Introduction
The primary objective of antenatal fetal surveillance is to mitigate the risk of stillbirth. For nearly 4 decades, techniques assessing fetal heart rate (FHR) patterns, alongside real-time ultrasonography and umbilical artery Doppler velocimetry, have been used to monitor fetal well-being. These methods are crucial for evaluating the risk of fetal death in pregnancies with preexisting maternal conditions, such as diabetes mellitus, or those complicated by issues such as fetal growth restriction. FHR patterns, activity levels, and muscle tone are indicators that can be affected by hypoxemia and acidemia. When a fetus experiences hypoxemia, blood flow redistribution can lead to reduced renal perfusion and oligohydramnios. Techniques such as cardiotocography, real-time ultrasonography, and monitoring maternal perception of fetal movements are used to detect potential uteroplacental compromise. Identifying fetal compromise allows for intervention before metabolic acidosis can progress to fetal death. However, sudden and severe changes in fetal status, such as placental abruption or umbilical cord accidents, are typically unpredictable and less preventable through these tests.[1][2]
The American College of Obstetricians and Gynecologists (ACOG) has provided general recommendations on when to initiate antenatal fetal surveillance based on the risk of stillbirth; however, strict guidelines have not been established due to the limited amount of evidence-based studies. Consequently, the ACOG encourages antenatal fetal surveillance to be individualized, including initiation, modalities utilized, and frequency, especially in high-risk cases where surveillance might begin at an age where delivery benefits perinatal outcomes. Antenatal fetal surveillance is indicated for conditions with a stillbirth incidence higher than 0.8 per 1000 and a relative risk or odds ratio for stillbirth >2.0 compared to unaffected pregnancies. In the absence of gestational age-adjusted data, ACOG suggests initiating surveillance at 32, 36, or 39 weeks of gestation. Shared decision-making between the patient and clinician is essential, particularly for pregnancies at a high risk of stillbirth or for those with multiple complicating factors. This approach is crucial when dealing with fetal anomalies or initiating surveillance near the threshold of viability, where patient preferences significantly influence care decisions.[1][2]
Various surveillance methods include maternal perception of fetal movement, contraction stress tests (CSTs), nonstress tests (NSTs), biophysical profiles (BPPs), modified BPPs, and umbilical artery Doppler velocimetry. Generally, normal results from these tests are reassuring due to their low false-negative rates. However, antenatal fetal surveillance using any modality may not accurately reflect a significantly affected fetus during acute distress and is less effective at predicting stillbirths resulting from acute maternal-fetal status changes. In addition, some maternal conditions may cause temporary abnormal results during fetal testing that improve as the maternal condition improves. Therefore, abnormal test results should be interpreted within the broader clinical context, with further testing or intervention guided by the overall maternal and fetal condition.[1][2]
In cases of decreased maternal perception of fetal movement, further assessment with NSTs, CSTs, BPPs, or modified BPPs is recommended. Abnormal findings typically lead to additional testing or consideration of delivery. The management of equivocal or abnormal BPP scores varies based on gestational age. For scores of 4 or lower, delivery is often indicated unless the pregnancy is less than 32 weeks, where extended monitoring may be appropriate. Ultimately, abnormal test results necessitate careful evaluation to avoid unnecessary interventions. Continuous intrapartum monitoring is advisable if delivery is attempted. Although fetal kick counting is a simple method to assess fetal well-being, its effectiveness in preventing stillbirth is not well-established and might lead to increased medical interventions.[1][2]
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Antenatal fetal surveillance is used to reduce the risk of stillbirth, particularly in high-risk pregnancies where conditions such as fetal hypoxemia and acidosis can lead to fetal death.[3] Surveillance methods such as maternal perception of fetal movement, CSTs, NSTs, BPPs, modified BPPs, and umbilical artery Doppler velocimetry are used to detect changes in amniotic fluid, fetal movements, and FHR characteristics.[4] Despite the lack of comprehensive, evidence-based guidelines due to challenges in conducting prospective trials, expert consensus and observational studies support the use of these methods.[1][2]
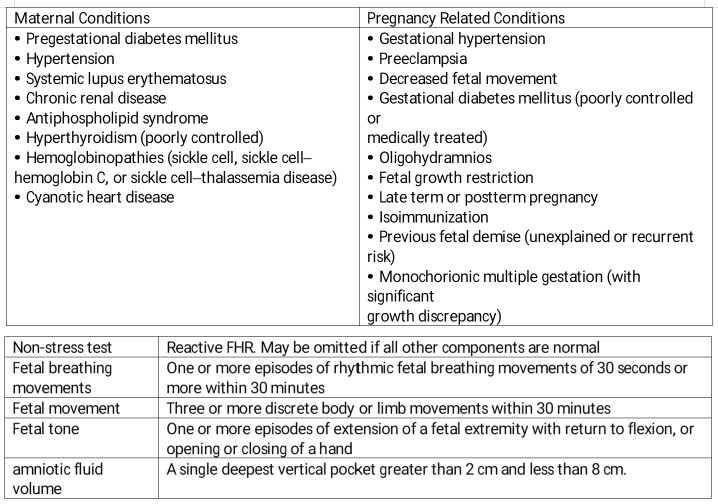
Indications for surveillance are individualized, considering the cumulative risk of stillbirth from multiple maternal, fetal, and placental complications, especially as the risk increases significantly in the final weeks of pregnancy. Shared decision-making between the patient and clinician is essential to balance the benefits and potential risks, such as false-positive results and associated costs. Ultimately, the guidance emphasizes tailoring the timing and frequency of surveillance to each unique clinical scenario.[2] Indications for antenatal fetal surveillance can be categorized as factors associated with the mother, fetus, or current and previous pregnancies, including:
Maternal Factors:
- Chronic hypertension
- Gestational hypertension or preeclampsia
- Diabetes
- Systemic lupus erythematosus
- Antiphospholipid syndrome
- Sickle cell disease
- Renal disease
- Thyroid disease
- Substance abuse
- In vitro fertilization
- Maternal age 35 or older
- Obesity [2]
Fetal Factors:
- Growth restriction
- Multiple gestations
- Fetal anomalies
- Decreased fetal movement [2]
Obstetric Factors:
- History of previous adverse pregnancy outcomes, such as preterm delivery due to preeclampsia or growth restriction
- Previous stillbirth
- Cholestasis
- Postterm pregnancy
- Abnormal serum markers
- Chronic placental abruption
- Vasa previa
- Velamentous cord insertion
- Polyhydramnios
- Single umbilical artery
- Oligohydramnios [2]
Contraindications
Relative contraindications for antenatal fetal surveillance emphasize the importance of individualized decision-making between the clinician and the pregnant individual, especially in high-risk pregnancies or those with multiple comorbidities. Initiating surveillance before 32 weeks should be approached with caution due to the high rate of nonreactive NSTs in preterm fetuses. The CST should be interpreted carefully, as frequent contractions or prolonged decelerations can lead to unnecessary delivery decisions. The NST, another standard surveillance method, also has limitations, particularly before 32 weeks of gestation, where nonreactive results are common due to fetal immaturity rather than distress.
Surveillance should be customized based on the overall clinical context, considering the high false-positive rates and the potential for unnecessary interventions. In the later stages of pregnancy, balancing the risks of surveillance against potential benefits, such as reducing stillbirth risk, is crucial. Furthermore, antenatal fetal surveillance is not useful in predicting outcomes or determining fetal well-being in patients with acute events, such as evolving placental abruption and cord prolapse. Such acute conditions require prompt clinical evaluation and may necessitate urgent delivery.[5][1][2]
Equipment
The equipment used to monitor fetal cardiac activity, maternal contractions, amniotic fluid volume, and vascular resistance without the umbilical cord for various antenatal fetal surveillance modalities include:
- External fetal monitor: This device, also called the cardiotocograph, records FHR and uterine contractions.
- Intravenous oxytocin: This device may be used during CSTs.
- Vibroacoustic stimulator: This device may be used during NST to elicit FHR accelerations.
- Ultrasound with Doppler velocimetry: This device is used for BPP, modified BPP, and umbilical artery Doppler velocimetry.[1]
Preparation
For NSTs, patients are typically positioned in the left lateral or semi-Fowler position to mitigate aortocaval compression frequently observed in the supine position. Studies have demonstrated conflicting findings regarding the optimal position, with some studies demonstrating reactive NSTs being achieved more quickly in the left lateral recumbent position. In contrast, others have shown the semi-Fowler position to be the most effective in attaining a reactive NST. A recent study concluded that the effectiveness of the left lateral or semi-Fowler position was similar. However, the patient's positional preference should be considered, as they may have a better sense of the position that maximizes fetal activity.[6] When performing a CST, the ACOG recommends placing patients in the lateral recumbent position.[1]
Technique or Treatment
Antenatal fetal surveillance includes several testing modalities, such as maternal perception of fetal movement, NSTs, CSTs, BPPs, modified BPPs, and umbilical artery Doppler velocimetry. In addition to selecting which testing method to use, the timing of initiation and frequency must also be determined. Although the ACOG offers some guidance, the optimal time to initiate antenatal fetal surveillance and testing frequency has not been established. Therefore, these surveillance methods must be tailored to the individual, weighing the potential risks, such as iatrogenic preterm delivery and increased healthcare costs, against the benefits. Shared decision-making is critical, particularly in high-risk pregnancies or those with multiple comorbidities, to ensure that the chosen surveillance approach aligns with the pregnant individual's goals and medical needs.[1][2]
Generally, 32 0/7 weeks of gestation is a reasonable point for most patients to begin antenatal fetal testing for well-being, as the NST is reactive in most fetuses by this time. Based on the indication for surveillance, this time frame may be adjusted. Similarly, the frequency of antenatal testing is influenced by the indication. Repeated surveillance is not necessary for conditions that may resolve, such as decreased fetal movement. For high-risk chronic conditions, a weekly frequency interval has been suggested for antenatal surveillance until delivery as long as the indication is present; however, this may be increased to twice a week or more for fetuses with the potential for rapid deterioration or in patients with abnormal findings during testing.[2]
In addition, clinicians should consider other clinical factors that may influence findings when interpreting antenatal fetal surveillance tests. In particular, maternal comorbidities, such as diabetes or fever, should be treated, and fetal surveillance should be repeated, as findings often improve in this setting.[1]
Maternal Perception of Fetal Movement
Many clinicians use maternal-fetal movement assessments, or kick counts, to monitor fetal well-being easily based on reports of a reduction in the mother's awareness of fetal movements preceding fetal demise by several days.[7][1] Moreover, findings of normal fetal movement are typically associated with normal development and function of fetal cardiovascular, neurologic, and musculoskeletal systems.[8] However, a meta-analysis of 5 randomized controlled trials involving over 450,000 fetuses revealed no significant difference in perinatal outcomes between those who performed kick counts and those who did not. However, slight increases in preterm delivery, labor induction, and cesarean delivery were noted in the kick counts group. Consequently, some authors have recommended further investigation to clarify this approach's benefits and potential harms.[1]
Various kick count protocols exist, with no established optimal number or duration. A standard method involves the mother lying on her side and counting movements until 10 distinct movements are felt within a 2-hour period, which was generally achieved in approximately 21 min. Another protocol advised counting movements for 1 hour 3 times a week, with counts considered reassuring if they met or exceeded the baseline count.[1]
Nonstress Test
Cardiotocography is the monitoring of FHR. NSTs monitor FHR through external abdominal transducers for a set amount of time, typically a minimum of 20 minutes. FHR is monitored using the Doppler ultrasound transducer, and the tocodynamometer is applied to detect uterine contractions or fetal movement. Fetal activity may be recorded by the patient using an event marker or noted by the staff performing the test. Clinicians have used this modality to assess fetal well-being for several decades. Variability, constant fluctuations from baseline FHR, decelerations, and a slowing of FHR can also be detected on NST. Moderate variability has an amplitude that ranges from 6 to 25 bpm.[9][10] An NST with FHR findings consistent with moderate variability and accelerations typically indicates normal development of the fetal central nervous and cardiovascular systems.[4]
The NST monitors FHR in response to fetal movements, which are evaluated as reactive or nonreactive. A reactive NST, indicating normal fetal autonomic function, is characterized by FHR accelerations that increase by at least 15 bpm above the baseline for a minimum of 15 seconds, suggesting fetal well-being. In contrast, a nonreactive NST, lacking sufficient accelerations, may require further evaluation.[1] Patients are typically placed in the left lateral or semi-Fowler position, and the NST is typically conducted for at least 20 minutes, sometimes extended to 40 minutes to account for fetal sleep cycles.[6] Vibroacoustic stimulation, using an artificial larynx, can be used to elicit fetal movement with FHR accelerations and reduce nonreactive NSTs. The ACOG recommends positioning the artificial larynx on the patient's abdomen for 1 to 2 seconds. If an FHR acceleration does not occur, the device may be repeated up to 3 times for up to 3 seconds.[1][6]
Contraction Stress Test
CSTs are typically performed to further evaluate patients with an abnormal NST. The CST assesses FHR response to uterine contractions to identify compromised fetal oxygenation, which can result in late FHR decelerations. Late decelerations are a decrease from the baseline FHR following a uterine contraction, with a duration of at least 30 seconds. Late decelerations are typified by their delayed onset, with the nadir of the deceleration observed following the contraction peak.[1]
During a CST, FHR and uterine contractions are recorded simultaneously. If spontaneous contractions are insufficient, they may be spontaneous or induced by nipple stimulation or intravenous oxytocin. An adequate uterine contraction pattern is characterized as at least 3 contractions within 10 minutes, with each contraction lasting a minimum of 40 seconds.[1] CSTs should typically be avoided in patients who have contraindications to labor or vaginal delivery.[1]
Biophysical Profile
The BPP has been studied since the 1970s. Initial studies were in fetal lambs to analyze cardiovascular and metabolic changes experienced under controlled environments that altered maternal pCO2 and cardiac output. Reflections in the fetal-placental, umbilical, and cardiovascular hemodynamics were monitored using vascular catheters and blood sampling. In addition, the ultrasound findings and FHR patterns were studied. In the 1990s, the correlation between these variables came together in a landmark study that identified a linear relationship between the BPP score and umbilical cord venous pH sampled through cordocentesis. Scores of 8 and 10 consistently had normal cord pH levels. However, a score of 0 indicated a mean cord pH of 7.07, prompting urgent intervention or delivery. Researchers further broke down the scores and found that abnormal amniotic fluid correlated with a lower pH compared to short-term abnormalities, such as an abnormal NST or absent fetal breathing movements.[11]
In the continuum of fetal distress, acidosis, hypoxia, and asphyxia, the fetal breathing movements are lost first, followed by body movements, then extremity tone. However, the precise point at which these behaviors cease is unclear.[1] Based on this principle, the BPP combines the following 5 components:
- A reactive NST
- Specific ultrasound findings
- Fetal breathing movements
- Fetal movements
- Fetal tone
- Amniotic fluid volume [1]
Each component is scored as either a 2 if observed to a sufficient degree or 0 if absent. The ultrasound observations are considered sufficient based on established criteria. For fetal breathing, ≥1 episode of fetal rhythmic breathing lasting ≥30 seconds must occur within 30 minutes. Sufficient fetal body movement is defined as at least 3 movements of the body or limbs within 30 minutes; fetal tone is considered to be present if ≥1 extension and flexion of a limb or opening and closing of a hand is observed during the test. A score of 2 is given for amniotic fluid volume if a single deepest vertical pocket that does not contain portions of the umbilical cord or fetal extremities of >2 cm can be measured (see Image. Ultrasound Biophysical Profile).
Modified Biophysical Profile
The modified BPP, often used in the late second or third trimester, includes an NST and amniotic fluid volume assessment to evaluate short-term fetal acid-base status and long-term placental function, respectively. The amniotic fluid volume assessment is based on placental physiological function, in which normal placental perfusion results in adequate blood flow to the fetal kidneys, as reflected by normal amniotic fluid volumes.[1] Therefore, decreased amniotic fluid volume in patients with intact membranes may indicate placental insufficiency.
Umbilical Artery Doppler Velocimetry
Umbilical artery Doppler velocimetry, typically used in pregnancies complicated by fetal growth restriction, assesses vascular resistance by analyzing flow velocity waveforms. This modality is not clinically valuable for fetuses with normal growth patterns. Doppler imaging is used in fetuses with suspected growth impairment in addition to NST or BPP. Studies have shown that umbilical vascular resistance progressively decreases in placentas with physiologically normal function as gestational age increases. However, in growth-restricted fetuses with placental insufficiency, the umbilical artery impedance increases until absent end-diastolic flow and reversed end-diastolic flow become apparent secondary to flow being redirected to the fetal brain.[1] Consequently, an umbilical artery Doppler velocimetry with these findings is associated with an increased perinatal mortality rate. Trained technicians evaluate umbilical artery Doppler velocimetry by measuring the peak systolic velocity, the frequency shift, the end-diastolic frequency shift, and the mean peak frequency shift over the cardiac cycle according to various ratios, including the systolic to diastolic ratio, resistance index, and pulsatility index. Umbilical placental insufficiency is reflected in these ratios, which increase abnormally as gestational age progresses instead of decreasing, as observed with normal placental function.[12]
Clinicians have also used Doppler velocimetry of other fetal vasculature, primarily the middle cerebral artery, due to several studies demonstrating the association between decreased vascular resistance in the middle cerebral artery and fetal growth restriction and hypoxemia. To increase oxygenation of the fetal brain, fetal blood flow is redistributed to the cerebral vessels at the expense of the fetal body. This redistribution is observed in the reduced pulsatility index caused by middle cerebral artery vasodilatation, which decreases as hypoxemia becomes more severe.[13] However, although professional societies do recommend the use of umbilical artery Doppler velocimetry, some, such as the Society for Maternal-Fetal Medicine, have not suggested the addition of middle cerebral Doppler velocimetry due to the lack of studies demonstrating its clinical relevance regarding improved fetal outcomes.[1][13]
Complications
Antenatal fetal surveillance carries the potential for several harms, including false-positive results that can lead to unnecessary additional evaluations or interventions, such as iatrogenic preterm birth. False-negative results, which fail to signal the need for further assessment, also pose a risk.[2] Some experts have also suggested that a risk of antenatal fetal surveillance may be the delivery of infants with severe conditions, such as cerebral palsy, due to nonreassuring test results, potentially resulting in the survival of fetuses with a permanent neurological impairment that might otherwise have resulted in fetal demise.[14]
In addition, the effects of antenatal testing on maternal mental health are not well understood; testing may induce complications such as anxiety but also provide reassurance if results are normal.[15] Costs associated with antenatal testing include financial expenditures, time spent by patients and healthcare practitioners, and potential maternal and infant morbidity from unnecessary interventions due to false-positive results.[16][3]
Generally, the use of ultrasound in obstetrics is considered safe. However, the ACOG guided the limited use of interventions in pregnancy.[17][18][19] The ACOG stated that although ultrasound is accepted as a safe study, the potential effects of prolonged or repeated exposure to ultrasound waves on a fetus can not be definitively determined. Therefore, the As Low As Reasonably Achievable (ALARA) principle is encouraged, in which interventions, such as ultrasound, are only implemented with specific indications.[20] Clinicians should be mindful of the thermal energy released by the probe, which shows in the margins of the ultrasound display as a thermal index, a ratio of the acoustic power emitted by the transducer to the power necessary to raise the temperature of the tissue 1 °C anywhere along the beam. Ideally, recommendations are for settings that have the lowest thermal index.
Clinical Significance
Antenatal fetal surveillance techniques are used to assess the risk of fetal death in pregnancies complicated by preexisting maternal conditions, such as diabetes, and those with developed complications, such as fetal growth restriction.[9] During testing using any of these modalities, deviation from the normal findings can be associated with fetal compromise and require further evaluation. In addition, the interpretation of nonreassuring findings should be informed by the entire clinical picture, including gestational age and maternal medications. For instance, central nervous system depressants, including narcotics, phenobarbital, magnesium sulfate, and beta-blockers, such as propranolol, can reduce FHR reactivity.[21] FHR accelerations also demonstrate decreases in some smokers.[22]
Maternal Perception of Fetal Movement
Several studies have shown that maternal perception of an overall decrease in fetal movement is more predictive of adverse outcomes compared to a cutoff of a specified number of kicks.[7] Therefore, with any fetal movement assessment used, if kick counts are not reassuring or a patient reports a qualitative decrease in fetal movement, further fetal assessment is recommended using an NST, CST, BPP, or modified BPP.[1]
Nonstress Test
The NST monitors FHR in response to fetal movements, assessing whether it is reactive or nonreactive. A reactive NST, indicating normal fetal autonomic function, is characterized by 2 FHR accelerations that each increase by at least 15 bpm above the baseline for a minimum of 15 seconds, suggesting fetal well-being, while a nonreactive NST, lacking sufficient accelerations, may require further evaluation with either a CST or BPP.[1]
Other findings, including variable and prolonged decelerations, may also be observed and must be interpreted in conjunction with other clinical factors. Variable decelerations, which are rapid decreases in the FHR lasting <30 seconds and are nonrecurring, typically do not necessitate intervention as they are not associated with impaired fetal status. However, repetitive variable decelerations, defined as 3 variable decelerations within 20 minutes, and prolonged FHR decelerations, lasting ≥1 minute, are associated with a nonreassuring intrapartum FHR pattern and potential fetal demise. Therefore, additional testing, such as BPP, is typically performed to assess these patients further.[1]
The NSTs of most preterm fetuses are frequently nonreactive; from 24 weeks, up to 50% of NSTs may be nonreactive, and from 28 to 32 weeks of gestation, 15% of NSTs are not reactive. For preterm fetuses between 24 and 32 weeks, the predictive value of NSTs is based on a lower threshold of acceleration of at least 10 beats per minute from baseline, and it has been found to predict fetal well-being sufficiently.[9]
Contraction Stress Test
The CST results are classified based on the presence or absence of late decelerations. The ACOG provides the following interpretations for CST findings:
- Negative CST: No late or variable decelerations are observed.
- Positive CST: Late decelerations are observed following ≥50% of contractions.
- Suspicious CST: Intermittent late decelerations or prolonged variable decelerations are observed.
- Equivocal CST: FHR decelerations more than every 2 minutes or lasting longer than 90 seconds associated with contractions.
- Unsatisfactory CST: <3 contractions within 10 minutes or FHR with inadequate tracing [1]
Biophysical Profile
The clinical significance of the BPP is based on clinical factors, such as gestational age, and the total score. A total score of 8 or 10 indicates fetal well-being. A BPP score of 6 is equivocal, and ≤4 is abnormal. However, isolated oligohydramnios associated with any score indicates further evaluation, including evaluation for premature rupture of membranes.[1]
Interventions may be considered based on BPP findings and gestational age.[1] For the following scores, the ACOG suggests the following management approaches:
- 6 out of 10: This score is considered equivocal; delivery should be strongly considered in patients 37 0/7 weeks of gestation or more. In patients less than 37 weeks of gestation, further evaluation through a repeat BPP within 24 hours is recommended.
- 4 out of 10: Delivery is recommended for patients 32 weeks of gestation or more; extended monitoring may be considered for those less than 32 weeks of gestation.
- 2 out of 10: Delivery is recommended in most cases; in patients where delivery is not being considered, the ACOG recommends the BPP not be repeated, as a decision of whether or not to deliver is not being made.[1]
If delivery is planned based on the BPP, induction of labor with continuous intrapartum monitoring is reasonable if no other vaginal delivery contraindications are present. Intervention recommendations vary slightly in cases of isolated oligohydramnios and no other abnormal BPP findings. The ACOG recommends delivery in patients at 36 0/7 weeks of gestation or more with persistent oligohydramnios not secondary to ruptured membranes. In patients at less than 36 0/7 weeks of gestation with intact membranes and oligohydramnios, delivery should be based on the risk of imminent maternal-fetal deterioration versus the risk of prematurity. If a clinician chooses to continue expectant management, serial amniotic fluid volume, NSTs, and fetal growth assessments should be performed.[1]
Modified Biophysical Profile
Normal findings for a modified BPP comprise a reactive NST and an amniotic fluid volume >2 cm in the deepest vertical pocket. If either of these components is abnormal, the modified BPP is reported as abnormal and should prompt further evaluation with a full BPP or CST.[1]
Umbilical Artery Doppler Velocimetry
Abnormal waveforms, such as absent or reversed end-diastolic flow, indicate increased perinatal mortality and morbidity and are linked to placental pathology and fetal hypoxemia. Multiple waveforms are evaluated to ensure accurate assessment.[1]
Enhancing Healthcare Team Outcomes
Effective antenatal fetal surveillance requires a collaborative approach involving various health professionals, each contributing unique skills and responsibilities to enhance patient-centered care, improve outcomes, ensure patient safety, and optimize team performance. Physicians play a pivotal role in diagnosing and managing conditions requiring fetal surveillance, interpreting complex data, and making critical decisions about the care plan. Advanced practitioners, such as nurses and physician assistants, support these efforts by conducting detailed patient assessments, counseling, and managing routine follow-up visits. Nurses are essential in educating and supporting patients, performing initial assessments, and monitoring fetal well-being through NSTs and other methods. Pharmacists contribute by ensuring the safe and effective use of medications required during antenatal care, such as those used to manage conditions such as hypertension or diabetes.
Interprofessional communication is crucial in this context, as timely and accurate information exchange among team members ensures coordinated and efficient care. For instance, nurses must promptly communicate any findings from fetal monitoring to physicians or advanced practitioners, who may then need to update the team on changes in the patient's condition or response to treatment. Care coordination involves scheduling and managing appointments, ensuring all necessary tests are performed, and maintaining comprehensive records accessible to all team members.
By leveraging the strengths and expertise of each team member, the healthcare team can provide holistic and responsive care that prioritizes the well-being of both the mother and the fetus. Ultimately, this integrated approach to antenatal fetal surveillance ensures that patients receive the highest quality of care, minimizes risks, prevents errors, and enhances patient safety by ensuring no aspect of care is overlooked, leading to better health outcomes and patient satisfaction.
Media
(Click Image to Enlarge)
References
. Antepartum Fetal Surveillance: ACOG Practice Bulletin, Number 229. Obstetrics and gynecology. 2021 Jun 1:137(6):e116-e127. doi: 10.1097/AOG.0000000000004410. Epub [PubMed PMID: 34011889]
. Indications for Outpatient Antenatal Fetal Surveillance: ACOG Committee Opinion, Number 828. Obstetrics and gynecology. 2021 Jun 1:137(6):e177-e197. doi: 10.1097/AOG.0000000000004407. Epub [PubMed PMID: 34011892]
Level 3 (low-level) evidenceDavies-Tuck ML, Davey MA, Hodges RL, Wallace EM. Fetal surveillance from 39 weeks' gestation to reduce stillbirth in South Asian-born women. American journal of obstetrics and gynecology. 2023 Sep:229(3):286.e1-286.e9. doi: 10.1016/j.ajog.2023.02.028. Epub 2023 Mar 11 [PubMed PMID: 36907532]
David AL, Spencer RN. Clinical Assessment of Fetal Well-Being and Fetal Safety Indicators. Journal of clinical pharmacology. 2022 Sep:62 Suppl 1(Suppl 1):S67-S78. doi: 10.1002/jcph.2126. Epub [PubMed PMID: 36106777]
Brecher A, Tharakan T, Williams A, Baxi L. Perinatal mortality in diabetic patients undergoing antepartum fetal evaluation: a case-control study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2002 Dec:12(6):423-7 [PubMed PMID: 12683655]
Level 2 (mid-level) evidencePatel R, Smitha MV, Jena SK, Jacob J, John J. Do different positions during a non-stress test affect the maternofetal physiological parameters and comfort in pregnant women? Journal of education and health promotion. 2022:11():386. doi: 10.4103/jehp.jehp_641_22. Epub 2022 Nov 26 [PubMed PMID: 36618471]
Niles KM, Jain V, Chan C, Choo S, Dore S, Kiely DJ, Lim K, Roy Lacroix ME, Sharma S, Waterman E. Guideline No. 441: Antenatal Fetal Health Surveillance. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2023 Sep:45(9):665-677.e3. doi: 10.1016/j.jogc.2023.05.020. Epub [PubMed PMID: 37661122]
Monari F, Menichini D, Salerno C, Gei V, Facchinetti F, Neri I. Women's perception of fetal movements and perinatal outcomes: results of a prospective cohort study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2023 Dec:36(1):2193664. doi: 10.1080/14767058.2023.2193664. Epub [PubMed PMID: 37040928]
Preboth M. ACOG guidelines on antepartum fetal surveillance. American College of Obstetricians and Gynecologists. American family physician. 2000 Sep 1:62(5):1184, 1187-8 [PubMed PMID: 10997537]
Keegan KA Jr. The nonstress test. Clinical obstetrics and gynecology. 1987 Dec:30(4):921-35 [PubMed PMID: 3319323]
Manning FA, Snijders R, Harman CR, Nicolaides K, Menticoglou S, Morrison I. Fetal biophysical profile score. VI. Correlation with antepartum umbilical venous fetal pH. American journal of obstetrics and gynecology. 1993 Oct:169(4):755-63 [PubMed PMID: 8238129]
Dixit S, Dixit NA, Rawat A, Bajpai A, Alelyani M, Sabah ZU, Raghuwanshi S. Color Doppler ultrasound in high-low risk pregnancies and its relationship to fetal outcomes: a cross-sectional study. Frontiers in pediatrics. 2023:11():1221766. doi: 10.3389/fped.2023.1221766. Epub 2024 Feb 20 [PubMed PMID: 38444769]
Level 2 (mid-level) evidenceLees CC, Romero R, Stampalija T, Dall'Asta A, DeVore GA, Prefumo F, Frusca T, Visser GHA, Hobbins JC, Baschat AA, Bilardo CM, Galan HL, Campbell S, Maulik D, Figueras F, Lee W, Unterscheider J, Valensise H, Da Silva Costa F, Salomon LJ, Poon LC, Ferrazzi E, Mari G, Rizzo G, Kingdom JC, Kiserud T, Hecher K. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: an evidence-based approach. American journal of obstetrics and gynecology. 2022 Mar:226(3):366-378. doi: 10.1016/j.ajog.2021.11.1357. Epub 2022 Jan 10 [PubMed PMID: 35026129]
Level 3 (low-level) evidenceMcIntyre S, Blair E, Badawi N, Keogh J, Nelson KB. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstetrics and gynecology. 2013 Oct:122(4):869-877. doi: 10.1097/AOG.0b013e3182a265ab. Epub [PubMed PMID: 24084547]
Saastad E, Winje BA, Israel P, Frøen JF. Fetal movement counting--maternal concern and experiences: a multicenter, randomized, controlled trial. Birth (Berkeley, Calif.). 2012 Mar:39(1):10-20. doi: 10.1111/j.1523-536X.2011.00508.x. Epub 2012 Jan 9 [PubMed PMID: 22369601]
Level 1 (high-level) evidenceCulliney KA, Parry GK, Brown J, Crowther CA. Regimens of fetal surveillance of suspected large-for-gestational-age fetuses for improving health outcomes. The Cochrane database of systematic reviews. 2016 Apr 5:4(4):CD011739. doi: 10.1002/14651858.CD011739.pub2. Epub 2016 Apr 5 [PubMed PMID: 27045604]
Level 1 (high-level) evidenceSimpson L, Khati NJ, Deshmukh SP, Dudiak KM, Harisinghani MG, Henrichsen TL, Meyer BJ, Nyberg DA, Poder L, Shipp TD, Zelop CM, Glanc P. ACR Appropriateness Criteria Assessment of Fetal Well-Being. Journal of the American College of Radiology : JACR. 2016 Dec:13(12 Pt A):1483-1493. doi: 10.1016/j.jacr.2016.08.028. Epub 2016 Oct 28 [PubMed PMID: 28029583]
Zelop CM, Javitt MC, Glanc P, Dubinsky T, Harisinghani MG, Harris RD, Khati NJ, Mitchell DG, Pandharipande PV, Pannu HK, Podrasky AE, Shipp TD, Siegel CL, Simpson L, Wall DJ, Wong-You-Cheong JJ, American College of Radiology. ACR Appropriateness Criteria® growth disturbances - risk of intrauterine growth restriction. Ultrasound quarterly. 2013 Sep:29(3):147-51. doi: 10.1097/RUQ.0b013e31829ea221. Epub [PubMed PMID: 23867573]
Maulik D. Management of fetal growth restriction: an evidence-based approach. Clinical obstetrics and gynecology. 2006 Jun:49(2):320-34 [PubMed PMID: 16721110]
. AIUM Practice Parameter for the Performance of Limited Obstetric Ultrasound Examinations by Advanced Clinical Providers. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2018 Jul:37(7):1587-1596. doi: 10.1002/jum.14677. Epub [PubMed PMID: 30133848]
Margulis E, Binder D, Cohen AW. The effect of propranolol on the nonstress test. American journal of obstetrics and gynecology. 1984 Feb 1:148(3):340-1 [PubMed PMID: 6695981]
Level 3 (low-level) evidencePhelan JP. Diminished fetal reactivity with smoking. American journal of obstetrics and gynecology. 1980 Jan 15:136(2):230-3 [PubMed PMID: 7352504]