Introduction
Optic disc edema and papilledema are critical examination findings as they can be the first sign of a variety of disease processes with potential for vision loss, neurological impairment, or death. Optic disc edema refers to swelling of the nerve fiber layer at the optic nerve head due to an optic neuropathy of any etiology (inflammatory, infiltrative, compressive, etc.) whereas the term papilledema refers to optic disc edema caused by raised intracranial pressure. True disc edema must be differentiated from pseudopapilledema, where there is an elevated appearance to the nerve head without edema of the nerve fiber layer, as pseudopapilledema has drastically different clinical implications. A variety of optic disc abnormalities can create the appearance of pseudopapilledema including optic disc drusen, congenital disc anomalies, myelinated nerve fibers, and peripapillary masses such as astrocytic hamartomas. This review will focus on optic disc drusen as careful examination, and ancillary testing can usually easily identify these other causes of pseudopapilledema.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
A variety of causes can create the appearance of pseudopapilledema. Congenitally small nerves with a crowded optic nerve head, often seen in hyperopic eyes with shorter axial lengths can appear to have some mild elevation of the optic nerve head. Conversely, in myopic eyes with longer axial lengths, the optic nerve can insert into the globe at an oblique angle so that the nasal part of the nerve is relatively elevated compared to the temporal nerve. Another congenital variant, Burgmeister papillae, originate from an incompletely regressed hyaloid artery that can obscure the optic nerve and give the impression of papilledema. Myelinated nerve fibers, which result from the anomalous migration of oligodendrocytes past the lamina cribosa, can also obscure the margin of the optic nerve.[1]
Optic disc drusen (ODD) are extracellular deposits consisting of calcium, hyaline, and other proteins located posterior to Bruch's membrane and anterior the lamina cribosa.[2] The pathogenesis of ODD is thought to be related to either altered axoplasmic flow resulting in extrusion of the drusen or degeneration of the retinal ganglion cell nerve fiber axons. The drusen deposits can progress from deep, buried drusen to superficial, visible drusen with age, resulting in an increase in elevation of the optic nerve head with time.[3]
Any other abnormalities around the optic nerve that obscure the nerve margins or elevate the nerve can produce pseudopapilledema; this would include vitreopapillary traction, non-calcified peripapillary astrocytic hamartomas or other peripapillary tumors.[4][5][6]
Epidemiology
Optical disc drusen have a reported prevalence of 0.2 to 3.1% of the population, with a higher prevalence reported on post-mortem cadaveric studies.[7][8] There is a familial component to ODD, but no causative gene has been identified.[9] ODD has correlations with retinitis pigmentosa, Alagille syndrome, and pseudoxanthoma elasticum (Grönblad–Strandberg syndrome).[10][11][12]
Histopathology
On histopathological examination, optical disc drusen can vary in size and number from 2 to 50 nodules, range in size from 5 to 1000µm, and stain for calcium.[13] Abnormal calcium deposits found within the mitochondria of intact axons which are thought to be extruded into extracellular space when there is disruption of axons. The calcium continues to be deposited in these extracellular mitochondria resulting in small extracellular calcium nidi that serve as a surface for the continuous deposition of calcium. These calcium microbodies grow into ODD.
History and Physical
It can be challenging to distinguish pseudopapilledema from true papilledema or disc edema based on the fundoscopic appearance of the optic nerve alone. A careful history of symptoms of raised intracranial pressure or other neurological symptoms, including positional headaches, nausea, pulsatile tinnitus, transient visual obscurations (TVOs), and diplopia is necessary. However, these features are non-specific and there is considerable overlap of headache symptoms between young females with idiopathic intracranial hypertension and those with ODD. Other signs of neurologic dysfunction on examination would not meet the modified Dandy criteria for IIH and, in the context of suspicious optic nerves, should raise the index of suspicion for acquired disc edema or papilledema.[14] If vision loss occurs with pseudopapilledema, it is painless and usually of insidious onset that is unnoticed by the patient. However, if the ODD cause a non-arteritic ischemic optic neuropathy, this can present as an episode of acute vision loss. Occasionally, patients with ODD will notice TVOs without raised intracranial pressure, attributed to transient ischemia from vascular compression by the space-occupying drusen.
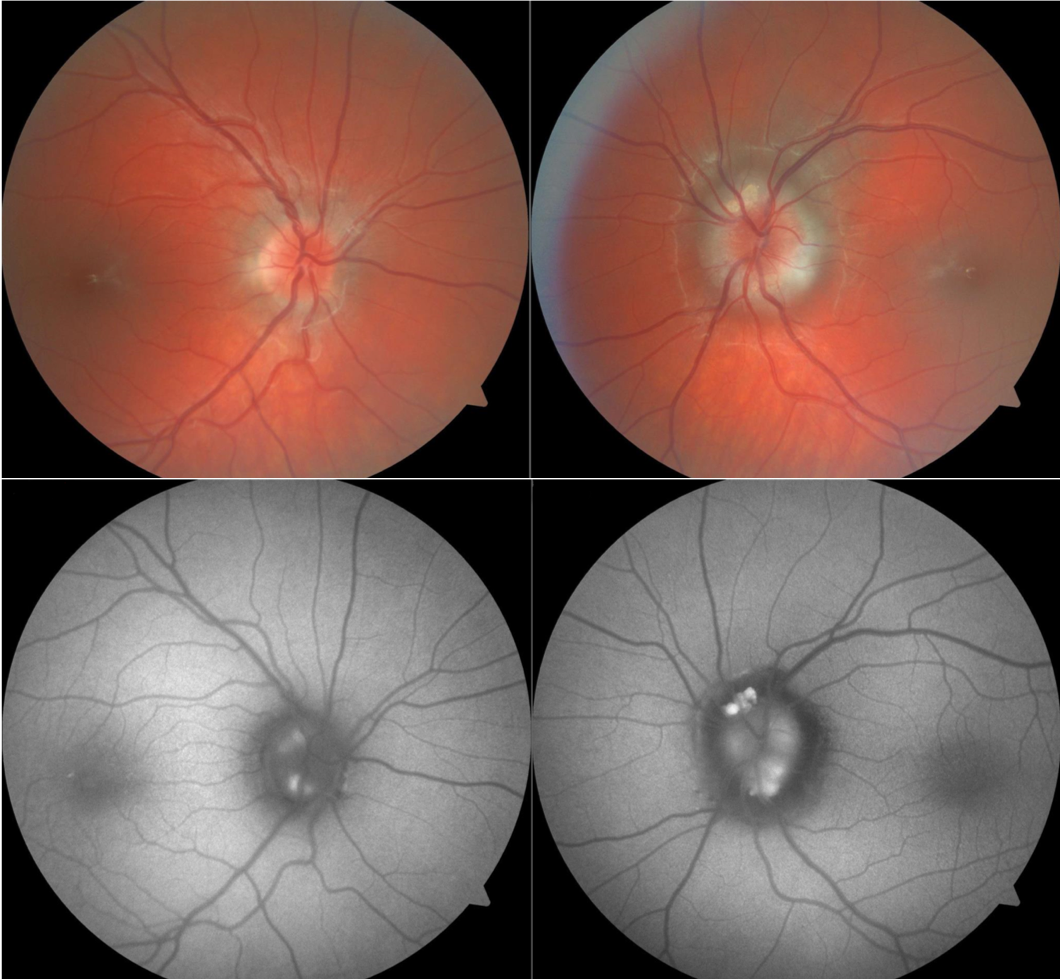
On fundus examination, optic nerves with pseudopapilledema will have an elevated appearance. In cases of ODD, the drusen may be superficial and easily visible on examination as refractile bodies or yellow drusen. The refractile appearance of drusen should not be confused with pseudodrusen that is present in longstanding papilledema. The diagnostic dilemma in ODD occurs when the drusen are deep in the nerve and obscured by the overlying retinal nerve fiber layer so that direct visualization is not possible. There may be blurred disc margins with nodular borders, but in optic nerves with buried drusen, there should not be any obscuration of the blood vessels, nor should there be any microvascular changes such as hemorrhages or exudates. The optic nerves will also tend to be small and cupless, with anomalous branching of the retinal vessels.[9] The presence of spontaneous venous pulsations is a reassuring sign that there is no raised intracranial pressure. However, venous pulsations are absent in 25% of the normal population and up to 75% of children with pseudopapilledema, so the absence of pulsations is not specific to raised intracranial pressure.[15][16] Signs of diseases associated with ODD should be ruled out: waxy pallor of the nerve, attenuated vessels, and peripheral bone spicules in retinitis pigmentosa; angioid streaks and peau d'orange skin changes in pseudoxanthoma elasticum; or posterior embryotoxon, anterior segment dysgenesis, and the characteristic facial appearance of Alagille syndrome.
Evaluation
The gold standard test for the diagnosis of true optic disc edema or papilledema is intravenous fluorescein angiography (IVFA), which will show leakage from the nerve if true disc edema is present.[17] Pseudopapilledema will only show late staining of the nerve on IVFA testing. However, an IVFA is an invasive test that requires IV access and a patient who is old enough to cooperate with serial fundus photography and is not readily accessible in most clinics. Thus, IVFA is typically only used in cases where other testing is equivocal. The absence of leakage does not necessarily mean that optical disc drusen are present, as the elevated optic nerve appearance could be due to one of the causes of pseudopapilledema mentioned above.
A variety of testing modalities can detect ODD that are buried too deep to be directly visualized on fundus examinations. CT scans are not routinely performed to diagnose ODD, but occasionally on CT scans performed for other indications, there will be an incidental hyperdensity at the optic nerve head corresponding to calcified drusen. Fundus autofluorescence, either via dedicated fundus photography or via pre-injection photos during an IVFA procedure, will highlight more superficial drusen which autofluoresce. B-scan ultrasonography can detect drusen as a hyperechoic signal at the nerve head that creates an acoustic shadow and a deep reduplication artifact.[18][19] The hyperechoic signal is strong enough that it will persist even when minimizing the gain on the ultrasound machine. Standardized A-scan ultrasound can also be used to measure the width of the optic nerve sheath, which increases in patients with papilledema. In papilledema, there is also a decrease in the optic nerve sheath width when the eye is rotated 30 degrees, whereas the diameter will be unchanged in patients with ODD.[20] This '30-degree test' requires an experienced ultrasonographer to perform reliably.
Optical coherence tomography (OCT) can be used to document the peripapillary retinal nerve fiber layer thickness, but this measurement cannot reliably distinguish disc edema from pseudopapilledema. The development of enhanced depth imaging OCT (EDI-OCT) and swept-source OCT (SS-OCT) technologies allows for visualization of the deep structures of the optic nerve, including deep drusen.[21][22] Drusen appear as hyporeflective masses with hyperreflective borders on EDI-OCT. Small hyperreflective bands have also been observed and postulated to represent very early drusen, but this theory has not yet had confirmation with longitudinal studies. When patients with drusen underwent study with spectral domain OCT or time domain OCT (which cannot image the deep structures of the nerve), peripapillary hyperreflective ovoid mass-like structures (PHOMS) were thought to be drusen. However, PHOMS are also present in patients with papilledema and do not exhibit any other of the imaging characteristics of drusen, so they more likely represent distended axons than optic nerve head drusen.[23] There are suggestions that EDI-OCT may be the most sensitive testing modality for ODD.[21] EDI-OCT also offers the advantage of showing the location of the drusen within the nerve head, unlike B-scan ultrasonography, so that any visual field defects can correlate with causative drusen.[24]
Treatment / Management
There are no recognized treatment options for pseudopapilledema caused by ODD. A case series described six patients with severe visual field loss who underwent radial optic neurotomy and found improvement or stabilization of visual function.[25] While the outcomes in the small series were positive, the risks of the surgery are such that this would only be a consideration in patients with very advanced visual loss which is an atypical finding in ODD. Excisions of superficial drusen have been published, but had poor outcomes and are not recommended.[26][27] Complications of ODD should receive treatment as they occur, as there are no preventative measures available.(B3)
There is no treatment necessary for pseudopapilledema if it is related to a congenital variant. If the pseudopapilledema appearance is due to a peripapillary tumor, the direction of treatment should be at the tumor as appropriate. In the case of vitreopapillary traction, the traction may spontaneously resolve with the completion of the posterior vitreous detachment, or if symptomatic, pars plana vitrectomy could be a therapeutic consideration.
Differential Diagnosis
The differential diagnosis for the elevated appearance of the optic nerve is broad and can group into either true disc edema or pseudopapilledema.
The differential for disc edema includes:
- Papilledema (Elevated intracranial pressure)
- Disc edema
- Inflammatory (demyelinating, granulomatous, uveitis, perineuritis, etc.)
- Ischemia/vascular (NAION, AION, CRVO, BRVO, diabetic papillitis, hypertension)
- Tumors/infiltration (meningioma, glioma, orbital masses, etc.)
- Intraocular hypotony or intracranial hypotension
- Inherited conditions (Leber hereditary optic neuropathy)
- Pseudopapilledema
- Optic disc drusen
- Hyperopic crowded nerve, myopic tilted nerve
- Bergmeister papilla
- Myelinated nerve fiber
- Vitrepapillary traction
- Peripapillary mass
It is important also to remember that a patient can have two diagnoses: the presence of ODD does not exclude true disc edema or papilledema as the two may co-exist.[28]
Prognosis
The prognosis of ODD in pseudopapilledema is very good. Individuals with drusen develop visual field defects with age: prevalences ranging from 11% in a pediatric population to 100% in an older population.[29] The visual field defects are usually mild, peripheral, and very slowly progressive.[30] Common patterns of field defects in ODD include enlarged blind spots, peripheral constriction, or glaucomatous nerve fiber bundle defects. Visual function loss correlates with the volume of drusen in the optic nerve head and is more severe within patients with superficial drusen, drusen larger than 500 micrometers, or confluent drusen.[31][24] Loss of central visual acuity is rare.
Complications
Apart from the peripheral visual field loss due to presumed compression of the retinal ganglion cell axons by the ODD, several other ocular conditions have associations with ODD. Non-arteritic ischemic optic neuropathy (NAION) occurs at a higher rate in individuals with ODD, and they have an increased risk (Hazard ratio 2.78) of developing a sequential NAION in their other eye.[32] Individuals with ODD are also at risk of central or branch retinal artery occlusions, central retinal vein occlusions, or peripapillary CNV.[33][34][35]
Deterrence and Patient Education
Pseudopapilledema and ODD reflect a congenital predisposition with no known preventative treatments or therapies. The most significant contribution one can make to a patients well-being in the diagnosis of ODD is to ensure that the patient and other health care practitioners are aware of the diagnosis.
Enhancing Healthcare Team Outcomes
Ensuring that the entire healthcare team is aware of the diagnosis of pseudopapilledema is critical to avoiding morbidity or mortality associated with misguided investigations or treatments for other causes of optic disc edema or papilledema. It is not unheard of patients with headaches and pseudopapilledema to undergo invasive testing with lumbar punctures, receive unnecessary radiation during CT venogram studies, or even have surgical interventions performed, such as dural venous sinus stenting for supposed idiopathic intracranial hypertension. With proper education of the patient and others in their healthcare team can avoid these diagnostic tests and treatments.
Patients with ODD should also work with their primary care physician to optimize their vasculopathic risk factors for NAION to minimize their chances of having a catastrophic acute anterior ischemic optic neuropathy.[36]
Media
(Click Image to Enlarge)
References
Tarabishy AB, Alexandrou TJ, Traboulsi EI. Syndrome of myelinated retinal nerve fibers, myopia, and amblyopia: a review. Survey of ophthalmology. 2007 Nov-Dec:52(6):588-96 [PubMed PMID: 18029268]
Level 3 (low-level) evidenceLam BL, Morais CG Jr, Pasol J. Drusen of the optic disc. Current neurology and neuroscience reports. 2008 Sep:8(5):404-8 [PubMed PMID: 18713576]
Spencer TS, Katz BJ, Weber SW, Digre KB. Progression from anomalous optic discs to visible optic disc drusen. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2004 Dec:24(4):297-8 [PubMed PMID: 15662245]
Level 3 (low-level) evidenceSimonett JM, Winges KM. Vitreopapillary Traction Detected by Optical Coherence Tomography. JAMA ophthalmology. 2018 May 10:136(5):e180727. doi: 10.1001/jamaophthalmol.2018.0727. Epub 2018 May 10 [PubMed PMID: 29800255]
Loukianou E, Kisma N, Pal B. Evolution of an Astrocytic Hamartoma of the Optic Nerve Head in a Patient with Retinitis Pigmentosa - Photographic Documentation over 2 Years of Follow-Up. Case reports in ophthalmology. 2011 Feb 2:2(1):45-9. doi: 10.1159/000324037. Epub 2011 Feb 2 [PubMed PMID: 21347192]
Level 3 (low-level) evidenceIovino C, Casini G, Peiretti E. Bilateral noncalcified astrocytic hamartomas in retinitis pigmentosa: Multimodal imaging evaluation over 8 years of follow-up. European journal of ophthalmology. 2019 Sep:29(5):NP18-NP21. doi: 10.1177/1120672118804386. Epub 2018 Oct 3 [PubMed PMID: 30280590]
Malmqvist L, Li XQ, Eckmann CL, Skovgaard AM, Olsen EM, Larsen M, Munch IC, Hamann S. Optic Disc Drusen in Children: The Copenhagen Child Cohort 2000 Eye Study. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2018 Jun:38(2):140-146. doi: 10.1097/WNO.0000000000000567. Epub [PubMed PMID: 28841585]
Friedman AH, Gartner S, Modi SS. Drusen of the optic disc. A retrospective study in cadaver eyes. The British journal of ophthalmology. 1975 Aug:59(8):413-21 [PubMed PMID: 1203227]
Level 2 (mid-level) evidenceAntcliff RJ, Spalton DJ. Are optic disc drusen inherited? Ophthalmology. 1999 Jul:106(7):1278-81 [PubMed PMID: 10406605]
Level 2 (mid-level) evidenceGrover S, Fishman GA, Brown J Jr. Frequency of optic disc or parapapillary nerve fiber layer drusen in retinitis pigmentosa. Ophthalmology. 1997 Feb:104(2):295-8 [PubMed PMID: 9052635]
Level 2 (mid-level) evidenceKim BJ, Fulton AB. The genetics and ocular findings of Alagille syndrome. Seminars in ophthalmology. 2007 Oct-Dec:22(4):205-10 [PubMed PMID: 18097983]
Georgalas I, Tservakis I, Papaconstaninou D, Kardara M, Koutsandrea C, Ladas I. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clinical & experimental optometry. 2011 Mar:94(2):169-80. doi: 10.1111/j.1444-0938.2010.00559.x. Epub 2010 Dec 29 [PubMed PMID: 21198842]
Tso MO. Pathology and pathogenesis of drusen of the optic nervehead. Ophthalmology. 1981 Oct:88(10):1066-80 [PubMed PMID: 7335311]
Smith JL. Whence pseudotumor cerebri? Journal of clinical neuro-ophthalmology. 1985 Mar:5(1):55-6 [PubMed PMID: 3156890]
Harder B, Jonas JB. Frequency of spontaneous pulsations of the central retinal vein. The British journal of ophthalmology. 2007 Mar:91(3):401-2 [PubMed PMID: 17322474]
Level 3 (low-level) evidenceEkdawi NS, Brodsky MC. Absence of spontaneous venous pulsations in children with pseudopapilloedema. The British journal of ophthalmology. 2011 Nov:95(11):1615-6. doi: 10.1136/bjophthalmol-2011-300363. Epub 2011 Jun 30 [PubMed PMID: 21719565]
Level 3 (low-level) evidenceChang MY, Velez FG, Demer JL, Bonelli L, Quiros PA, Arnold AC, Sadun AA, Pineles SL. Accuracy of Diagnostic Imaging Modalities for Classifying Pediatric Eyes as Papilledema Versus Pseudopapilledema. Ophthalmology. 2017 Dec:124(12):1839-1848. doi: 10.1016/j.ophtha.2017.06.016. Epub 2017 Jul 18 [PubMed PMID: 28732589]
McNicholas MM, Power WJ, Griffin JF. Sonography in optic disk drusen: imaging findings and role in diagnosis when funduscopic findings are normal. AJR. American journal of roentgenology. 1994 Jan:162(1):161-3 [PubMed PMID: 8273656]
Almog Y, Nemet A, Nemet AY. Optic disc drusen demonstrate a hyperechogenic artifact in B mode ultrasound. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016 Jan:23():111-119. doi: 10.1016/j.jocn.2015.08.005. Epub 2015 Sep 26 [PubMed PMID: 26412252]
Saenz R, Cheng H, Prager TC, Frishman LJ, Tang RA. Use of A-scan Ultrasound and Optical Coherence Tomography to Differentiate Papilledema From Pseudopapilledema. Optometry and vision science : official publication of the American Academy of Optometry. 2017 Dec:94(12):1081-1089. doi: 10.1097/OPX.0000000000001148. Epub [PubMed PMID: 29120977]
Merchant KY, Su D, Park SC, Qayum S, Banik R, Liebmann JM, Ritch R. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013 Jul:120(7):1409-14. doi: 10.1016/j.ophtha.2012.12.035. Epub 2013 Mar 24 [PubMed PMID: 23531353]
Level 2 (mid-level) evidenceMalmqvist L, Bursztyn L, Costello F, Digre K, Fraser JA, Fraser C, Katz B, Lawlor M, Petzold A, Sibony P, Warner J, Wegener M, Wong S, Hamann S. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2018 Sep:38(3):299-307. doi: 10.1097/WNO.0000000000000585. Epub [PubMed PMID: 29095768]
Malmqvist L, Sibony PA, Fraser CL, Wegener M, Heegaard S, Skougaard M, Hamann S, Optic Disc Drusen Studies Consortium. Peripapillary Ovoid Hyperreflectivity in Optic Disc Edema and Pseudopapilledema. Ophthalmology. 2018 Oct:125(10):1662-1664. doi: 10.1016/j.ophtha.2018.04.036. Epub 2018 Jun 8 [PubMed PMID: 29891127]
Traber GL, Weber KP, Sabah M, Keane PA, Plant GT. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen: A Comparison of Cases with and without Visual Field Loss. Ophthalmology. 2017 Jan:124(1):66-73. doi: 10.1016/j.ophtha.2016.09.022. Epub 2016 Nov 3 [PubMed PMID: 27817914]
Level 3 (low-level) evidenceNentwich MM, Remy M, Haritoglou C, Kampik A. Radial optic neurotomy to treat patients with visual field defects associated with optic nerve drusen. Retina (Philadelphia, Pa.). 2011 Mar:31(3):612-5. doi: 10.1097/IAE.0b013e318209b748. Epub [PubMed PMID: 21336072]
Kapur R, Pulido JS, Abraham JL, Sharma M, Buerk B, Edward DP. Histologic findings after surgical excision of optic nerve head drusen. Retina (Philadelphia, Pa.). 2008 Jan:28(1):143-6. doi: 10.1097/IAE.0b013e31815e98d8. Epub [PubMed PMID: 18185151]
Level 3 (low-level) evidencePfriem M, Hoerauf H. Unsuccessful surgical excision of optic nerve drusen. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Oct:249(10):1583-5. doi: 10.1007/s00417-011-1693-x. Epub 2011 Jun 3 [PubMed PMID: 21638031]
Level 3 (low-level) evidencePineles SL, Arnold AC. Fluorescein angiographic identification of optic disc drusen with and without optic disc edema. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2012 Mar:32(1):17-22. doi: 10.1097/WNO.0b013e31823010b8. Epub [PubMed PMID: 21926917]
Level 2 (mid-level) evidenceHamann S, Malmqvist L, Costello F. Optic disc drusen: understanding an old problem from a new perspective. Acta ophthalmologica. 2018 Nov:96(7):673-684. doi: 10.1111/aos.13748. Epub 2018 Apr 16 [PubMed PMID: 29659172]
Level 3 (low-level) evidenceLee AG, Zimmerman MB. The rate of visual field loss in optic nerve head drusen. American journal of ophthalmology. 2005 Jun:139(6):1062-6 [PubMed PMID: 15953437]
Level 2 (mid-level) evidenceSkaat A, Muylaert S, Mogil RS, Furlanetto RL, Netto CF, Banik R, Liebmann JM, Ritch R, Park SC. Relationship Between Optic Nerve Head Drusen Volume and Structural and Functional Optic Nerve Damage. Journal of glaucoma. 2017 Dec:26(12):1095-1100. doi: 10.1097/IJG.0000000000000783. Epub [PubMed PMID: 29045333]
Chang MY, Keltner JL. Risk Factors for Fellow Eye Involvement in Nonarteritic Anterior Ischemic Optic Neuropathy. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2019 Jun:39(2):147-152. doi: 10.1097/WNO.0000000000000715. Epub [PubMed PMID: 30300257]
Farah SG, Mansour AM. Central retinal artery occlusion and optic disc drusen. Eye (London, England). 1998:12 ( Pt 3a)():480-2 [PubMed PMID: 9775256]
Level 3 (low-level) evidenceChern S, Magargal LE, Annesley WH. Central retinal vein occlusion associated with drusen of the optic disc. Annals of ophthalmology. 1991 Feb:23(2):66-9 [PubMed PMID: 2029117]
Level 3 (low-level) evidenceSilva R, Torrent T, Loureiro R, Travassos A, de Abreu JR. Bilateral CNV associated with optic nerve drusen treated with photodynamic therapy with verteporfin. European journal of ophthalmology. 2004 Sep-Oct:14(5):434-7 [PubMed PMID: 15506607]
Level 3 (low-level) evidenceBerry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye and brain. 2017:9():23-28. doi: 10.2147/EB.S125311. Epub 2017 Sep 27 [PubMed PMID: 29033621]