Definition/Introduction
The heart is a specialized vascular structure with an intrinsic pumping mechanism to direct oxygenated blood and nutrients to the tissues of the body. Basic physics informs us that pressure gradients provide the physical impetus for fluid flow and the force generated by myocardial contraction drives blood flow into systemic and pulmonary circulation. Myocardial relaxation generates a drop in pressure that promotes retrograde blood flow. Prevention of this retrograde blood flow is by the presence of heart valves which open and close due to changes in pressure. The heart has four valves: the mitral valve, aortic valve, tricuspid valve, and pulmonic valve. The mitral and tricuspid valves are collectively known as the atrioventricular valves due to their anatomical location at the junction of the atrium and ventricle. Their primary function is to prevent retrograde blood flow into the atria during ventricular systole. The aortic and pulmonic valves are collectively known as the semilunar valves, and their primary function is to prevent retrograde blood flow into the ventricles during diastole. They are at the junction of the ventricle and their respective great artery.
The atrioventricular valves are composed of flat and non-homogenous tissue leaflets (or cusps) with the mitral valve consisting of two leaflets while the tricuspid valve is composed three leaflets. The leaflets of the atrioventricular valves extend into the ventricle via fibrous chordae tendinae (literally “heart-strings”). The chordae tendinae attach the apical aspects of the atrioventricular valves to muscular projections along the ventricular wall. These muscular projections are called papillary muscles, and they serve as a point of anchoring stability for the atrioventricular valves.
The presence of heart valves is to facilitate unidirectional blood flow. The driver behind the functionality of the heart valves is pressure gradients rather than electrical conduction. Heart valve disease can broadly classify as either valvular stenosis and valvular regurgitation (also known as incompetence or insufficiency). Valvular stenosis is the narrowing of the heart valve orifice. As such, valvular stenosis can accurately be described as an incomplete opening of the valvular orifice. In contrast, valvular regurgitation is an incomplete closure of the valvular orifice. Patients often describe this condition as having a “leaky valve.”
The cardiac cycle is the best context by which to understand heart valve disease. During ventricular diastole, the heart is in a relaxed state, and the mitral valve is open, allowing passive blood flow from the left atrium into the left ventricle. The increased blood flow into the left ventricle increases the interventricular pressure. With atrial systole, additional blood is actively directed into the ventricle, further increasing the ventricular pressure. The start of ventricular contraction leads to an abrupt increase in interventricular pressure. As the ventricular volume of blood increases, the volume pushes against the inferior aspects of the mitral valve, effectively closing them as the valve cusps coapt. The closure of the mitral valve leads to an abrupt and physiologic increase in interventricular pressure that, when coupled with the ventricular systole, exceeds the pressure in the aorta. As the interventricular pressure exceeds the aortic pressure, the aortic valve opens, leading to a blood jet into the ascending aorta. As ventricular systole ends, the driving pressure that directs blood flow into the ascending aorta decreases. When the interventricular pressure drops below the aortic pressure, the aortic valve closes. Considering only the left side of the heart, the aortic valve should be fully open during systole and completely closed during diastole. The mitral valve, on the other hand, should be fully closed during systole and open during diastole. Any deviation from this mechanism is likely representative of a heart valve pathology.
As the heart continuously beat throughout a lifetime, there is cyclic stress and strain on the native heart valves. The left side of the heart, in particular, is associated with higher pressures which range between approximately 100 mmHg to 150 mmHg. As a result, the left-sided valves (i.e., aortic and mitral) have a higher predisposition to disease compared to their right-sided counterparts. A diseased valve that can cause hemodynamic compromise or affect the quality of life will often warrant evaluation regarding possible prosthesis.
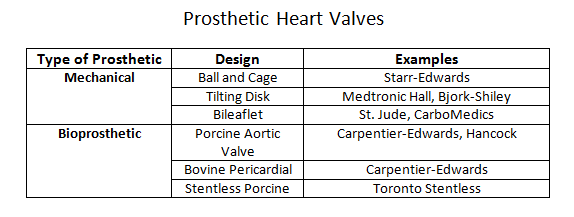
Prosthetic heart valves can broadly classify into a few general categories: mechanical heart valves, bioprosthetic heart valves, and homograft valves.Mechanical valves are composed of three major components: an occluder, an occluder restraint, and a sewing ring. Throughout the evolution of mechanical valve design, the artificial mechanical valves fall into three basic classes: ball and cage, disc valves (with tilting and non-tilting variants), and bileaflet valves. The prototypical ball and cage valve is the Starr-Edwards valve which, despite its longevity of use from the early 1960s, was retired in 2007. The occluder in this valve is a ball retained in a silicone-coated stainless steel cage. The sewing cuff is composed a Teflon ring with the intent minimizing thrombosis. During diastole (or systole for the mitral valve), the occluder ball would situate itself into the sewing ring, forming a seal to prevent retrograde flow. The major drawback of this design is the subpar hemodynamics. The ball occluder of the ball and cage design disrupted the central flow of blood found in a native heart valve. Further designs were developed to help improve the hemodynamics. The non-tilting disc valve, composed of a flat, circular disc within a cage, did not necessarily offer improved fluid dynamics but later bileaflet design performed better in emulating the natural hemodynamics of the native heart valve.Bioprosthetic valves are composed of three xenograft tissue leaflets of either porcine valvular leaflets or bovine pericardial tissue. Both xenografts are treated with glutaraldehyde solution to reduce thromboembolic risk due as glutaraldehyde inhibits collagen denaturation. In a stented bioprosthetic valve, the leaflets get sewn to a cloth-covered (typically dacron) metallic support structure, similar to their mechanical counterparts. The non-stented bioprosthetics require the prosthetic leaflets to attach directly into the aortic annulus. The bioprosthetic valves are hemodynamically improved compared to the mechanical valves but generate greater pressure gradients due to their smaller annulus.Homograft valves are valves obtained from human cadavers. As such valves no longer have regenerative capabilities, they are more susceptible to cyclic stress. Homograft valves are usable in conjunction with an autograft valve. An autograft is a heart valve transplant from one anatomic point to a separate location within the same individual. The Ross procedure is an example of a surgical intervention utilizing a homograft/autograft procedure.[1] The Ross procedure uses a cadaver valve (homograft) to replace a functional pulmonary valve. The healthy pulmonary valve is then transferred to replace the diseased aortic valve (autograft). The rationale behind the autograft component of the Ross procedure is due to the higher pressure system of the left side of the heart. A healthy pulmonary valve has enough regenerative capability to withstand the high-pressure environment compared to a homograft.The choice of the prosthetic type is patient-centered and depends on two risk factors: the concern for anticoagulation-associated bleeding risk, and valve deterioration.[2] The durability of the tissue is inversely correlated with age, reflecting the increased level of deterioration in younger patients compared to the older population. Thus, bioprosthetics tend to be a more suitable choice in older patients who may be less tolerant of anticoagulation while mechanical valves may be a better option in younger patients where bioprosthetic valves may have accelerated deterioration. Although older patients may be opted for a bioprosthetic valve to avoid complications with anticoagulation, the development of atrial fibrillation in the same demographic may effectively cancel any benefit of this management approach.[3]The delivery method of the prosthetic valve (surgical vs. transcatheter) should also play a role in the discussion of management. SAVR (surgical aortic valve replacement) is a highly invasive procedure that is accompanied by the usual risk associated with an open thoracotomy. TAVR (transcatheter aortic valve replacement) is considerably less invasive but offers its particular share of disadvantages, including valve oversizing, the difficulty of intravascular manipulation, and positioning. Furthermore, any injury occurring during prosthesis deployment may have adverse effects on the lifespan of the prosthesis.[4] Perioperative imaging can help minimize these adverse effects although they do not comletely eliminate the risks. CT imaging has utility as an imaging tool for all stages of TAVR.[5]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Design of prosthetic heart valves focuses on four components: improved optimal hemodynamics, mechanical/biological durability, minimizing biological response to the prosthetic heart valve, and optimal delivery system.
Although prosthetic heart valves functionally provide adequate hemodynamics, the hemodynamics are still suboptimal, and the introduction of a foreign body into the body increases the risk of thrombogenicity. The latter statement is of particular importance and is the rationale behind anticoagulant therapy in post-heart valve replacement patients. The most severe complications associated with prosthetic heart valves include thrombosis, anticoagulant-related hemorrhage, infection, valvular failure, tissue hyperplasia, and overgrowth.
As mentioned previously, exposure of human blood to a prosthetic implant leads to an increased risk of thrombogenicity, thus warranting appropriate anticoagulant therapy. However, anticoagulant therapy has its own share of risks which clinicians must address with a patient. Anticoagulation will be covered further under the "Clinical Significance" topic.
The Hufnagel prosthesis is the first historical heart valve prosthesis and served as a precursor for future developments in mechanical prostheses. The original Hufnagel prosthesis consisted of a ball within a non-collapsible tube. The ball would move respective to the cardiac cycle but was restrained by the decreasing radius of its plastic tubing. As one would expect, the Hufnagel prosthesis led to more turbulent flow due to inadequate flow dynamics. Mechanical valves exhibit poor hemodynamics when compared to biological prostheses. The Starr-Edwards valve, the first orthotropic prosthesis, consists of a ball occluder within a cage. The ball occluder would form a seal with an appropriately sized sewing ring during a particular period of the cardiac cycle (systole for the mitral valve and diastole for the aortic valve). In the native valve, central flow through the valvular orifice is essentially undisturbed and can be, for all practical purposes, considered laminar flow. The mechanical ball occluder in the Starr-Edwards valve induced turbulent flow, leading to suboptimal hemodynamics. Efforts directed at improving the fluid dynamics of mechanical valve prosthesis led to bi-leaflet designs. The main difference between the Starr-Edwards and the subsequent bi-leaflet designs was the improved central flow, leading to decreased turbulent flow. The issue of prosthetic durability clearly delineates along the lines of mechanical versus biological prosthesis. Mechanical heart valve prostheses have a considerably longer life duration relative to biological prosthesis. The typical lifespan of a mechanical prosthesis is on the order of decades while the biological prostheses have a lifespan on the order of 10-12 years.
Clinical Significance
Cyclic stress loading on native heart valves contributes to eventual wear and subsequent pathology. A diseased valve, whether it is stenosis or regurgitant, has impaired hemodynamic performance compared to a non-diseased valve. Placement of a prosthetic never entirely duplicates the natural hemodynamics of the native heart valve. Anticoagulation therapy is essential for the management of heart valve prosthetic patients. As of date, warfarin (with heparin bridging) is the most widely used anticoagulant for mechanical valves. The direct factor Xa inhibitors such as apixaban, rivaroxaban, and dabigatran currently have no approved indications for anticoagulation therapy with mechanical valves. The 2013 RE-ALIGN trial was an open-label study which compared dabigatran vs. standard of care warfarin as anticoagulant therapy for mechanical valves. Conclusions of the study determined that dabigatran conveyed a greater thromboembolic risk and risk of bleeding complications compared to warfarin.[6] Current FDA recommendations state that dabigatran is contraindicated in patients with mechanical valves. Per AHA/ACC 2017 guidelines, the goal INR of 3.0 was the recommended value for mitral mechanical prostheses or aortic valve prostheses plus additional thromboembolic risks. Furthermore, the addition to aspirin 75 mg to 100 mg among patients with a mechanical valve prosthesis in addition to VKA therapy was the recommendation; this was unchanged compared to the AHA/ACC 2014 guidelines.[7] The guidelines additionally recommended the use of a vitamin K antagonist (VKA) after bioprosthetic valve replacement for both aortic and mitral bioprosthesis for a duration 3-6 months after surgery, provided the patient had no excessive risk of bleeding, which represents a change from previous 2014 AHA/ACC recommendationsThe medical decision of a mechanical valve versus a biological valve should account for the patient’s willingness and ability to undergo potential lifelong anticoagulation. Anticoagulation therapy itself carries a significant risk, the most significant being possible life-threatening hemorrhage.
Pregnancy with concurrent heart valve disease poses its share of medical challenges. Physiologic changes accompanying pregnancy include increased glomerular filtration rate and increased intravascular response. Subsequently, there is an increased elimination of pharmacological agents and an increased volume of distribution, respectively. The lack of larger randomized clinical trials addressing anticoagulation and pregnancy leave very few evidence-based options. Although VKA therapy is the gold standard for anticoagulation in patients with mechanical valves, warfarin is a well-known teratogen and is contraindicated in pregnancy. In one 2018 study, there was an observed increased in the rate of pregnancy loss in mechanical versus bioprosthetic (61% vs. 15%) and an increased rate of hemorrhage in those receiving mechanical valves (16% vs. 6%, p < 0.01).[8] An analysis of the patient demographics in the study, however, reveals a proportionately higher number of patients with coagulopathy in the mechanical valve group compared to the bioprosthetic valve group, likely contributing to increased hemorrhage rates in the mechanical valve group.The cardiac output necessary to satisfy the metabolic and oxygen demands of the body is dependent on the patency of the prosthetic valve. A prosthesis-patient mismatch is a condition in which a normally functioning prosthetic heart valve possesses an effective orifice area (EOA) too small relative to the patient’s body surface area. Normalization of the indexed effective orifice area (EOAi) is with respect to body surface area. A severe prosthesis-patient mismatch is an EOAi less than 0.65 cm^2/m^2. A moderate prosthesis-patient mismatch is an EOAi between 0.65 and 0.85 cm^2/m^2.[9] Prevention of prosthesis-patient mismatch includes a calculation of predicted EOAi. Low flow dynamics can lead to falsely elevated EOAi. In such states, the low flow does not allow for full opening of the prosthetic leaflets; this results in what is known as “pseudo-prosthesis-patient mismatch.” A prosthesis-patient mismatch is not a benign entity, and there exists a notable effect on mortality. In the PARTNER cohort study, the rate of severe prosthesis-patient mismatch was higher in those undergoing SAVR (surgical aortic valve replacement) compared to those undergoing TAVR (transcatheter aortic valve replacement).[10]
Media
(Click Image to Enlarge)
References
Mazine A, El-Hamamsy I, Verma S, Peterson MD, Bonow RO, Yacoub MH, David TE, Bhatt DL. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2018 Dec 4:72(22):2761-2777. doi: 10.1016/j.jacc.2018.08.2200. Epub [PubMed PMID: 30497563]
Musumeci L, Jacques N, Hego A, Nchimi A, Lancellotti P, Oury C. Prosthetic Aortic Valves: Challenges and Solutions. Frontiers in cardiovascular medicine. 2018:5():46. doi: 10.3389/fcvm.2018.00046. Epub 2018 May 14 [PubMed PMID: 29868612]
Poli D, Antonucci E, Pengo V, Grifoni E, Maggini N, Testa S, Lodigiani C, Insana A, Marongiu F, Barcellona D, Paparo C, Bucherini E, Pignatelli P, Palareti G. Risk of reoperation in bioprosthetic valve patients with indication for long-term anticoagulation. Results from the observational retrospective multicentre PLECTRUM study. Open heart. 2018:5(2):e000837. doi: 10.1136/openhrt-2018-000837. Epub 2018 Aug 27 [PubMed PMID: 30228907]
Level 2 (mid-level) evidenceSalaun E, Clavel MA, Rodés-Cabau J, Pibarot P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart (British Cardiac Society). 2018 Aug:104(16):1323-1332. doi: 10.1136/heartjnl-2017-311582. Epub 2018 May 7 [PubMed PMID: 29735584]
Wilson R, McNabney C, Weir-McCall JR, Sellers S, Blanke P, Leipsic JA. Transcatheter Aortic and Mitral Valve Replacements. Radiologic clinics of North America. 2019 Jan:57(1):165-178. doi: 10.1016/j.rcl.2018.08.001. Epub 2018 Oct 31 [PubMed PMID: 30454811]
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F, RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. The New England journal of medicine. 2013 Sep 26:369(13):1206-14. doi: 10.1056/NEJMoa1300615. Epub 2013 Aug 31 [PubMed PMID: 23991661]
Level 1 (high-level) evidenceNishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017 Jul 11:70(2):252-289. doi: 10.1016/j.jacc.2017.03.011. Epub 2017 Mar 15 [PubMed PMID: 28315732]
Level 1 (high-level) evidenceBatra J, Itagaki S, Egorova NN, Chikwe J. Outcomes and Long-term Effects of Pregnancy in Women With Biologic and Mechanical Valve Prostheses. The American journal of cardiology. 2018 Nov 15:122(10):1738-1744. doi: 10.1016/j.amjcard.2018.07.020. Epub 2018 Aug 3 [PubMed PMID: 30449326]
Pibarot P, Clavel MA. Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement: It Is Neither Rare Nor Benign. Journal of the American College of Cardiology. 2018 Dec 4:72(22):2712-2716. doi: 10.1016/j.jacc.2018.09.045. Epub [PubMed PMID: 30497556]
Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T, Kodali SK, Mack MJ, Thourani VH, Miller DC, Svensson LG, Herrmann HC, Smith CR, Rodés-Cabau J, Webb J, Lim S, Xu K, Hueter I, Douglas PS, Leon MB. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. Journal of the American College of Cardiology. 2014 Sep 30:64(13):1323-34. doi: 10.1016/j.jacc.2014.06.1195. Epub [PubMed PMID: 25257633]
Level 2 (mid-level) evidence