can be combined with all the surgical methods.
Cautery
Since the growth of the blood vessels at the operation site leads to the recurrence of a pterygium, some people have recommended the intensive use of intraoperative cautery, particularly at the limbus, to improve the pterygium's surgical removal. With this approach, however, there is no data available about the risk of recurrence. Excessive cautery can trigger the development of hypertrophic scar tissue, scleral thinning, and necrosis.[50]
Laser Therapy
The use of the Argon laser may be done in postoperative cases. 50µm spots are applied to early neovascular fronds to minimize conjunctival epithelial damage.[25] The recurrence rate is around 12%.[25] Complications such as scleral necrosis, scleromalacia, secondary iritis, and cataract may be seen.
The excimer laser was used to ablate irregularities in the bare scleral surface after pterygium excision. The recurrence rate was 91% (20 cases) at 1 year.[25]
MMC (Mitomycin C)
Because of its potential to suppress fibroblasts, it was used as an adjunctive therapy. MMC is an alkylating agent, which prevents the synthesis of DNA. By inhibiting DNA synthesis, this results in the death of cells triggered by the failure to fix the alkylation-caused genotoxic injury. DNA synthesis inhibition results in a reduction in the number of mitoses, particularly when MMC comes into contact with cells that are in the cell cycle's late G1 and early S stages. It can be administered locally or in the form of eye drops before or after pterygium surgery. The delivery of the injection directly on the pterygium has the advantage of preserving the corneal endothelium and epithelium.
Subconjunctival injection enables a more effective dosage to be administered to the patient's eye. The application of sponges directly on the sclera during surgery with MMC doesn't have the same efficacy. Its action to prevent the recurrence of pterygium occurs by inhibition of fibroblast proliferation in the episcleral region. The minimum acceptable and successful dosing rates, however, are yet to be established. MMC may be used in different ways, including the intraoperative application of MMC directly to the scleral bed following excision with pterygium, and postoperative use of topical MMC eye drops. Kunimoto et al. originally used MMC for the first time as a topical treatment postoperatively, but it caused detrimental complications.[51]
Topical MMC use after pterygium surgery was popularized in the USA by Singh et al.[52] The risk of recurrence was about 2.3%. But after installation of 0.04 percent MMC postoperatively, Rubinfeld et al. documented problems such as scleral thinning, corneal edema, secondary glaucoma, corneal perforation, iritis, and cataract development.[53] High MMC concentrations (0.04 percent 3 to 4 times daily for 7 days) result in a substantial reduction in pterygium recurrence relative to bare sclera excision.[53] (B3)
Thiotepa
It is an alkylating agent comparable to nitrogen mustard. It inhibits mitosis and division within the rapidly multiplying tissues. After excising the pterygium, it is used for 6–8 weeks in a frequency of four times a day, in the form of a drop in 0.05 % concentration. The recurrence rates have been reported to be between 0 and 28 %. [54] Ngoy et al. [55]reported a recurrence rate by 28% with the use of thiotepa, whereas and Wu et al. reported the recurrence rates as 10 %.[56] Thiotepa has many side effects, which include black pigment deposits on the eyelids and hyperpigmentation of the skin as well as allergic reactions and local irritations.(A1)
Fluorouracil
This is a pyrimidine analog that interferes with deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) synthesis and inhibits fibroblast proliferation, thus acting as an anti-metabolite. [57] Akarsu et al. reported recurrence by 25 %. [58](A1)
Topical Monoclonal Antibodies Against Vascular Endothelial Growth Factor (anti-VEGF)
More recently, the use of topical monoclonal antibodies against vascular endothelial growth factors (anti-VEGF) has been advocated as an adjunctive therapy postoperatively, either in drop form or as sub-conjunctival injections. VEGF level in the pterygium tissue was found to be higher than normal conjunctiva. This suggests the fact that the use of anti-VEGF drugs during the treatment could be effective. Ranibizumab and bevacizumab, as the anti-VEGF drugs, are applied to the eye. In a meta-analysis, topical bevacizumab was relatively safe, associated only with an increased risk of the subconjunctival hemorrhage, but it had no significant effect on preventing pterygium recurrence.[59] The role of such agents as a primary therapy without adjunctive surgery is equivocal.[59](A1)
Radiotherapy
High-energy, beta-emission radioactive substances are used in this technique. The radiation suppresses fibroblast proliferation. Ruthenium 106 and Sr 90 are more commonly used as radioactive substances. Radiotherapy is one of the oldest methods used as adjuvant therapy in the treatment of pterygium. In this technique, after the excision of pterygium, the radioactive plaque is sutured on the sclera and is kept there until the desired dose is reached. The radiotherapy can be given in different regimens and dosages as described in the literature. The recurrence rates reported in the literature vary between 0 and 50 %.[60] In this practice, various serious complications such as cataract, scleral necrosis, corneal perforation, and endophthalmitis may develop. Due to the high cost of these techniques, risks, and difficulty in application, this method is not widely used. [61](B2)
Tenon's Layer Excision
In the 1980s, Barraquer introduced the concept that removal of Tenon's layer may be important in reducing the recurrence rate after pterygium removal.[62] This also was emphasized by Solomon et al., who combined this technique with mitomycin and amniotic membrane to achieve low recurrence rates.[63] Most studies do not record the size of the pterygium, although a study by Tan et al. did attempt to correlate the morphologic features of the pterygium to recurrence rates.[64] However, the obscuration of the episcleral vessels by the pterygium, which is the measure adopted by Tan et al., is an indirect measure of the thickness of the Tenon's layer underlying the pterygium body.[64](A1)
In the currently described method of excision, this area of Tenon's layer is excised no matter what it is thickness.[64] Further studies are required to evaluate if extensive Tenon's layer removal, to up to 10 mm beyond the conjunctival edges superiorly and inferiorly, is required to prevent recurrence when the grading is T1. Studies of growth factor and angiogenesis modulation in vitro of pterygial fibroblasts may explain in part why extensive Tenon's layer removal may inhibit the regrowth of pterygia.[24] It has been seen that the fibroblasts may be stimulated to produce hyaluronate or can be activated by growth factors to cause scarring after glaucoma procedures.[25] These studies may cast some light on why the extensive removal of the Tenon's layer for approximately 10 mm beyond the conjunctival edges, and the long-term and high-dose of topical steroid use in the P.E.R.F.E.C.T. for PTERYGIUM technique, results in a low recurrence rate with this procedure.(A1)
P.E.R.F.E.C.T. [pterygium extended removal followed by extended conjunctival transplantation]
This methodology produces an exceptional outcome, with a recurrence rate close to zero and a reasonable complication rate, according to Lawrence W. Hirst. [65] P.E.R.F.E.C.T. results in no conjunctival recurrences. A pure white appearance in the region of the nasal bulbar area with no macroscopically noticeable suture line is seen. This is presumably rendered feasible by a variety of surgical considerations. The surgery consists of removal of the conjunctiva and Tenon, including the semilunar fold nasally. Superiorly and inferiority, the conjunctiva, and Tenon were removed almost to the superior and inferior recti, leaving the bare sclera. The medial and lateral recti were bridled for moving the eye. The superotemporal conjunctival autograft was dissected, leaving a frill near the limbs. The dissection of conjunctiva from the underlying Tenon was done by injecting a balanced salt solution just beneath the conjunctiva. This autograft was sutured at the area of bare sclera with the margin of the conjunctival host area and sclera using interrupted 9-0 polyglactin. The para-caruncular area was repaired using a running suture.[66]
There is tacking of the graft to the sclera without regrowth of the layer of Tenon or vascularization under the graft. There is limited concealment of the upper and lower wounds under the lids. It is possible because of the large size of the graft and intensive postoperative steroid administration. [65] In 20 eyes, a prospective intervention series was conducted and was followed up for 1 year or more with recurrence and cosmetic appearance examination. The first pterygium to be removed was generally the larger of the two, and this was preceded at least 6 to 12 months later by the other pterygium. Hirst chose 6 months as the early slit-lamp changes visible in the upper bulbar conjunctive had disappeared within 6 months, leaving a conjunctival surface which could not be distinguished from the normal conjunctiva. No recurrences have been recorded. Cosmesis has been rated as normal or excellent by all patients. In 19 of the eyes, the superior bulbar conjunctiva from which the 2 grafts were obtained revealed no scarring, while 1 eye had a central scar.
P.E.R.F.A.M.T. (pterygium extended removal followed by amniotic membrane transplantation)[67]
H. Liu et al. conducted extensive removal of pterygium with thorough removal of Tenon's layer as in P.E.R.F.E.C.T. [67]After the application of mitomycin C, amniotic membrane transplantation aided by conjunctival autograft fibrin was inserted instead. At a median follow-up of 12 months, it had a recurrence rate of 6.89 percent. Additionally, P.E.R.F.A.M.T. was also associated with a number of complications, including postoperative pyogenic granuloma, transient diplopia, permanent motility restriction, steroid-induced glaucoma, fat prolapse, subamniotic membrane hemorrhage, and early detachment of amniotic membrane. However, the only advantage over the P.E.R.F.E.C.T. technique is the use of a reduced number of sutures or no sutures and the avoidance of complicated manipulation around the caruncular region.[67]
Allam WA published a study in 2016 on recurrence and complications of P.E.R.F.E.C.T. for primary pterygia in Europe.[68] His methodology differs in that he used limbal tissue with the donor graft. However, Hirst had left the donor site with limbal tissue, and also 1 to 2 mm of the perilimbal conjunctiva.No recurrences have been recorded. One case, however, developed tenon granuloma 2 weeks after surgery at the donor site. Transient graft swelling was seen in 22 percent of cases, and transient diplopia was seen in 17.6 percent of cases.[68]
In 2017, Cornelius carried out an independent analysis of the efficacy of P.E.R.F.E.C.T. for pterygium removal in avoiding recurrence after pterygium surgery.[69] Using this procedure, sixty consecutive cases of primary pterygium excision were conducted. Cornelius used a retrobulbar rather than a peribulbar anesthetic. Instead of the medial rectus and lateral rectal fixation sutures, limbal bridle sutures were used. There was no suture passing into the lateral rectum. Subconjunctival lidocaine was used in lieu of BSS at the donor site to extract the graft. The semilunar fold was not often excised in this study, although, in every case, it was removed in Hirst's series. This step shift made the procedure less offensive than the first. The grafts were actually smaller in this study than Hirst's. There were no intermittent diplopia, postoperative corneal erosions, granulomas, dellens, corneal ulcers, or vision impairment. Long operating time, use of retrobulbar or peribulbar block, need for assistance, and greater surgical trauma are just a few of his technique's drawbacks. The reported rate of recurrence is as low as 1.6%.
Differential Diagnosis
While evaluating a case of pterygium, the following conditions should be ruled out:
Pseudo-pterygium
It is a fold of bulbar conjunctiva which is attached to the cornea. It is an inflammatory reaction where adhesions are formed between bulbar conjunctiva and cornea. A 'probe' test can be performed where the probe can be passed beneath the tissue in pseudo-pterygium but not in pterygium. It can appear in any quadrant of the eye and is non-progressive in nature.
Pinguecula
It is a yellowish colored growth on bulbar conjunctiva close to the limbus. It is said to be a deposit of fat, protein or calcium. It is commonly found nasally. The patient is usually asymptomatic but may present with mild redness and foreign body sensation when inflamed.
Nodular Episcleritis
A discrete area of inflammation in episcleral tissue is seen, which is elevated. Only vascular congestion may be present in cases of simple episcleritis. If phenylephrine 2.5% is instilled, there will be blanching of the superficial episcleral vascular network. Usually, this condition is mildly painful, unlike pterygium.
Marginal Keratitis
It is an inflammatory disease of the peripheral cornea. Stromal infiltrates with the breakdown of the epithelium, and ulceration is seen. Infiltrate on the corneal surface is separated from limbus by a clear zone.
Phlycten
It is a result of delayed-type immune reaction leading to nodular inflammation of the conjunctiva.
Conjunctival carcinoma in situ/ Bowen epithelioma
It is a rare presentation. It presents as a gelatinous-appearing mass.
Ocular surface squamous neoplasia (OSSN)
Staging
Various grading systems have been studied to grade the extent of pterygium preoperatively.
The simplest method is to measure the length of the fibrovascular stroma of pterygium, which over-hangs the cornea.
Verma et al. gave the classification based on the size of pterygium being measured from the limbus.[64]
- Grade 1: 0-2 mm
- Grade 2: 2-4 mm
- Grade 3: >4 mm
Tan et al. gave a morphological classification of pterygium based on its transparency under the microscope.[64]
- Grade T1: consists of atrophic pterygia, whose episcleral blood vessels which are present under the body of pterygium are clearly visible
- Grade T2: consists of intermediate pterygium which has characteristics between atrophic and fleshy pterygium
- Grade T3: consists of fleshy pterygia with episcleral vessels completely obscured
According to Sejal Maheshwari, the grading is as follows[70]:
- Grade I: pterygium between the limbus and a point midway between the limbus and the pupillary margin (temporal pupillary margin for temporal pterygium, and nasal pupillary margin for nasal pterygium)
- Grade II: head of the pterygium is present between a point midway between the limbus and the pupillary margin
- Grade II: pterygium that crosses the pupillary margin
Complications
Various complications of pterygium surgery can be divided into intra-operative complications and postoperative complications.
Intraoperative complications include excessive bleeding, injury to the medial rectus muscle, wide excision of the pterygium, button-holing of graft, thick graft, loss of orientation of the graft, smaller harvested graft, and perforation of the globe with the suture needle.
The main postoperative complication is RECURRENCE.
Other postoperative complications include graft edema, dellen, persistent epithelial defects, pyogenic granuloma, symblepharon, graft necrosis, graft retraction, epithelial inclusion cysts, subconjunctival hematoma, sub-conjunctival fibrosis, corneoscleral thinning, sutural granuloma, etc.
Scleral necrosis, infectious scleritis, severe secondary glaucoma, and iritis are the complications associated with adjunctive use of MMC and beta radiations.
Deterrence and Patient Education
Patients with pterygium should be informed about the chances of recurrence after surgery. Postoperative mitomycin drops or steroid strops should be strictly used under the supervision of the surgeon. Unsupervised use may lead to the melting of the cornea or sclera, resulting in loss of vision and may even result in medicolegal suits. In elderly patients, OSSN may masquerade as pterygium, and histopathological examination of such tissue is important in ruling out OSSN.
Enhancing Healthcare Team Outcomes
Management of pterygium still remains an enigma. Literature is full of a variety of surgical procedures with variable success rates. However, the recurrence of pterygium still remains the most inexplicable complication. 90% recurrences occur between the first and the third month. Recurrent pterygium cases are more difficult to handle than the primary pterygium, and it is of great importance to determine the treatment method with the lowest recurrence rate. Prompt referral by primary care providers to ophthalmologists is needed. A coordinated effort between physicians, optometrists, nurses, and pharmacists can benefit patients. [Leve 5]
Media
(Click Image to Enlarge)
References
ROSENTHAL JW. Chronology of pterygium therapy. American journal of ophthalmology. 1953 Nov:36(11):1601-16 [PubMed PMID: 13104571]
Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. The British journal of ophthalmology. 1984 May:68(5):343-6 [PubMed PMID: 6712914]
Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Current opinion in ophthalmology. 2007 Jul:18(4):308-13 [PubMed PMID: 17568207]
Level 3 (low-level) evidenceNangia V, Jonas JB, Nair D, Saini N, Nangia P, Panda-Jonas S. Prevalence and associated factors for pterygium in rural agrarian central India. The central India eye and medical study. PloS one. 2013:8(12):e82439. doi: 10.1371/journal.pone.0082439. Epub 2013 Dec 4 [PubMed PMID: 24324789]
Luthra R, Nemesure BB, Wu SY, Xie SH, Leske MC, Barbados Eye Studies Group. Frequency and risk factors for pterygium in the Barbados Eye Study. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Dec:119(12):1827-32 [PubMed PMID: 11735795]
Ang M, Li X, Wong W, Zheng Y, Chua D, Rahman A, Saw SM, Tan DT, Wong TY. Prevalence of and racial differences in pterygium: a multiethnic population study in Asians. Ophthalmology. 2012 Aug:119(8):1509-15. doi: 10.1016/j.ophtha.2012.02.009. Epub 2012 Apr 10 [PubMed PMID: 22494631]
Shiroma H, Higa A, Sawaguchi S, Iwase A, Tomidokoro A, Amano S, Araie M. Prevalence and risk factors of pterygium in a southwestern island of Japan: the Kumejima Study. American journal of ophthalmology. 2009 Nov:148(5):766-771.e1. doi: 10.1016/j.ajo.2009.06.006. Epub 2009 Aug 6 [PubMed PMID: 19664753]
Lu P, Chen X, Kang Y, Ke L, Wei X, Zhang W. Pterygium in Tibetans: a population-based study in China. Clinical & experimental ophthalmology. 2007 Dec:35(9):828-33. doi: 10.1111/j.1442-9071.2007.01630.x. Epub [PubMed PMID: 18173411]
Lu J, Wang Z, Lu P, Chen X, Zhang W, Shi K, Kang Y, Ke L, Chen R. Pterygium in an aged Mongolian population: a population-based study in China. Eye (London, England). 2009 Feb:23(2):421-7 [PubMed PMID: 17948037]
Level 2 (mid-level) evidenceLiu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ open. 2013 Nov 19:3(11):e003787. doi: 10.1136/bmjopen-2013-003787. Epub 2013 Nov 19 [PubMed PMID: 24253031]
Level 1 (high-level) evidenceDi Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Progress in retinal and eye research. 2004 Mar:23(2):195-228 [PubMed PMID: 15094131]
Level 3 (low-level) evidenceHamed-Azzam S, Edison N, Briscoe D, Mukari A, Elmalah I. Identification of human papillomavirus in pterygium. Acta ophthalmologica. 2016 May:94(3):e195-7. doi: 10.1111/aos.12729. Epub 2015 Apr 12 [PubMed PMID: 25864511]
Reid TW, Dushku N. Does human papillomavirus cause pterygium? The British journal of ophthalmology. 2003 Jul:87(7):806-8 [PubMed PMID: 12812871]
Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, Tseng SC. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Jan:119(1):71-80 [PubMed PMID: 11146729]
Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Investigative ophthalmology & visual science. 2000 Mar:41(3):671-9 [PubMed PMID: 10711680]
Zeng W, Liu Z, Dai H, Yan M, Luo H, Ke M, Cai X. Anti-fibrotic, anti-VEGF or radiotherapy treatments as adjuvants for pterygium excision: a systematic review and network meta-analysis. BMC ophthalmology. 2017 Nov 25:17(1):211. doi: 10.1186/s12886-017-0601-5. Epub 2017 Nov 25 [PubMed PMID: 29178848]
Level 1 (high-level) evidenceVerma N,Garap JA,Maris R,Kerek A, Intraoperative use of mitomycin C in the treatment of recurrent pterygium. Papua and New Guinea medical journal. 1998 Mar; [PubMed PMID: 10741176]
Level 1 (high-level) evidenceRong SS, Peng Y, Liang YB, Cao D, Jhanji V. Does cigarette smoking alter the risk of pterygium? A systematic review and meta-analysis. Investigative ophthalmology & visual science. 2014 Sep 4:55(10):6235-43. doi: 10.1167/iovs.14-15046. Epub 2014 Sep 4 [PubMed PMID: 25190665]
Level 1 (high-level) evidenceHirst LW, Sebban A, Chant D. Pterygium recurrence time. Ophthalmology. 1994 Apr:101(4):755-8 [PubMed PMID: 8152771]
Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997 Jun:104(6):974-85 [PubMed PMID: 9186439]
Level 3 (low-level) evidenceHirst LW. Cosmesis after pterygium extended removal followed by extended conjunctival transplant as assessed by a new, web-based grading system. Ophthalmology. 2011 Sep:118(9):1739-46. doi: 10.1016/j.ophtha.2011.01.045. Epub 2011 Jun 2 [PubMed PMID: 21640383]
Level 1 (high-level) evidenceZolli CL. Experience with the avulsion technique in pterygium surgery. Annals of ophthalmology. 1979 Oct:11(10):1569-76 [PubMed PMID: 400324]
GIBSON JB. Brisbane survey of pterygium. Transactions of the Ophthalmological Society of Australia. 1956:16():125-34 [PubMed PMID: 13467988]
Level 3 (low-level) evidenceYoungson RM. Recurrence of pterygium after excision. The British journal of ophthalmology. 1972 Feb:56(2):120-5 [PubMed PMID: 5010313]
Krag S, Ehlers N. Excimer laser treatment of pterygium. Acta ophthalmologica. 1992 Aug:70(4):530-3 [PubMed PMID: 1414302]
D'Ombrain A. THE SURGICAL TREATMENT OF PTERYGIUM. The British journal of ophthalmology. 1948 Feb:32(2):65-71 [PubMed PMID: 18170420]
Alpay A, Uğurbaş SH, Erdoğan B. Comparing techniques for pterygium surgery. Clinical ophthalmology (Auckland, N.Z.). 2009:3():69-74 [PubMed PMID: 19668546]
Kareem AA, Farhood QK, Alhammami HA. The use of antimetabolites as adjunctive therapy in the surgical treatment of pterygium. Clinical ophthalmology (Auckland, N.Z.). 2012:6():1849-54. doi: 10.2147/OPTH.S38388. Epub 2012 Nov 8 [PubMed PMID: 23152665]
Yanyali AC, Talu H, Alp BN, Karabas L, Ay GM, Caglar Y. Intraoperative mitomycin C in the treatment of pterygium. Cornea. 2000 Jul:19(4):471-3 [PubMed PMID: 10928760]
Level 1 (high-level) evidenceDemirok A, Simsek S, Cinal A, Yasar T. Intraoperative application of mitomycin C in the surgical treatment of pterygium. European journal of ophthalmology. 1998 Jul-Sep:8(3):153-6 [PubMed PMID: 9793768]
Level 2 (mid-level) evidenceAnduze AL, Merest sclera technique for primary pterygium surgery. Ophthalmic surgery. 1989 Dec; [PubMed PMID: 2630971]
Staz L. A MODIFICATION AND EXTENSION OF THE McREYNOLDS' OPERATION FOR PTERYGIUM*. The British journal of ophthalmology. 1945 Apr:29(4):193-6 [PubMed PMID: 18170108]
Wilson SE, Bourne WM. Conjunctival Z-plasty in the treatment of pterygium. American journal of ophthalmology. 1988 Sep 15:106(3):355-7 [PubMed PMID: 3421299]
Lewallen S. A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology. 1989 Nov:96(11):1612-4 [PubMed PMID: 2694049]
Level 1 (high-level) evidenceTi SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. The British journal of ophthalmology. 2000 Apr:84(4):385-9 [PubMed PMID: 10729295]
Level 2 (mid-level) evidenceKenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985 Nov:92(11):1461-70 [PubMed PMID: 4080320]
Level 3 (low-level) evidenceCohen RA, McDonald MB. Fixation of conjunctival autografts with an organic tissue adhesive. Archives of ophthalmology (Chicago, Ill. : 1960). 1993 Sep:111(9):1167-8 [PubMed PMID: 8363455]
Level 3 (low-level) evidenceKumar S, Singh R. Pterygium excision and conjunctival autograft: A comparative study of techniques. Oman journal of ophthalmology. 2018 May-Aug:11(2):124-128. doi: 10.4103/ojo.OJO_6_2017. Epub [PubMed PMID: 29930445]
Level 2 (mid-level) evidenceHaider TA,Al-Amin LM,Bamashmus MA,Ali SF, Ninety degree conjunctival rotation autograft versus zero and 180 degree rotation autograft for pterygium surgery. Saudi medical journal. 2009 Apr; [PubMed PMID: 19370279]
Alp BN, Yanyali A, Ay GM, Keskin O. Conjunctival rotation autograft for primary pterygium. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2002 Sep-Oct:216(5):333-6 [PubMed PMID: 12424398]
Gris O, Güell JL, del Campo Z. Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology. 2000 Feb:107(2):270-3 [PubMed PMID: 10690823]
Malik KP, Goel R, Gutpa A, Gupta SK, Kamal S, Mallik VK, Singh S. Efficacy of sutureless and glue free limbal conjunctival autograft for primary pterygium surgery. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2012 Jul-Dec:4(2):230-5. doi: 10.3126/nepjoph.v4i2.6537. Epub [PubMed PMID: 22864027]
Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002 Sep:109(9):1752-5 [PubMed PMID: 12208727]
Level 1 (high-level) evidenceMasters JS, Harris DJ Jr. Low Recurrence Rate of Pterygium After Excision With Conjunctival Limbal Autograft: A Retrospective Study With Long-Term Follow-Up. Cornea. 2015 Dec:34(12):1569-72. doi: 10.1097/ICO.0000000000000597. Epub [PubMed PMID: 26382900]
Level 2 (mid-level) evidenceFernandes M, Sangwan VS, Bansal AK, Gangopadhyay N, Sridhar MS, Garg P, Aasuri MK, Nutheti R, Rao GN. Outcome of pterygium surgery: analysis over 14 years. Eye (London, England). 2005 Nov:19(11):1182-90 [PubMed PMID: 15543190]
Level 2 (mid-level) evidenceSyam PP, Eleftheriadis H, Liu CS. Inferior conjunctival autograft for primary pterygia. Ophthalmology. 2003 Apr:110(4):806-10 [PubMed PMID: 12689907]
Level 2 (mid-level) evidenceEssex RW, Snibson GR, Daniell M, Tole DM. Amniotic membrane grafting in the surgical management of primary pterygium. Clinical & experimental ophthalmology. 2004 Oct:32(5):501-4 [PubMed PMID: 15498062]
Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. The British journal of ophthalmology. 2000 Sep:84(9):973-8 [PubMed PMID: 10966947]
Level 2 (mid-level) evidenceLaughrea PA, Arentsen JJ. Lamellar keratoplasty in the management of recurrent pterygium. Ophthalmic surgery. 1986 Feb:17(2):106-8 [PubMed PMID: 3960467]
Level 2 (mid-level) evidenceRich AM, Kietzman B, Payne T, McPherson SD Jr. A simplified way to remove pterygia. Annals of ophthalmology. 1974 Jul:6(7):739-42 [PubMed PMID: 4843887]
Murakami M, Mori S, Kunitomo N. [Studies on the pterygium. V. Follow-up information of mitomycin C treatment]. Nippon Ganka Gakkai zasshi. 1967 Apr:71(4):351-8 [PubMed PMID: 6070608]
Singh G, Wilson MR, Foster CS. Mitomycin eye drops as treatment for pterygium. Ophthalmology. 1988 Jun:95(6):813-21 [PubMed PMID: 3211484]
Level 3 (low-level) evidenceRubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, Stoleru S, Talley AR, Speaker MG. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992 Nov:99(11):1647-54 [PubMed PMID: 1454338]
Level 3 (low-level) evidenceTassy A, Ribe D. [Thiotepa eyedrops for prevention of pterygium recurrence: 18 years of use]. Journal francais d'ophtalmologie. 1999 Mar:22(2):215-9 [PubMed PMID: 10327354]
Ngoy D, Kayembe L. [A comparative study of thio-tepa and mitomycin C in the treatment of pterygium. Preliminary results]. Journal francais d'ophtalmologie. 1998 Feb:21(2):96-102 [PubMed PMID: 9759389]
Level 1 (high-level) evidenceWu H, Chen G. [Cyclosporine A and thiotepa in prevention of postoperative recurrence of pterygium]. Yan ke xue bao = Eye science. 1999 Jun:15(2):91-2 [PubMed PMID: 12579708]
Bekibele CO, Baiyeroju AM, Olusanya BA, Ashaye AO, Oluleye TS. Pterygium treatment using 5-FU as adjuvant treatment compared to conjunctiva autograft. Eye (London, England). 2008 Jan:22(1):31-4 [PubMed PMID: 16778821]
Level 1 (high-level) evidenceAkarsu C, Taner P, Ergin A. 5-Fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary report. Cornea. 2003 Aug:22(6):522-6 [PubMed PMID: 12883344]
Kasetsuwan N, Reinprayoon U, Satitpitakul V. Prevention of recurrent pterygium with topical bevacizumab 0.05% eye drops: a randomized controlled trial. Clinical therapeutics. 2015 Oct 1:37(10):2347-51. doi: 10.1016/j.clinthera.2015.08.023. Epub 2015 Sep 26 [PubMed PMID: 26409291]
Level 1 (high-level) evidenceYamada T, Mochizuki H, Ue T, Kiuchi Y, Takahashi Y, Oinaka M. Comparative study of different β-radiation doses for preventing pterygium recurrence. International journal of radiation oncology, biology, physics. 2011 Dec 1:81(5):1394-8. doi: 10.1016/j.ijrobp.2010.07.1983. Epub 2010 Oct 1 [PubMed PMID: 20889266]
Level 2 (mid-level) evidenceAli AM, Thariat J, Bensadoun RJ, Thyss A, Rostom Y, El-Haddad S, Gérard JP. The role of radiotherapy in the treatment of pterygium: a review of the literature including more than 6000 treated lesions. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2011 Apr:15(2):140-7. doi: 10.1016/j.canrad.2010.03.020. Epub 2010 Jul 31 [PubMed PMID: 20674450]
Level 2 (mid-level) evidenceSingh SK, Pterygium: epidemiology prevention and treatment. Community eye health. 2017 [PubMed PMID: 29849437]
Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001 Mar:108(3):449-60 [PubMed PMID: 11237898]
Level 3 (low-level) evidenceTan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Archives of ophthalmology (Chicago, Ill. : 1960). 1997 Oct:115(10):1235-40 [PubMed PMID: 9338666]
Level 1 (high-level) evidenceHirst LW, Smallcombe K. Double-Headed Pterygia Treated With P.E.R.F.E.C.T for PTERYGIUM. Cornea. 2017 Jan:36(1):98-100 [PubMed PMID: 27749449]
Hirst LW. Recurrence and complications after 1,000 surgeries using pterygium extended removal followed by extended conjunctival transplant. Ophthalmology. 2012 Nov:119(11):2205-10. doi: 10.1016/j.ophtha.2012.06.021. Epub 2012 Aug 11 [PubMed PMID: 22892149]
Liu HY, Chen YF, Chen TC, Yeh PT, Hu FR, Chen WL. Surgical result of pterygium extended removal followed by fibrin glue-assisted amniotic membrane transplantation. Journal of the Formosan Medical Association = Taiwan yi zhi. 2017 Jan:116(1):10-17. doi: 10.1016/j.jfma.2015.10.013. Epub 2016 Feb 8 [PubMed PMID: 26868168]
Allam WA. Recurrence and complications of pterygium extended removal followed by extended conjunctival transplant for primary pterygia. European journal of ophthalmology. 2016 May-Jun:26(3):203-8. doi: 10.5301/ejo.5000685. Epub 2015 Oct 6 [PubMed PMID: 26449259]
Cornelius CR. Recurrence Rate and Complications of Pterygium Extended Removal Followed by Extended Conjunctival Transplant. Cornea. 2017 Jan:36(1):101-103 [PubMed PMID: 27749451]
Maheshwari S. Pterygium-induced corneal refractive changes. Indian journal of ophthalmology. 2007 Sep-Oct:55(5):383-6 [PubMed PMID: 17699952]
Level 2 (mid-level) evidence