Introduction
Preload, also known as left ventricular end-diastolic pressure (LVEDP), measures the degree of the ventricular stretch when the heart is at the end of diastole. Preload, in addition to afterload and contractility, is one of the 3 main factors that directly influence stroke volume (SV), the amount of blood pumped out of the heart in 1 cardiac cycle.[1] Affected by changes in venous tone and circulating blood volume, changes in preload directly affect stroke volume, influencing cardiac output and the heart's overall function. Understanding preload, its effects, and how pharmacological treatments can manipulate it is essential to understanding overall cardiac physiology (see Diagram. Cardiac Preload).[2]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
On a cellular level, preload is related to the intrinsic properties of the actin and myosin filaments that make up the myocardial muscle. Preload determines the resting length of the cardiac muscle fibers at a given LVEDP. Initially, when preload increases, the starting length of the muscle fibers also increases; consequentially, the resting tension increases. However, the amount of muscle fibers that shorten during contraction also increases correspondingly. As a result, the final length of the muscle fibers does not change dramatically.[2]
Mechanism
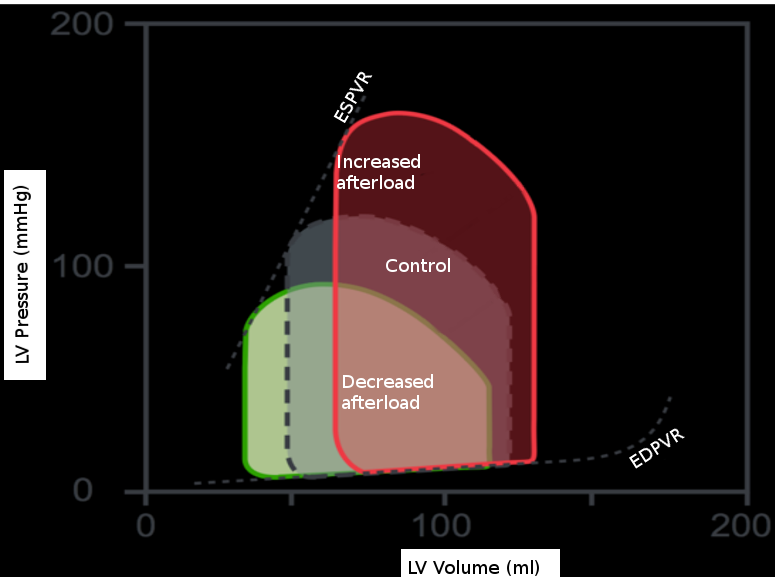
The effects of isolated changes in preload are best demonstrated on the pressure-volume (P-V) loop, which relates ventricular volume to the pressure inside the ventricle throughout the cardiac cycle. The P-V loop plots volume along the x-axis and pressure on the y-axis. The loop area is equal to the stroke volume, which refers to the amount of blood pumped out of the left ventricle in 1 cardiac cycle. This number is calculated as the end-diastolic volume (EDV), point B, minus the end-systolic volume (ESV), point A, on the graph. The effects of intramyocardial and extra myocardial events are plottable on the P-V loop. Changes in preload are visible as movements along the line show the end-diastolic P-V relationship. If afterload and contractility are held constant, an increase in preload results in a rightward shift along this line. As a result, the EDV increases, thus increasing stroke volume. Also, as EDV increases, the proportion of blood ejected by the heart increases slightly; this is the ejection fraction (EF) calculated by the equation (EDV-ESV)/EDV. The reverse is also true. A decrease in preload results in a leftward shift down the end-diastolic P-V line, decreasing EDV, stroke volume, and a slight decrease in ejection fraction.[3]
The P-V loop doesn’t account for the neurohormonal and reflex responses that can affect preload. For example, activating beta-adrenergic receptors leads to an increase in renin and antidiuretic hormone. Consequently, there is an increase in preload via salt and water retention. Further, stimulation of beta 1–adrenergic receptors, specifically, increases both the inotropy and lusitropy of the heart, which results in a shift of the end-diastolic P-V curve down and to the right as the time the heart spends in diastole decreases. The sympathetic stimulation of the alpha-1 receptors in the veins causes vasoconstriction and forces more blood in the veins to return to the heart, increasing preload. Additionally, the release of angiotensin II stimulates the release of aldosterone from the adrenal cortex; this causes additional sodium and water retention.[3]
Related Testing
One can estimate cardiac preload using a catheter by measuring the pulmonary capillary wedge pressure (PCW).[1] By advancing a catheter into the right or left pulmonary artery, the catheter can be fed into the smaller pulmonary artery branches and briefly block blood flow. This blockage creates a stagnant blood flow between the catheter tip and the pulmonary venous system, which feeds into the left atrium. Thus, the pressure recorded from the catheter in this placement estimates the left atrium pressure, known as the PCW. In a healthy heart, since the left atrium and left ventricle share a similar pressure during diastole, as blood flows freely across the mitral valve from the LA to the LV, the PCW can also be used to estimate the LV diastolic pressure; this is a measurement of preload.[4]
Pathophysiology
As demonstrated by the pressure-volume loops, left ventricular myocardial function is determined by the combination of preload, afterload, and contractility. Thus, preload changes are associated with many different clinical scenarios.[3]
Increases in preload, as demonstrated through an elevated PCW, are seen in several conditions, such as heart failure, mitral stenosis, and mitral regurgitation. At higher preloads, the heart also has an increased oxygen demand, further debilitating the already diseased heart. In cases of heart failure, eventually, the heart cannot keep up with the increased load, and deleterious ventricular remodeling and loss of function ensue.[2]
Abnormally low preload is associated with several related pathologies, including distributive and hypovolemic shock. For example, in the beginning phases of sepsis, a hypovolemic state induced by the capillary leak and low vascular resistance can lead to low preload and afterload. A similar response occurs in the setting of hemorrhage. Severe blood loss leads to a decrease in circulating blood volume. Consequently, it decreases the amount of blood returning to the heart, accounting for reduced stroke work and cardiac output in this setting.[5]
Clinical Significance
A variety of commonly used medications affect cardiac preload. These are some of the first-line treatments for heart failure, myocardial ischemia, and hypertension. Drugs that decrease preload and the mechanism by which they work include:
- Angiotensin-converting enzyme (ACE) inhibitors: Interrupt the renin-angiotensin-aldosterone system (RAAS) system
- Angiotensin receptor blockers (ARBs): Interrupt the RAAS system
- Nitrates: Causes nitric oxide-induced vasodilation
- Diuretics: Promote the elimination of salt and water, decreasing overall intravascular volume
- Calcium Channel Blockers: Block calcium-induced vasoconstriction and decrease cardiac contractility [1][6][7][8][9]
These drugs have utility in cases such as the acute management of heart failure, where the goal is to reduce the blood volume the failing heart has to pump. By decreasing the volume overload experienced by the patient, using one or more of the above-listed medications, symptoms such as dyspnea and edema can improve rapidly. Long-term use of ACE Inhibitors or ARBs has been shown to lower mortality in chronic heart failure patients by decreasing the amount of filling pressure in the heart and downregulating the compensatory neurohormonal stimulation.[6] In treating myocardial ischemia, nitrates can decrease the amount of blood returning to the left ventricle and preload by causing venous dilation. As a result, the oxygen demand of the heart decreases. This process is crucial in the treatment of ischemia.[9] A third clinical scenario in which drugs may help to reduce preload is in treating hypertension. Specifically, one of the most effective IV medications for the treatment of hypertensive emergencies is sodium nitroprusside.[7]
Additionally, several nonpathological states may result in increased preload, including:
- Pregnancy
- Exercise
- Excessive sodium intake
- IV fluid [3]
Finally, using several bedside maneuvers and manipulations in preload may help diagnose murmurs associated with several conditions. For example, rapid squatting causes an increased volume in the LV at the end of diastole. This increase in preload increases the intensity of murmurs such as aortic stenosis, mitral regurgitation, and ventricular septal defect. Signified by a click, a later onset can be heard in mitral valve prolapse. In contrast, maneuvers that decrease preload, such as the Valsalva maneuver or standing up, increase the intensity of hypertrophic cardiomyopathy and cause an earlier onset in the click heard in mitral valve prolapse.[10]
Media
References
Fukuta H, Little WC. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart failure clinics. 2008 Jan:4(1):1-11. doi: 10.1016/j.hfc.2007.10.004. Epub [PubMed PMID: 18313620]
Sheth PJ, Danton GH, Siegel Y, Kardon RE, Infante JC Jr, Ghersin E, Fishman JE. Cardiac Physiology for Radiologists: Review of Relevant Physiology for Interpretation of Cardiac MR Imaging and CT. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015 Sep-Oct:35(5):1335-51. doi: 10.1148/rg.2015140234. Epub 2015 Jul 17 [PubMed PMID: 26186546]
Villars PS, Hamlin SK, Shaw AD, Kanusky JT. Role of diastole in left ventricular function, I: Biochemical and biomechanical events. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2004 Sep:13(5):394-403; quiz 404-5 [PubMed PMID: 15470855]
Holzheimer RG, Mannick JA. Surgical Treatment: Evidence-Based and Problem-Oriented. 2001:(): [PubMed PMID: 21028753]
Kreimeier U. Pathophysiology of fluid imbalance. Critical care (London, England). 2000:4 Suppl 2(Suppl 2):S3-7 [PubMed PMID: 11255592]
Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2019 Apr:29(2):231-243. doi: 10.1007/s10286-018-0572-5. Epub 2018 Nov 9 [PubMed PMID: 30413906]
Walter U, Waldmann R, Nieberding M. Intracellular mechanism of action of vasodilators. European heart journal. 1988 Jun:9 Suppl H():1-6 [PubMed PMID: 3049092]
Level 3 (low-level) evidenceOh GC, Lee HY, Chung WJ, Youn HJ, Cho EJ, Sung KC, Chae SC, Yoo BS, Park CG, Hong SJ, Kim YK, Hong TJ, Choi DJ, Hyun MS, Ha JW, Kim YJ, Ahn Y, Cho MC, Kim SG, Shin J, Park S, Sohn IS, Kim CJ. Comparison of effects between calcium channel blocker and diuretics in combination with angiotensin II receptor blocker on 24-h central blood pressure and vascular hemodynamic parameters in hypertensive patients: study design for a multicenter, double-blinded, active-controlled, phase 4, randomized trial. Clinical hypertension. 2017:23():18. doi: 10.1186/s40885-017-0074-0. Epub 2017 Sep 4 [PubMed PMID: 28879040]
Level 1 (high-level) evidenceLu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial Infarction: Symptoms and Treatments. Cell biochemistry and biophysics. 2015 Jul:72(3):865-7. doi: 10.1007/s12013-015-0553-4. Epub [PubMed PMID: 25638347]
Frank JE, Jacobe KM. Evaluation and management of heart murmurs in children. American family physician. 2011 Oct 1:84(7):793-800 [PubMed PMID: 22010618]