Introduction
Posttraumatic syringomyelia (PTS) is a progressive neurological condition characterized by the formation of a fluid-filled cavity, or syrinx, within the spinal cord following spinal cord injury (SCI). The pathophysiology of PTS is multifactorial, involving altered cerebrospinal fluid (CSF) dynamics, inflammation, gliosis, and mechanical stress leading to syrinx formation and expansion. The condition may develop months to years after the initial trauma, with a prevalence reported between 3% and 30% among patients with SCI. PTS is a significant cause of delayed neurological deterioration, often manifesting as pain, spasticity, sensory disturbances, autonomic dysfunction, and motor weakness.
The diagnosis of PTS relies on clinical suspicion and imaging studies, with magnetic resonance imaging being the gold standard for detecting syrinx formation and associated spinal cord changes. Management strategies range from conservative symptomatic treatment to surgical intervention, such as syrinx drainage, CSF flow restoration, and spinal cord untethering. Despite advancements in surgical techniques, PTS remains a challenging condition with a high recurrence rate and variable outcomes. A comprehensive understanding of its pathophysiology, risk factors, and management strategies is crucial for improving patient care and preventing long-term morbidity in individuals with posttraumatic SCI.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The development of PTS is multifactorial, arising from a complex interplay of mechanical, vascular, and inflammatory factors following SCI. The primary inciting event is typically trauma leading to spinal cord contusion, hemorrhage, or ischemia, which initiates a cascade of secondary injury mechanisms, including disruption of CSF flow, gliosis, and inflammation. Over time, these processes form and expand a syrinx, a fluid-filled cavity within the spinal cord.
A significant contributing factor in PTS is the obstruction of normal CSF dynamics, which can result from vertebral fractures, scar tissue, arachnoid adhesions, penetrating injuries, and arachnoid scarring that occurs even in the absence of overt trauma. Posttraumatic scarring and adhesions within the subarachnoid space can create a physical block in CSF circulation, leading to abnormal pressure gradients that force fluid into the spinal cord parenchyma. This process, often described by the "piston theory," suggests that increased subarachnoid CSF pressure transmits force into the cord through perivascular spaces, gradually enlarging the syrinx.[1] Additionally, spinal canal stenosis and bony deformities such as kyphosis increase the risk of syrinx formation by further disrupting CSF flow dynamics.
Ischemia-reperfusion injury following SCI leads to microvascular damage, promoting chronic inflammation and gliotic scarring, which further disrupts CSF circulation and contributes to syrinx formation. Biomechanical alterations in the injured spinal cord, such as tethering from posttraumatic fibrosis, exert longitudinal traction on the cord and exacerbate syrinx expansion. Persistent low-grade inflammation in the injured spinal cord environment leads to extracellular matrix remodeling and barrier dysfunction, allowing fluid accumulation.
Traffic accidents are the leading cause of PTS, with the thoracic spine being the most commonly affected region.[2] Genetic susceptibility, individual variations in CSF dynamics, and preexisting spinal abnormalities may predispose certain patients to syrinx formation. Ultimately, PTS arises from the convergence of these pathological mechanisms, resulting in progressive syrinx expansion and neurological deterioration.
Epidemiology
PTS is a relatively common yet often underdiagnosed complication of SCI, affecting approximately 3% to 9% of patients with a history of spinal trauma. However, radiological studies suggest that the prevalence may be as high as 30% over 30 years of follow-up, particularly as advanced imaging techniques such as magnetic resonance imaging improve detection rates. Despite this, fewer than 10% of these cases exhibit clinical symptoms.[3][4][5][6][7] The onset of PTS is typically delayed, with symptoms developing months to decades after the initial injury, with a median time to diagnosis ranging between 9 and 15 years. Onset has been reported as early as 1 month and as late as 45 years postinjury.[8] The incidence tends to be higher in studies with longer follow-ups, as patients now survive longer after spinal cord injuries due to advancements in acute and rehabilitative care.
PTS is more frequently observed in individuals with complete or high-grade incomplete spinal cord injuries, particularly those classified as ASIA 'A' neurological status, who are at the highest risk of developing syrinx formation.[2][9][10][11] The thoracic spine is the most commonly affected region, likely due to its relatively rigid structure and vulnerability to traumatic forces, though cervical syrinxes are also prevalent. Men are more commonly affected, reflecting their higher involvement in motor vehicle accidents and extreme activities leading to spinal trauma. While no known racial or ethnic predisposition has been identified, the primary etiologies of spinal trauma leading to PTS vary geographically, with motor vehicle accidents, falls, sports injuries, and penetrating trauma being the leading causes. In developed countries, high-energy blunt trauma remains the predominant mechanism, while penetrating injuries contribute significantly in regions with higher rates of violence. Patients with associated spinal canal stenosis, posttraumatic kyphosis, or a history of prior spinal surgeries are at an increased risk of developing syrinx formation.
Pathophysiology
PTS develops due to altered CSF dynamics, spinal cord ischemia, and progressive tissue degeneration following SCI. The primary mechanism involves obstruction of normal CSF flow, leading to fluid accumulation within the spinal cord parenchyma, ultimately forming a syrinx. This obstruction can arise from scar tissue, arachnoid adhesions, meningeal fibrosis, or spinal deformities such as posttraumatic kyphosis and stenosis. Over time, the trapped CSF expands the syrinx, compressing surrounding neural structures and causing progressive neurological deterioration.
The exact pathophysiology of PTS is not fully understood, but several theories have been proposed, all of which relate to abnormalities in CSF flow dynamics.[6][12] Initially, a presyrinx state characterized by medullary edema may occur, followed by cavity formation at the injury site due to inflammation, autolysis, liquefaction of hematoma, cord infarction, and myelomalacia.[5][6][7][13] Key molecular contributors include betaine (an osmoprotectant), aquaporin channels, and damage to the blood-spinal cord barrier (BSCB).[3][14][15][16] Additionally, arachnoiditis, subarachnoid adhesions, fibrosis, and tethering can obstruct CSF outflow from the syrinx cavity, further exacerbating fluid accumulation.[17][18][19][20]
The "slosh effect" and "suck effect" are 2 interrelated mechanisms that contribute to syrinx expansion. CSF may be forced into the spinal cord due to obstruction from dural adhesions or scarring, creating a 1-way valve phenomenon. During actions like coughing and sneezing, CSF pressure changes, which are typically dissipated, become concentrated at the injury site, causing fluid to be driven into the spinal cord (slosh effect).[17] Additionally, differential pulse pressures above and below the injury site create a suction-like effect that further expands the syrinx.[5] The combination of these effects results in progressive cavitation.[13][21]
Furthermore, differential CSF pressures between 2 noncommunicating compartments (above and below the injury) lead to pulsation occurring only above the injury, forcing CSF into the spinal cord and promoting syrinx expansion. The osmotic gradient between hypertonic fluid surrounding the syrinx and CSF may further contribute to cavity enlargement. Some theories propose a "Venturi effect," where rapid CSF flow in a region of dural stenosis pulls the spinal cord outward, worsening the obstruction. Additionally, posttraumatic kyphosis and spinal canal stenosis can exacerbate the progression of PTS.[6][7][13]
Histopathology
The histopathological features of PTS reflect a chronic pathological process involving gliosis, cavitation, inflammatory changes, and vascular abnormalities within the spinal cord. Syrinx cavities can take on various forms, with some individuals having a single cavity while others develop multiple, often multiloculated cavities separated by tissue septations. These cavities are not filled solely with CSF but contain varying amounts of cellular elements and debris, including inflammatory cells, proteinaceous material, and hemosiderin from prior hemorrhages.
The syrinx cavity is typically lined by reactive astrocytes rather than a true epithelial lining, distinguishing it from congenital syringomyelia. The surrounding spinal cord tissue often exhibits a combination of demyelination, axonal loss, and necrosis, indicative of progressive damage due to chronic compression and ischemia.[14] Gliosis is a prominent feature, particularly at the margins of the syrinx, where reactive astrocytes form a glial scar. This scarring contributes to further obstruction of CSF flow and limits the potential for neural regeneration. The perisyringeal tissue often shows evidence of chronic inflammation, with infiltration of lymphocytes and macrophages. Microglial activation is frequently observed, further contributing to ongoing neuroinflammation and tissue degradation.
Vascular abnormalities are another hallmark of PTS. Disruption of the BSCB leads to increased vascular permeability, perivascular edema, and hemosiderin deposition, indicative of previous microhemorrhages.[16] Chronic hypoxia and ischemia in the affected spinal segments contribute to myelomalacia and further neural tissue loss. In some cases, areas of cystic degeneration and liquefactive necrosis can be seen within the syrinx cavity, particularly in long-standing cases.
Furthermore, the extracellular matrix surrounding the syrinx cavity often undergoes significant remodeling, with increased fibrous tissue and collagen deposition. This fibrosis, along with arachnoid adhesions and leptomeningeal scarring, contributes to further obstruction of CSF dynamics, perpetuating the syrinx formation and expansion cycle. Traditionally, it has been believed that syrinx cavities expand under pressure by dissecting through intramedullary tissue. Understanding these histopathological changes is critical in developing targeted therapeutic interventions, particularly those aimed at mitigating inflammation, restoring CSF flow, and preventing progressive spinal cord damage.
History and Physical
History
PTS has an unpredictable natural history, with a median time from injury to diagnosis ranging from 9 to 15 years.[22] However, cases have been reported as early as 1 month and as late as 45 years postinjury. The clinical presentation depends on the location, size, and extent of the syrinx.[13] Clinically, it is characterized by dissociated sensory loss, lower motor neuron involvement, the involvement of descending and ascending tract functions, and alterations of skin tropism.[13] Sometimes, even large syringes that involve the brainstem may be paradoxically asymptomatic.[13]
The most common initial symptom is neuropathic pain, which is often localized to the zone of injury or diffusely below the injury level. The pain is typically aggravated by activities that increase intrathoracic pressure, such as coughing, sneezing, or straining.[5][21] The pain can be aching, burning, stabbing, or tender to light touch or pressure.[23] Some patients may report an ascending sensory level, though they may not always be aware of this progression. Tissue tenderness in the zone of injury may feel like bruising despite the absence of visible bruising. Patients may report a loss in a previously present voiding reflex, bowel function, or erections. This is followed by sensory loss and motor weakness, respectively.[21]
Sensory loss is another hallmark of PTS, often presenting as a dissociated pattern due to spinothalamic tract involvement. The classic "cape-like" sensory loss occurs when the decussating spinothalamic fibers in the anterior white commissure are affected.[24][25] In some cases, motor weakness follows sensory loss, although it is typically a late finding. Some patients may retain motor function despite the presence of large syrinx cavities.[26] Previously present muscle stretch reflexes may be lost, and the dorsolateral location of the syrinx can sometimes cause atypical symptoms.[24] When the syrinx extends to the brainstem (syringobulbia), patients may develop involvement of lower cranial nerves, autonomic instability, and symptoms related to dysfunction of the reticular activating system.[13][18] Additionally, the involvement of sympathetic preganglionic neurons may lead to hyperhidrosis.[21] Despite the potential for significant neurological deficits, some patients with even large syringes remain paradoxically asymptomatic.
Physical Examination
The neurological examination in PTS varies depending on the extent of the syrinx but typically demonstrates a combination of upper and lower motor neuron signs, dissociated sensory loss, and autonomic dysfunction:
- Motor function
- Weakness in the upper or lower extremities, often asymmetric
- Lower motor neuron signs (muscle atrophy, fasciculations, and reduced reflexes) at the level of the syrinx
- Upper motor neuron signs (spasticity, hyperreflexia, clonus, and Babinski sign) below the level of the syrinx due to corticospinal tract involvement
- Sensory deficits
- Dissociated sensory loss with impaired pain and temperature sensation but preserved proprioception and vibration sense
- Classic "cape-like" distribution of sensory loss if the cervical spine is involved
- An ascending sensory level may be present, though not always noticed by the patient
- Reflexes
- Absent or diminished deep tendon reflexes at the level of the syrinx
- Hyperreflexia and pathological reflexes (eg, Babinski sign) below the lesion
- Spasticity and tone
- Increased muscle tone and resistance to passive movement in affected limbs
- Clonus (may be present in the ankles)
- Autonomic dysfunction
- Hyperhidrosis due to the involvement of sympathetic preganglionic neurons
- Blood pressure dysregulation, including orthostatic hypotension
- Bowel and bladder dysfunction (urinary retention or incontinence)
- Trophic changes
- Skin changes, hair loss, and ulceration in areas of chronic denervation
- Muscle atrophy, particularly in the hands, if the syrinx extends into the cervical cord
A thorough neurological examination is critical for detecting PTS early, particularly in patients with prior SCIs who develop new or worsening symptoms. Serial assessments are necessary, as the condition can progress over time. If PTS is suspected, further evaluation with imaging is warranted to confirm the diagnosis.
Evaluation
Laboratory Tests
While no specific laboratory tests confirm PTS, certain tests may be performed to rule out other conditions that mimic its presentation:
- Complete blood count and C-reactive protein
- To evaluate for infection if there is concern for an inflammatory or infectious etiology, such as arachnoiditis or meningitis
- Erythrocyte sedimentation rate
- To assess for chronic inflammation
- Vitamin B12 and folate levels
- To exclude nutritional deficiencies that can cause myelopathy
- Autoimmune panel
- To rule out demyelinating disorders, autoimmune myelitis, or neuromyelitis optica
- CSF analysis via lumbar puncture
- Rarely indicated but may be used if an infectious or inflammatory etiology (eg, chronic meningitis, tuberculosis, sarcoidosis) is suspected. Elevated protein and lymphocytosis may suggest chronic inflammation, while oligoclonal bands may indicate multiple sclerosis.
Imaging Studies
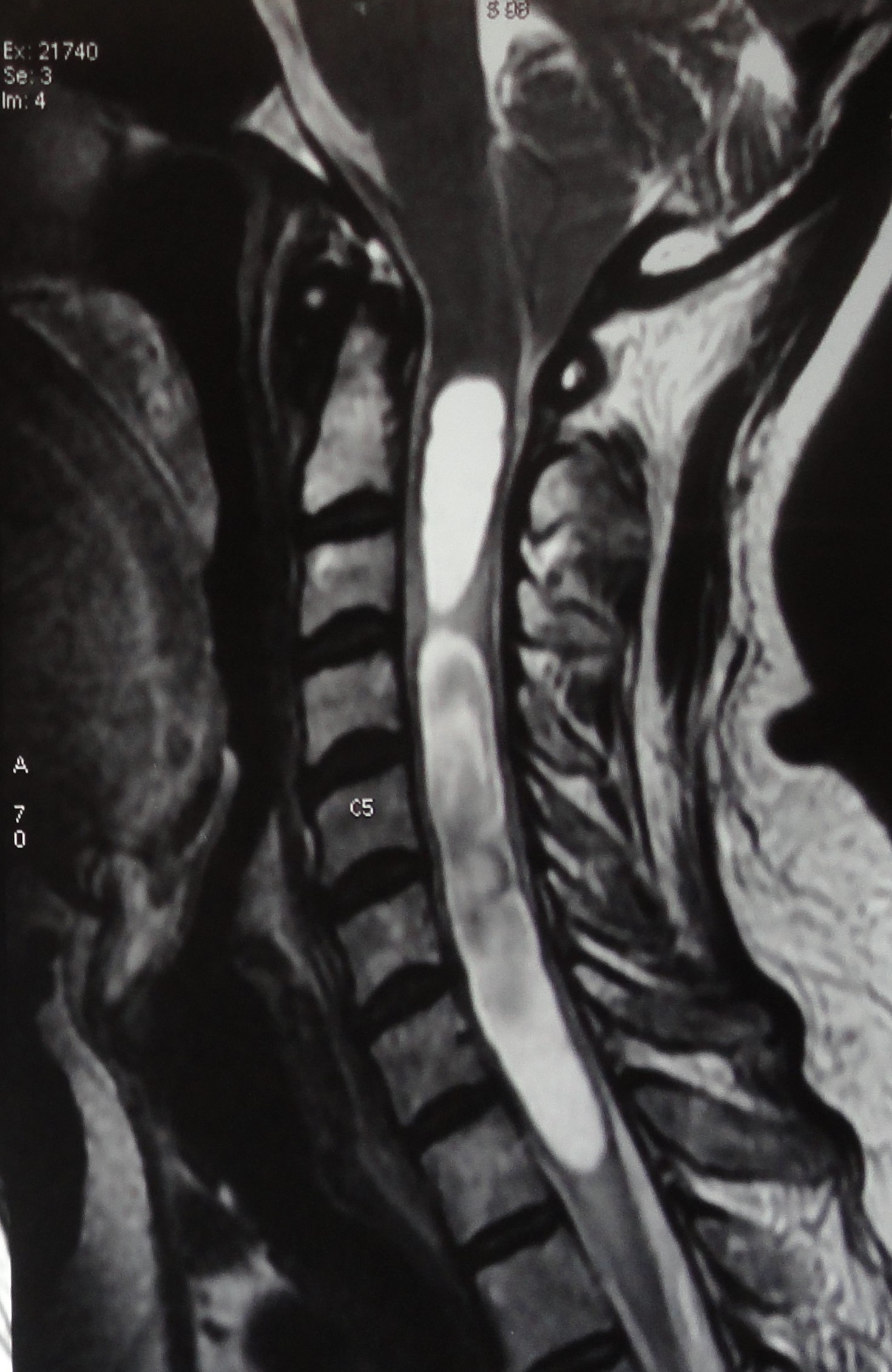
The gold standard for diagnosing PTS is a magnetic resonance image (MRI) of the spine, supplemented by other imaging modalities when necessary (see Image. Syringomyelia on Magnetic Resonance Imaging).[18]
-
MRI with and without contrast
- T1-weighted MRI
- Syrinx appears as a well-defined, hypointense cavity within the spinal cord.
- T2-weighted MRI
- Syrinx is hyperintense, best delineating its size and extent.
- Contrast-enhanced MRI
- Evaluates for associated arachnoiditis, spinal tumors, or inflammatory processes
- Cine MRI (CSF Flow Studies)
- Assesses CSF dynamics and obstruction at the level of prior spinal trauma or post-surgical adhesions. A lack of normal CSF pulsation around the syrinx suggests impaired CSF flow and supports a 1-way valve mechanism contributing to syrinx expansion.
- MRI is also used to monitor disease progression and evaluate postoperative outcomes.[22] Despite its high sensitivity, 10% to 50% of syringes may remain undetected.[18]
- T1-weighted MRI
-
Computed tomography myelography
- Used when MRI is contraindicated (eg, pacemakers, metallic implants) [18]
- Can detect arachnoid scarring, subarachnoid adhesions, cord tethering, and spinal canal stenosis
- Delineates obstruction to dye flow, helping evaluate dynamic CSF blockages
- Often advocated in cases where MRI is insufficient or unavailable
-
X-rays and CT scan of the spine
- These are useful for assessing spinal alignment, posttraumatic kyphosis, vertebral fractures, dislocations, or bony abnormalities contributing to CSF flow obstruction.
- Flexion-extension views help evaluate spinal instability.
Electrodiagnostic and Other Functional Tests
-
Somatosensory and motor evoked potentials
- This assesses conduction through the spinal cord and detects subclinical dysfunction in patients with mild or progressive deficits.
- Motor-evoked potentials can demonstrate central motor conduction delays and should be used intraoperatively, though this technology is not widely available.
-
Electromyography and nerve conduction studies
- Differentiate PTS from peripheral nerve compression or radiculopathy
- May reveal chronic denervation in affected myotomes and various forms of abnormal spontaneous activity
Emerging Imaging Techniques
Propagation-based synchrotron radiation microtomography
- A novel imaging technique used in animal models of PTS
- Provides 3-dimensional morphological and peri-syringeal microvasculature mapping, offering insights into disease pathogenesis [27]
Additional Testing
- Urodynamic studies
- For patients with suspected neurogenic bladder dysfunction
- Autonomic testing (eg, quantitative sudomotor axon reflex test, tilt-table test)
- In cases of suspected autonomic instability or hyperhidrosis
Treatment / Management
PTS is difficult to treat.[28] Some authors feel that motor loss is infrequent or late; therefore, conservative management is indicated.[10] This can stabilize or even improve symptomatology.[18] The syrinx may sometimes spontaneously recede.[29][30] Paradoxically, up to 68% of patients may experience neurologic worsening within a year. (A1)
Treating syrinx cavities is primarily surgical if they produce disabling pain or neurological deterioration (most common being motor weakness).[2][18] Most advocate for early surgery to reduce the progressively delayed deficit. Correction of the underlying cause is the main goal of surgical management.[6] The prime dictum of surgical treatment is restoring normal CSF flow dynamics (adhesiolysis, extradural decompression, or duraplasty) and surgical drainage of the syrinx (syringostomy, syringosubarachnoid, syringopleural, and syringoperitoneal shunts or cordectomy).[17][18][21][22] (B2)
Historically, syringostomy was the preferred treatment option for syringomyelia in the 1980s.[7] However, this was later replaced by drain placement.[7] Due to complications from these initial strategies, procedures such as decompression, adhesiolysis, and untethering were advocated.[7][18] Shunting the syrinx cavity (including syringoperitoneal and syringopleural shunts) is often attempted as a first-line treatment.[31][32][33] However, these shunts can become clogged with debris and may require replacement. The rate of shunt failure, along with the recurrence of the cavity and shunt-related complications, is notably high.[34] Other drainage procedures, such as needle aspiration, myelotomy, and cord opening, are less common.(B2)
More recently, surgical approaches that aim to restore normal CSF flow—especially through areas narrowed by dural scar tissue, adhesions, and spinal cord tethering—have gained favor. These techniques include laminectomy with intradural exploration, lysis of adhesions, and duraplasty to widen the CSF space.[33][35][36][37] While this is a posterior surgical approach, evaluating and addressing potential anterior tethering is important. Recurrence of syrinx formation is common, with rates of about 50% for adhesiolysis and 40% for syringopleural shunting.[2][14] While surgery may halt the progression of the syrinx, it often does not reverse neurological deficits.[14] The surgical management of PTS remains suboptimal, and long-term deterioration is frequently observed.[3] Some study results have reported that changes in syrinx size do not correlate with clinical outcomes.[11] Additionally, kyphotic correction plays a crucial role in the overall management of the condition.[6](B2)
Stem cell therapy, which includes treatments using autologous bone marrow-derived mesenchymal stromal cells, neuroepithelial-like stem cells, and fetal neural precursor cells, has shown promising results in reducing the size of syrinxes.[4][38][39][40] Physical therapy and exercise play a crucial role in enhancing the quality of life for patients with PTS.[25] Regular neurological examinations are essential for monitoring these patients. Handheld dynamometry of key muscle groups can serve as a valuable objective supplement to manual muscle testing.(B3)
Patients’ self-reports on changes in their functional abilities—such as walking, wheelchair propulsion, and transfers—are often the most significant indicators of the condition's progression. Additionally, individuals with cervical syringomyelia should be monitored for pulmonary function to assess any decline in vital capacity. Interdisciplinary evaluations by rehabilitation teams can help determine adjustments needed for mobility devices, seating, walking activities, and daily living tasks. This team approach aims to reduce the risks of complications such as pressure ulcers, decreased mobility, and falls.
Differential Diagnosis
The differential diagnoses for PTS include:
- Spinal instability
- Tethered spinal cord
- Subacute progressive ascending myelopathy (SPAM)
- Chronic relapsing ascending myelopathy (CRAM) syndrome [17][41]
SPAM, CRAM, or PTS constitute the extreme spectrum of the same disease process and only depend upon the time frame and the pace for the obliteration of the CSF space to take place.[17]
Prognosis
PTS has a gradual and deceptive progression, typically developing around 9 years after a traumatic SCI, although it can vary from 6 to 34 years.[17] While there is a correlation between the size of the syrinx and the severity of clinical symptoms, discrepancies between clinical presentation and imaging findings can occur due to the brain's plasticity and the extremely slow progression of PTS.[5] Over 10 years without treatment, 17% to 50% of patients may remain stable.[17] Research results indicate that the length of the syrinx, rather than its diameter, is a more significant factor in neurological decline.[13] Additionally, increased fractional anisotropy observed in tractography can heighten the risk of PTS formation.[42]
The presence of intradural adhesions plays a crucial role in determining patient outcomes.[16] Surgical intervention primarily benefits patients experiencing radicular symptoms but is less effective for improving autonomic symptoms or spasticity.[11] Changes in syrinx size do not necessarily correlate with clinical outcomes, and long-term neurological improvement is seen in fewer than 50% of patients undergoing surgery.[18][22] Among the different types of syringomyelia, the moniliform variant tends to have better surgical outcomes than the distended subtype.[43] Both anterior and posterior surgical approaches show similar outcomes.[44] Moreover, detethering accompanied by duraplasty has proven more effective than shunting in enhancing neurological outcomes.[4] Shunt failure or nonfunctional shunts can occur in nearly 50% of patients within 6 years of operation.[6]
Complications
Complications of PTS include:
- Bleeding into the syrinx
- Acute syringomyelia
- Syringobulbia
- Autonomic instability
- Burns to senseless areas
- Sensory-motor deterioration
- Loss of bowel and bladder function
- Neuropathic arthropathies (Charcot joint)
- Fall incidents
- Progressive scoliosis
- Emotional instability [13][21][22]
Complications of surgery for PTS include:
- Shunt failure
- Shunt dislocation
- Infection
- Recurrence [21]
Consultations
A neurosurgical consultation is recommended when surgery is being considered. An electrodiagnostic consultation may be necessary to rule out other differential diagnoses. SCI specialists and physical and occupational therapists should be involved to address issues related to mobility, seating, self-care, and neurogenic bowel function. Additionally, pain specialists should be included in the treatment plan. If a neurogenic bladder develops, consulting a neurourologic specialist is also advised.[45]
Deterrence and Patient Education
Deterrence of PTS primarily focuses on preventing SCI, early recognition of symptoms, and timely intervention to minimize long-term complications. Public health measures, such as seatbelt laws, helmet use, fall prevention strategies, and workplace safety protocols, play a critical role in reducing the incidence of traumatic spinal injuries that can predispose individuals to PTS. In patients with known spinal trauma, routine clinical and radiographic follow-up is essential to monitor for delayed syrinx formation. High-risk individuals, particularly those with ASIA A spinal cord injuries, kyphotic deformities, or prior spine surgeries, should be closely observed for signs of progressive neurological dysfunction.
Patient education is crucial in empowering individuals to recognize early symptoms of PTS, such as neuropathic pain, sensory loss, autonomic dysfunction, or progressive weakness. Patients should be informed that symptoms may emerge years to decades after the initial injury and that prompt evaluation can prevent irreversible neurological damage. Educating patients on avoiding activities that increase spinal strain, maintaining proper posture, and adhering to routine follow-ups can reduce the risk of progression. Additionally, lifestyle modifications, including weight management, smoking cessation, and physical therapy, may help mitigate complications.
For individuals diagnosed with PTS, education should emphasize treatment options, self-care strategies, and the importance of adhering to medical recommendations. Patients undergoing surgical intervention should understand the risks, benefits, and expected outcomes of procedures such as cord untethering, syrinx-subarachnoid shunting, or decompressive surgery. They should also be informed about potential recurrence and the need for long-term monitoring. By fostering patient engagement and shared decision-making, clinicians can enhance adherence to follow-up care and improve overall outcomes in PTS management.
Enhancing Healthcare Team Outcomes
Effective management of PTS requires a multidisciplinary approach to optimize patient-centered care, improve outcomes, and enhance patient safety. Clinicians, including neurosurgeons, physiatrists, and neurologists, play a central role in diagnosing, monitoring, and determining the need for surgical intervention. Advanced clinicians and nurses are essential in early symptom recognition, patient education, wound care, and postoperative monitoring. Rehabilitation specialists, including physical and occupational therapists, help manage motor dysfunction and prevent complications associated with prolonged immobility. Pharmacists contribute by optimizing pain management strategies and ensuring the safe use of neuropathic pain medications. A modest improvement in neurological function can significantly enhance the quality of life for patients and reduce overall healthcare costs.[24]
Interprofessional communication and care coordination is crucial for long-term follow-up, complication prevention, and patient adherence to treatment. Regular case discussions, shared electronic health records, and team-based rounds improve continuity of care. Nurses and therapists act as patient advocates, ensuring concerns are addressed promptly and that care plans are adjusted based on functional status. Clear documentation and communication across disciplines help mitigate delays in diagnosis or treatment and reduce the risk of preventable complications. A coordinated care approach ensures that patients receive holistic, individualized treatment that improves quality of life and minimizes long-term disability associated with PTS.
Media
(Click Image to Enlarge)
References
Roy AK, Slimack NP, Ganju A. Idiopathic syringomyelia: retrospective case series, comprehensive review, and update on management. Neurosurgical focus. 2011 Dec:31(6):E15. doi: 10.3171/2011.9.FOCUS11198. Epub [PubMed PMID: 22133183]
Level 3 (low-level) evidenceViana Bonan de Aguiar V, Batista G, Gepp R, Falavigna A. Epidemiological aspects of syringomyelia in a 19-year old cohort of spinal cord injury patients. Neurocirugia (English Edition). 2024 Nov-Dec:35(6):311-318. doi: 10.1016/j.neucie.2024.09.004. Epub 2024 Sep 30 [PubMed PMID: 39357742]
Level 2 (mid-level) evidenceJohnson L, Bartlett-Tomasetig F, Fok S, Whan R, Berliner J, Hemley SJ, Stoodley MA, Bilston LE. A novel method to quantify perivascular space enlargement near the syrinx in a rodent model of post-traumatic syringomyelia. Scientific reports. 2023 Sep 12:13(1):15043. doi: 10.1038/s41598-023-42275-y. Epub 2023 Sep 12 [PubMed PMID: 37700036]
Xu N, Xu T, Mirasol R, Holmberg L, Vincent PH, Li X, Falk A, Benedikz E, Rotstein E, Seiger Å, Åkesson E, Falci S, Sundström E. Transplantation of Human Neural Precursor Cells Reverses Syrinx Growth in a Rat Model of Post-Traumatic Syringomyelia. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2021 Apr:18(2):1257-1272. doi: 10.1007/s13311-020-00987-3. Epub 2021 Jan 19 [PubMed PMID: 33469829]
Pucci GF, Akita J, Berkowitz AL. Clinical-Radiologic Dissociation in Post-traumatic Syringomyelia. World neurosurgery. 2021 Sep:153():9-10. doi: 10.1016/j.wneu.2021.06.063. Epub 2021 Jun 18 [PubMed PMID: 34153481]
Gurjar H, Abhilash Reddy D, Tungish B. Complete resolution of syrinx after circumferential adhesiolysis without deformity correction in a case of 6 year old neglected cervical facet dislocation. Journal of clinical orthopaedics and trauma. 2023 Mar:38():102126. doi: 10.1016/j.jcot.2023.102126. Epub 2023 Feb 12 [PubMed PMID: 36866195]
Level 3 (low-level) evidenceKleindienst A, Engelhorn T, Roeckelein V, Buchfelder M. Development of pre-syrinx state and syringomyelia following a minor injury: a case report. Journal of medical case reports. 2020 Nov 18:14(1):223. doi: 10.1186/s13256-020-02568-6. Epub 2020 Nov 18 [PubMed PMID: 33203466]
Level 3 (low-level) evidenceSchurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. Journal of neurology, neurosurgery, and psychiatry. 1996 Jan:60(1):61-7 [PubMed PMID: 8558154]
Krebs J, Koch HG, Hartmann K, Frotzler A. The characteristics of posttraumatic syringomyelia. Spinal cord. 2016 Jun:54(6):463-6. doi: 10.1038/sc.2015.218. Epub 2015 Dec 1 [PubMed PMID: 26620880]
Bonfield CM, Levi AD, Arnold PM, Okonkwo DO. Surgical management of post-traumatic syringomyelia. Spine. 2010 Oct 1:35(21 Suppl):S245-58. doi: 10.1097/BRS.0b013e3181f32e9c. Epub [PubMed PMID: 20881468]
Level 1 (high-level) evidenceKaram Y, Hitchon PW, Mhanna NE, He W, Noeller J. Post-traumatic syringomyelia: outcome predictors. Clinical neurology and neurosurgery. 2014 Sep:124():44-50. doi: 10.1016/j.clineuro.2014.06.007. Epub 2014 Jun 17 [PubMed PMID: 25016238]
Lam S, Batzdorf U, Bergsneider M. Thecal shunt placement for treatment of obstructive primary syringomyelia. Journal of neurosurgery. Spine. 2008 Dec:9(6):581-8. doi: 10.3171/SPI.2008.10.08638. Epub [PubMed PMID: 19035753]
Level 2 (mid-level) evidenceScivoletto G, Masciullo M, Pichiorri F, Molinari M. Silent post-traumatic syringomyelia and syringobulbia. Spinal cord series and cases. 2020 Mar 13:6(1):15. doi: 10.1038/s41394-020-0264-y. Epub 2020 Mar 13 [PubMed PMID: 32170091]
Level 3 (low-level) evidencePukale DD, Adkins-Travis K, Aryal SR, Shriver LP, Patti GJ, Leipzig ND. Investigating post-traumatic syringomyelia and local fluid osmoregulation via a rat model. Fluids and barriers of the CNS. 2024 Feb 26:21(1):19. doi: 10.1186/s12987-024-00514-y. Epub 2024 Feb 26 [PubMed PMID: 38409031]
Berliner JA, Lam MA, Najafi E, Hemley SJ, Bilston LE, Stoodley MA. Aquaporin-4 expression and modulation in a rat model of post-traumatic syringomyelia. Scientific reports. 2023 Jun 14:13(1):9662. doi: 10.1038/s41598-023-36538-x. Epub 2023 Jun 14 [PubMed PMID: 37316571]
Yuan C, Xia P, Duan W, Wang J, Guan J, Du Y, Zhang C, Liu Z, Wang K, Wang Z, Wang X, Wu H, Chen Z, Jian F. Long-Term Impairment of the Blood-Spinal Cord Barrier in Patients With Post-Traumatic Syringomyelia and its Effect on Prognosis. Spine. 2024 Mar 15:49(6):E62-E71. doi: 10.1097/BRS.0000000000004884. Epub 2023 Nov 28 [PubMed PMID: 38014747]
Visagan R, Bandi S, Robinson L, Gadhok A, Saadoun S, Papadopoulos MC. Chronic relapsing ascending myelopathy: a treatable progressive neurological syndrome following traumatic spinal cord injury. British journal of neurosurgery. 2022 Dec:36(6):792-795. doi: 10.1080/02688697.2022.2102146. Epub 2022 Jul 22 [PubMed PMID: 35867035]
Azad TD, Materi J, Hwang BY, Mathios D, Lehner KR, Hansen L, Bernhardt LJ, Xia Y, Shah PP, Kannapadi NV, Theodore N. Spinal cord untethering and midline myelotomy for delayed, symptomatic post-traumatic syringomyelia due to retained ballistic fragments: case report. Spinal cord series and cases. 2022 Jul 12:8(1):66. doi: 10.1038/s41394-022-00533-7. Epub 2022 Jul 12 [PubMed PMID: 35831274]
Level 3 (low-level) evidenceGrassner L, Riemenschneider MJ, Altendorfer B, Grillhösl A, Arevalo-Martin A, Garcia-Ovejero D, Mach O, Maier D, Bierschneider M, Strowitzki M, Thomé C, Aigner L. Subarachnoid Fibrosis in Human Post-Traumatic Syringomyelia: A Prospective Observational Clinical Study. Journal of neuropathology and experimental neurology. 2022 Jan 29:81(2):149-153. doi: 10.1093/jnen/nlab121. Epub [PubMed PMID: 35094074]
Berliner JA, Woodcock T, Najafi E, Hemley SJ, Lam M, Cheng S, Bilston LE, Stoodley MA. Effect of extradural constriction on CSF flow in rat spinal cord. Fluids and barriers of the CNS. 2019 Mar 26:16(1):7. doi: 10.1186/s12987-019-0127-8. Epub 2019 Mar 26 [PubMed PMID: 30909935]
Mousele C, Georgiopoulos M, Constantoyannis C. Syringobulbia: A delayed complication following spinal cord injury - case report. The journal of spinal cord medicine. 2019 Mar:42(2):260-264. doi: 10.1080/10790268.2018.1439437. Epub 2018 Feb 27 [PubMed PMID: 29485364]
Level 3 (low-level) evidenceLi YD, Therasse C, Kesavabhotla K, Lamano JB, Ganju A. Radiographic assessment of surgical treatment of post-traumatic syringomyelia. The journal of spinal cord medicine. 2021 Nov:44(6):861-869. doi: 10.1080/10790268.2020.1743086. Epub 2020 Mar 30 [PubMed PMID: 32223591]
Kim HG, Oh HS, Kim TW, Park KH. Clinical Features of Post-Traumatic Syringomyelia. Korean journal of neurotrauma. 2014 Oct:10(2):66-9. doi: 10.13004/kjnt.2014.10.2.66. Epub 2014 Oct 31 [PubMed PMID: 27169036]
Lodhi MU, Kuzel AR, Syed IA, Rahim M. An Atypical Clinical Presentation of Post-traumatic Syringomyelia: A Case Report and Brief Review of the Literature. Cureus. 2017 Nov 16:9(11):e1852. doi: 10.7759/cureus.1852. Epub 2017 Nov 16 [PubMed PMID: 29372126]
Level 3 (low-level) evidenceGade SP, Harjpal P, Kovela RK. Novelty in Impact of Neurorehabilitation in a Classic Case of Syringomyelia. Cureus. 2022 Sep:14(9):e29126. doi: 10.7759/cureus.29126. Epub 2022 Sep 13 [PubMed PMID: 36258946]
Level 3 (low-level) evidenceGoldstein B, Hammond MC, Stiens SA, Little JW. Posttraumatic syringomyelia: profound neuronal loss, yet preserved function. Archives of physical medicine and rehabilitation. 1998 Jan:79(1):107-12 [PubMed PMID: 9440427]
Level 3 (low-level) evidenceLiao S, Ni S, Cao Y, Yin X, Wu T, Lu H, Hu J, Wu H, Lang Y. The 3D characteristics of post-traumatic syringomyelia in a rat model: a propagation-based synchrotron radiation microtomography study. Journal of synchrotron radiation. 2017 Nov 1:24(Pt 6):1218-1225. doi: 10.1107/S1600577517011201. Epub 2017 Oct 4 [PubMed PMID: 29091065]
Lee TT, Alameda GJ, Gromelski EB, Green BA. Outcome after surgical treatment of progressive posttraumatic cystic myelopathy. Journal of neurosurgery. 2000 Apr:92(2 Suppl):149-54 [PubMed PMID: 10763684]
Killeen T, Rosner J, Jutzeler CR, Hupp M, Heilbronner R, Curt A. Spontaneous resolution of an extensive posttraumatic syrinx. Neurology. 2016 Sep 20:87(12):1299-301. doi: 10.1212/WNL.0000000000003130. Epub 2016 Aug 19 [PubMed PMID: 27543642]
Maharaj MM, Phan K, Mobbs R. Spontaneous regression of post-traumatic syringomyelia: A case report and literature review. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017 Oct:44():249-253. doi: 10.1016/j.jocn.2017.06.065. Epub 2017 Jul 14 [PubMed PMID: 28712735]
Level 3 (low-level) evidenceSrikantha U, Hari A, Lokanath YK, Varma RG. Syringo-Subarachnoid Shunt Placement: A Minimally Invasive Technique Using Fixed Tubular Retractors-Three Case Reports and Literature Review. International journal of spine surgery. 2020 Apr:14(2):133-139. doi: 10.14444/7020. Epub 2020 Apr 30 [PubMed PMID: 32355617]
Level 3 (low-level) evidenceHussain I, Greenfield JP. Ultrasound-guided Syringosubarachnoid Shunt Insertion for Cervicothoracic Syringomyelia. Clinical spine surgery. 2020 Jun:33(5):185-191. doi: 10.1097/BSD.0000000000000835. Epub [PubMed PMID: 31220040]
Aghakhani N, Baussart B, David P, Lacroix C, Benoudiba F, Tadie M, Parker F. Surgical treatment of posttraumatic syringomyelia. Neurosurgery. 2010 Jun:66(6):1120-7; discussion 1127. doi: 10.1227/01.NEU.0000369609.30695.AB. Epub [PubMed PMID: 20495426]
Level 2 (mid-level) evidenceBatzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. Journal of neurosurgery. 1998 Sep:89(3):382-8 [PubMed PMID: 9724111]
Falci SP, Indeck C, Lammertse DP. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. Journal of neurosurgery. Spine. 2009 Oct:11(4):445-60. doi: 10.3171/2009.4.SPINE09333. Epub [PubMed PMID: 19929342]
Holly LT, Johnson JP, Masciopinto JE, Batzdorf U. Treatment of posttraumatic syringomyelia with extradural decompressive surgery. Neurosurgical focus. 2000 Mar 15:8(3):E8 [PubMed PMID: 16676931]
Level 3 (low-level) evidenceKlekamp J. Treatment of posttraumatic syringomyelia. Journal of neurosurgery. Spine. 2012 Sep:17(3):199-211. doi: 10.3171/2012.5.SPINE11904. Epub 2012 Jul 13 [PubMed PMID: 22794351]
Vaquero J, Hassan R, Fernández C, Rodríguez-Boto G, Zurita M. Cell Therapy as a New Approach to the Treatment of Posttraumatic Syringomyelia. World neurosurgery. 2017 Nov:107():1047.e5-1047.e8. doi: 10.1016/j.wneu.2017.08.019. Epub 2017 Aug 10 [PubMed PMID: 28804041]
Xu T, Li X, Guo Y, Uhlin E, Holmberg L, Mitra S, Winn D, Falk A, Sundström E. Multiple therapeutic effects of human neural stem cells derived from induced pluripotent stem cells in a rat model of post-traumatic syringomyelia. EBioMedicine. 2022 Mar:77():103882. doi: 10.1016/j.ebiom.2022.103882. Epub 2022 Feb 16 [PubMed PMID: 35182996]
Vaquero J, Zurita M, Rico MA, Aguayo C, Fernandez C, Rodriguez-Boto G, Marin E, Tapiador N, Sevilla M, Carballido J, Vazquez D, Garcia-Olmo D, Guadalajara H, Leon M, Valverde I, Neurological Cell Therapy Group From Puerta De Hierro-Majadahonda Hospital. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. 2018 Jun:20(6):796-805. doi: 10.1016/j.jcyt.2018.04.006. Epub 2018 May 18 [PubMed PMID: 29784434]
Levi V, Franzini A, Di Cristofori A, Bertani G, Pluderi M. Subacute posttraumatic ascending myelopathy (SPAM): A potential complication of subarachnoid shunt for syringomyelia? The journal of spinal cord medicine. 2020 Sep:43(5):714-718. doi: 10.1080/10790268.2018.1512735. Epub 2018 Aug 29 [PubMed PMID: 30156977]
Zhang C, Chen K, Han X, Fu J, Douglas P, Morozova AY, Abakumov MA, Gubsky IL, Li D, Guo J, Zhang X, Wang G, Chekhonin VP. Diffusion Tensor Imaging in Diagnosis of Post-Traumatic Syringomyelia in Spinal Cord Injury in Rats. Medical science monitor : international medical journal of experimental and clinical research. 2018 Jan 9:24():177-182 [PubMed PMID: 29311540]
Lu C, Guan J, Ding C, Wang X, Wang Z, Chen Z, Wu H, Jian F. Post-traumatic syringomyelia resolution following surgical treatment: the moniliform syrinx with a better prognosis. Acta neurologica Belgica. 2023 Jun:123(3):1061-1071. doi: 10.1007/s13760-023-02233-x. Epub 2023 Mar 17 [PubMed PMID: 36930392]
Fadhil M, Wilson PJ, Reddy R. Does Direct Surgical Decompression After Traumatic Spinal Cord Injury Influence Post-Traumatic Syringomyelia Rates? An 18-Year Single-Center Experience. World neurosurgery. 2022 May:161():e664-e673. doi: 10.1016/j.wneu.2022.02.074. Epub 2022 Feb 21 [PubMed PMID: 35202879]
Caremel R, Hamel O, Gerardin E, Lenormand L, Parker F, Lefort M, Grise P, Perrouin-Verbe B. [Post-traumatic syringomyelia: What should know the urologist?]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2013 Jan:23(1):8-14. doi: 10.1016/j.purol.2012.09.009. Epub 2012 Oct 15 [PubMed PMID: 23287478]