Introduction
Porcelain aorta (PA) is a structural aortic wall disease characterized by extensive heavy calcification of the ascending thoracic aorta extending to the aortic arch and descending aorta. The calcification occurs in a diffuse complete or near-complete circumferential pattern involving, predominantly, the ascending aorta's anterior wall and the aortic arch's superior wall.[1][2]
The definition of porcelain aorta is not clear or standard, and it varied between authors who described it. The common denominator that best describes the clinical problem is aortic calcification that interferes with aortic cannulation, aortic clamping, aortotomy, or central coronary bypass anastomosis, necessitating modification of the surgical technique to avoid complications.[1] The presence of PA complicates the successful performance of surgical and interventional procedures, and the aortic calcification has been associated with an increased risk of periprocedural complications and is an independent predictor of mortality in surgical patients.
Classification
Calcium deposition in porcelain aorta can be located in the tunica intima, starting at the base of atherosclerotic plaques, known as the atherosclerotic type. While in the non-atherosclerotic type, calcification usually occurs in the tunica media of the aortic wall.[2]
Furthermore, porcelain aorta can also be classified into two main types based on the site of calcification in the thoracic aorta as follows:
- Type I - implies the localization of circumferential calcification of the ascending aorta independent of further extensions. This type is subdivided into two subtypes according to assessing the aorta's clamping possibility during cardiac surgery by a calcification score proposed by Nishi et al.[3] and defined as the ratio of the circumferential length of calcification to the entire ascending aortic circumference.[2]
- Type IA in which the calcification score is above 75%, impeding the possibility of aorta clamping during cardiac surgeries[2]
- Type IB shows a calcification score below 75%, allowing the option to clamp the aorta but with increased risk[2]
- Type II - refers to the calcification localized only in the aortic arch and descending aorta.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Porcelain aorta is associated with several disease processes, including chronic kidney disease, mediastinal radiation, chronic systemic inflammatory diseases such as Takayasu arteritis, systemic lupus erythematosus, and rheumatoid arthritis. Multiple risk factors have been deemed responsible in PA in literature reviews. The factors commonly involved in both types are a familial factor, aging, diabetes mellitus, radiation, uremia, and Takayasu arteritis. The atherosclerotic type is associated with additional factors such as hypertension, hyperlipidemia, smoking, alcoholism, sedentary lifestyle, obesity, systemic lupus erythematosus, and rheumatoid arthritis.[1]
Epidemiology
Porcelain aorta is a relatively rare entity in the general population, but recently it has been increasingly recognized in patients 60 years and older, particularly those with coronary artery disease, chronic renal disease, and calcific aortic stenosis (AS). The reported incidence rate ranges from 1% to 20% based on different studies. Leyh et al. found that 23 out of 1861 (1.2%) patients who underwent coronary artery bypass grafting (CABG) had porcelain aorta.[4] PA was also detected in 15.1% (54 of 358) of patients enrolled in an inoperable cohort of PARTNER (placement of aortic transcatheter valves) trial.[5] PA was present in 18% (61 of 339) patients undergoing transcatheter aortic valve replacement (TAVR).[6] The FRANCE-2 TAVI registry and German TAVI registry published porcelain aorta incidence rates of 5% and 11%.[7][8] Faggiano et al. also evaluated 240 patients for AS and noted that 7.5% (18/240) had porcelain aorta.[9]
Several studies have reported discordant results for gender differences in the prevalence of porcelain aorta. The evaluation of PA among the heterogenic population has revealed some sex distribution discrepancies, showing a male predominance in some[10][11] and a female predominance in others.[12][13][14] Moreover, a series of patients with PA who underwent transaortic valve replacement (TAVR) for severe aortic stenosis demonstrated a female predominance (52.8% to 75.4%).[6][8][15] On the other hand, there is a male predominance in porcelain aorta prevalence among patients who underwent coronary revascularization surgery.[16]
Pathophysiology
The exact cause of porcelain aorta is still elusive, but two pathophysiological processes have been described in the pathogenesis of this condition. These mechanisms allowed us to divide porcelain aorta into the following two entities:
Atherosclerotic Porcelain Aorta
It is characterized by calcification of tunica intima of the aortic wall due to atherosclerotic plaques development. These atheromatous lesions are induced by an inflammatory endothelial injury that activates the endothelial cells to express adhesion molecules that attract inflammatory cells, mainly monocytes and T lymphocytes, into the subendothelial intima. These monocytes undergo a specific differentiation into macrophages, which phagocytose the modified lipoproteins producing foam macrophages. The activated macrophages secrete cytokines to recruit more inflammatory cells and growth factors that induce the proliferation of inflammatory cells and vascular smooth muscle cells (VSMCs) and endothelial cells. In turn, these factors stimulate the migration of VSMCs from the tunica media into the tunica intima.
The intimal VSMCs proliferate and ingest lipoproteins forming lipid-laden VSMCs. The layers of foam macrophages and lipid-laden VSMCs are collectively known as fatty streaks, which are recognized as an early sign of stable atherosclerosis. The advanced, stable atherosclerosis is characterized by further enlargement of fatty streaks and secretion of extracellular matrix proteins by VSMCs, forming a fibrous cap covering the lesion. This matrix is followed by apoptosis of foam macrophages, leading to necrosis of VSMCs and degradation of extracellular matrix proteins, thus producing a necrotic core associated with the advanced unstable lesion. The destabilization of atheromatic plaques increases the liability of rupture, resulting in platelet accumulation at the site of rupture and the formation of atherothrombosis that occludes the artery.[1]
Nonatherosclerotic Porcelain Aorta
In this type, calcification occurs in the tunica media of the aortic wall in the absence of atherosclerosis. It is induced by vascular inflammation, radiation, and uremia via triggering a metaplastic transformation of VSMCs into osteoblasts leading to the production of bone-associated proteins such as alkaline phosphatase, bone sialoprotein, bone GLa protein, and bone morphogenic protein 2. In cases of vascular inflammation and aging, apoptotic vesicles arising from dead VSMCs, and elastin degradation mediated by matrix metalloprotease will act as a nidus for medial calcification by sending a paracrine osteogenic signal that will provoke the aortic calcium deposition as well as the collagen deposition and loss of the elastic fibers causing an arterial wall stiffness.[1][17]
History and Physical
Pertinent aspects of history and physical examination in patients with porcelain aorta are related to underlying medical conditions and risk factors.
Evaluation
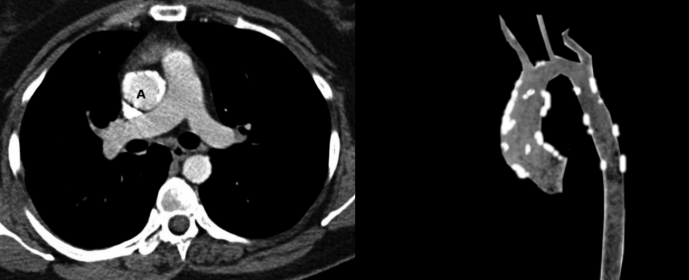
Porcelain aorta is an asymptomatic condition that usually appears as an incidental finding in patients evaluated for cardiovascular or pulmonary diseases.[1] It is commonly identified on chest radiographs or computerized tomographic scans done for other indications.
Different diagnostic tools have been suggested to diagnose porcelain aorta and evaluate its extent and exact location before any cardiac intervention. The most accurate modality is a multislice computed tomography (MSCT). It is used to accurately diagnose porcelain aorta and give valuable information about the exact level and site of aortic calcification, thereby distinguishing PA (circumferential calcification) from a less extensive aortic calcification. Furthermore, a 3-dimensional (3D), volume-rendered reconstructions of MSCT images can be done to allow 3D mapping of the aortic wall, thus providing us with useful information about the three-dimensional distribution of the calcification.[1][2][18][19][20]
Other modalities include:
- A chest X-ray may occasionally reveal a calcification in the thoracic aorta, but it is not accurate in defining PA.
- Fluoroscopy during coronary angiography is somehow sensitive in detecting an aortic calcification that suggests PA; however, it lacks the precision in assessing the distribution and localization of this calcification.
On the other hand, PA is recognizable during the cardiac surgery by performing an epiaortic echocardiographic scanning of the aorta in conjunction with manual palpation after sternotomy and exposure of the aorta. Such testing confirms the presence of PA, as well as its extent and exact location.[1][2][18][19][20]
Pre-operative identification of porcelain aorta is important because the procedural plan often changes if PA is discovered. When porcelain aorta is suspected based on incidental findings on other imaging modalities, pre-procedural computed tomography can evaluate PA.
Treatment / Management
During any cardiac procedure, the need for advanced surgical techniques in patients with concomitant porcelain aorta (PA) is mandatory to avoid manipulating the heavily calcified aorta, thus preventing unfavorable consequences from the calcified aorta leading to stroke and systemic embolism.
Porcelain Aorta During Coronary Bypass Surgery
Patients with porcelain aorta demonstrate an increased morbidity and mortality rate during CABG operation because of the elevated risk of embolic stroke from the atheromatous heavily calcified aorta. Such complication usually takes place during manipulation of the diseased aorta in three different maneuvers in CABG:
- Cannulation of the aorta
- Cross-clamping
- Partial clamping for the construction of the proximal anastomosis[4]
Several amendments have been described to avoid cannulation and clamping of the porcelain aorta, including:
- A no-touch technique or aortic off-pump CABG [1][21][22]
- CABG performed during deep hypothermic circulatory arrest [1][4][16][23]
- Femoral or axillary artery cannulation for cardiopulmonary bypass [1][4][16][24]
- Placement of proximal saphenous vein grafts onto the internal mammary artery, onto the innominate artery, onto the axillary artery, or the carotid artery[1][4][16][25][26]
- Single clamp technique[1][4][16][27]
- Intraluminal balloon catheter as a replacement for external clamps[1][4][16][28]
- Ascending aorta endarterectomy[1][4][16][29][30]
- Patch aortoplasty[1][4][16][31]
- Graft replacement of the ascending aorta[1][4][16] (B2)
The most common and effective recent modality is "no-touch technique" or aortic off-pump CABG in which cannulation and clamping of the ascending aorta are averted. This technique is performed on the beating heart without using the heart-lung machine and, so-called, off-pump, or beating heart CABG. It is achieved by an arterial grafting using bilateral internal mammary grafts in addition to a radial artery or reverse saphenous venous grafts, forming a T, or Y shaped graft that will be connected to the innominate artery or common carotid artery that will act as the inflow source, thereby bypassing the blockage area without touching the diseased aorta.[1][18][21][16][22] Lev-Ran et al.[16] performed a retrospective analysis and comparison of CABG results with femoral artery cannulation in fifteen patients and off-pump CABG in 41 porcelain aorta patients. He found that the off-pump CABG group has only 1 case of perioperative mortality (2.4%) and no perioperative stroke or transient ischemic attack cases. While in CABG with femoral cannulation, there was one case of perioperative mortality (6.6%) and three cases of perioperative strokes or transient ischemic attacks (20%). These results reveal that the off-pump technique has a beneficial impact on reducing perioperative mortality and stroke risk compared to the conventional CABG procedure. However, this technique's major drawback in their study was the failure to achieve complete revascularization in 24.3% of patients compared to the lower rate in the normal CABG group. (B2)
If there is a small non-calcified area confirmed by epiaortic ultrasound, a safe proximal anastomosis construction can be done using a proximal seal system [1][25][21][16] to allow the surgeon to perform an anastomosis between the graft and the aorta without the need to do an aortic clamping. Hilker and his colleagues[32] published a study that reveals a stroke rate of 0.48% of 412 patients in which 542 had proximal anastomoses using a Heartstring device during CABG was performed. Dohmen G et al.[21] reported 25 successful anastomoses in 17 patients undergoing CABG. It was demonstrated that eight patients had porcelain aorta. Two of them developed neurological deficits after the procedure.(B2)
The other beneficial method is performing CABG during deep hypothermic circulatory arrest. In this practice, myocardial revascularization is performed using moderate hypothermia with a core temperature of 28-32°C for up to 40 minutes without harming the patient. In this case, sufficient time is given for the surgeon to perform safe proximal anastomoses without placing clamps on the aorta, minimizing the rate of cerebral complications. Salenger et al.[23] studied the outcome of this approach in 71 patients and found that one patient (1.4%) developed a mild stroke that resolved afterward, [26] analyzed six patients not suitable for clamping due to the severely calcified aorta. A deep hypothermic circulatory arrest for proximal anastomosis was made on all patients with complete recovery free of neurological problems.
Ascending aorta is the usual cannulation site in cardiac surgery.[18] The existence of a porcelain aorta precludes the conventional arterial cannulation.[18] Therefore, a suitable cannulation site should be chosen for safe surgery. Several places have been suggested, and the classic approach is performing arterial perfusion via the common femoral artery (CFA) because it can be quickly done but carries the risk of embolization from the atherosclerotic thoracic and abdominal aorta due to retrograde perfusion. If the CFA is atherosclerotic and cannot be used for cannulation, the alternative location will be through the axillary artery.[25] Perfusion through the axillary artery has the following advantages:(B2)
- It carries a lower risk of cerebral embolization.
- The rate of atherosclerosis is less than that of the ascending aorta or the femoral artery.
- The risk of severe distal ischemic-reperfusion injury or embolization after cannulation is reduced due to the presence of abundant collaterals.
However, axillary artery cannulation can be accompanied by several local complications, including axillary artery dissection, thrombosis, and brachial plexus injury.[25]
Svensson et al.[24] conducted arterial perfusion via the axillary side graft in 299 patients and the femoral artery in 375 patients. They observed that the stroke occurred in 4% (12/299) of the axillary side graft group and 6.7% (25/375 patients) of the femoral artery group. The risk of hospital mortality was higher with femoral perfusion (11%, 42/375) than axillary side graft perfusion (7.0%, 21/299).
Cannulation via the brachiocephalic artery could also be a feasible option in patients with porcelain aorta. Banbury et al. endorsed that brachiocephalic arterial cannulation is a good alternative in cases whose aorta cannot be manipulated. This option can avoid making a second incision in axillary artery cannulation or retrograde perfusion issues in femoral artery cannulation.[25]
Porcelain Aorta During Mitral Valve Surgery
Hypothermia and a fibrillating heart can avoid aortic cross-clamping in mitral valve surgeries.[1][25][27] Loulmet et al. reported outstanding hypothermia results and a fibrillating heart method during mitral valve surgeries in patients with PA.[28](B2)
Porcelain Aorta and Aortic Stenosis
Strategies like deep hypothermic circulatory arrest, the apico-aortic conduit technique have been proposed in the scholarly literature to avoid a cross-clamping of the aorta during surgical aortic valve surgery in patients with aortic stenosis and porcelain aorta.[1][25][21] Unfortunately, these technically challenging methods require significant surgical experience and don't completely avoid manipulating the porcelain aorta.[1](B2)
Another new promising alternative is transcatheter aortic valve replacement (TAVR). It is the least invasive procedure to replace the aortic valve in high surgical risk patients with severe aortic stenosis. Patients having porcelain aorta will benefit from this approach by replacing the aortic valve without an aortic cross-clamping. In a study of around 60 patients with severe AS and PA, TAVR was successful in 98.4% of the patients with PA. The stroke and thirty-day mortality rates were 1.6% (1/61 patients) and 11.5% (7/61 patients), respectively, showing no differences from patients without PA. Pascual et al. stated that 449 patients underwent TAVR, of which 36 patients (8%) had a porcelain ascending aorta. The procedure was successful in 94.4%. The stroke and thirty-day mortality rates were 2.8% (1/36 patients) and 5.6% (2/36 patients), respectively, similar to patients without PA.
Differential Diagnosis
Porcelain aorta is a radiographic diagnosis with few or no differential diagnosis. Often, minor forms of thoracic aortic calcification are found in older patients. PA is the most severe form of thoracic aortic calcification.[33]
Prognosis
Porcelain aorta is typically an incidental finding associated with diffuse atherosclerotic cardiovascular disease, chronic kidney disease, or prior chest radiation therapy. General prognosis in patients with PA is usually related to the underlying medical conditions.
Complications
Porcelain aorta is usually an incidental finding in patients being evaluated for cardiovascular or pulmonary diseases.[1] Porcelain aorta is frequently associated with valvular and coronary calcification, thus increasing the risk of stenotic valvular disorders and ischemic heart diseases due to coronary atherosclerosis in these patients. Furthermore, severe calcification of the aorta and resultant luminal narrowing increases the resistance to the blood flow ejected from the left ventricle, resulting in the development of cardiac hypertrophy, congestive heart failure, and arrhythmias over time. A few studies have also linked an elevated risk of long-term mortality and adverse cardiovascular events and stroke in patients with aortic calcification.[1]
Cardiac surgery is more complicated in patients with porcelain aorta, and their management can be challenging due to the increased risk of perioperative atheroembolism and aortic dissection.[25]Patients with a PA are at high risk for embolic stroke due to manipulating aortic atheroma during surgery.[33]
Pearls and Other Issues
A porcelain aorta finding is important to recognize in the preoperative evaluation of cardiac surgery as it potentially can complicate cardiac surgeries that require cross-clamping or involving the aorta. Modification of surgical technique may be needed with this condition.
Enhancing Healthcare Team Outcomes
Porcelain aorta significantly increases the morbidity and mortality in patients undergoing cardiac surgeries.[18] It is considered a challenging issue for cardiac surgeons and interventional cardiologists because it precludes and complicates aortic cannulation, aortic clamping, aortotomy, and central coronary bypass anastomosis, thereby raising substantially the complexity and the risks associated with cardiac procedures, particularly cardiopulmonary bypass graft and aortic valve replacement procedures.
Careful pre-operative evaluation can help identify the presence of PA in patients planned for interventional and surgical procedures and can aid in pre-operative planning to minimize complications associated with PA.
Media
References
Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Porcelain aorta: a comprehensive review. Circulation. 2015 Mar 3:131(9):827-36. doi: 10.1161/CIRCULATIONAHA.114.011867. Epub [PubMed PMID: 25737502]
Amorim PA, Penov K, Lehmkuhl L, Haensig M, Mohr FW, Rastan AJ. Not all porcelain is the same: classification of circular aortic calcifications (porcelain aorta) according to the impact on therapeutic approach. The Thoracic and cardiovascular surgeon. 2013 Oct:61(7):559-63. doi: 10.1055/s-0032-1333204. Epub 2013 Mar 8 [PubMed PMID: 23475797]
Nishi H, Mitsuno M, Ryomoto M, Miyamoto Y. Comprehensive approach for clamping severely calcified ascending aorta using computed tomography. Interactive cardiovascular and thoracic surgery. 2010 Jan:10(1):18-20. doi: 10.1510/icvts.2009.216242. Epub 2009 Oct 27 [PubMed PMID: 19861326]
Leyh RG, Bartels C, Nötzold A, Sievers HH. Management of porcelain aorta during coronary artery bypass grafting. The Annals of thoracic surgery. 1999 Apr:67(4):986-8 [PubMed PMID: 10320239]
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010 Oct 21:363(17):1597-607. doi: 10.1056/NEJMoa1008232. Epub 2010 Sep 22 [PubMed PMID: 20961243]
Level 1 (high-level) evidenceRodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. Journal of the American College of Cardiology. 2010 Mar 16:55(11):1080-90. doi: 10.1016/j.jacc.2009.12.014. Epub 2010 Jan 22 [PubMed PMID: 20096533]
Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrié D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, Delpine S, Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, Van Belle E, Laskar M, FRANCE 2 Investigators. Registry of transcatheter aortic-valve implantation in high-risk patients. The New England journal of medicine. 2012 May 3:366(18):1705-15. doi: 10.1056/NEJMoa1114705. Epub [PubMed PMID: 22551129]
Zahn R, Schiele R, Gerckens U, Linke A, Sievert H, Kahlert P, Hambrecht R, Sack S, Abdel-Wahab M, Hoffmann E, Senges J, German Transcatheter Aortic Valve Interventions Registry Investigators. Transcatheter aortic valve implantation in patients with "porcelain" aorta (from a Multicenter Real World Registry). The American journal of cardiology. 2013 Feb 15:111(4):602-8. doi: 10.1016/j.amjcard.2012.11.004. Epub 2012 Nov 27 [PubMed PMID: 23195040]
Level 1 (high-level) evidenceFaggiano P, Frattini S, Zilioli V, Rossi A, Nistri S, Dini FL, Lorusso R, Tomasi C, Cas LD. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. International journal of cardiology. 2012 Aug 23:159(2):94-9. doi: 10.1016/j.ijcard.2011.02.026. Epub 2011 Mar 3 [PubMed PMID: 21376407]
Kälsch H, Lehmann N, Möhlenkamp S, Hammer C, Mahabadi AA, Moebus S, Schmermund A, Stang A, Bauer M, Jöckel KH, Erbel R, Investigator Group of the Heinz Nixdorf Recall Study. Prevalence of thoracic aortic calcification and its relationship to cardiovascular risk factors and coronary calcification in an unselected population-based cohort: the Heinz Nixdorf Recall Study. The international journal of cardiovascular imaging. 2013 Jan:29(1):207-16. doi: 10.1007/s10554-012-0051-3. Epub 2012 Apr 22 [PubMed PMID: 22527262]
Level 2 (mid-level) evidenceItani Y, Watanabe S, Masuda Y. Aortic calcification detected in a mass chest screening program using a mobile helical computed tomography unit. Relationship to risk factors and coronary artery disease. Circulation journal : official journal of the Japanese Circulation Society. 2004 Jun:68(6):538-41 [PubMed PMID: 15170088]
Yamamoto H, Shavelle D, Takasu J, Lu B, Mao SS, Fischer H, Budoff MJ. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. American heart journal. 2003 Jul:146(1):153-9 [PubMed PMID: 12851625]
Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, Budoff MJ. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). American heart journal. 2008 Apr:155(4):765-71. doi: 10.1016/j.ahj.2007.11.019. Epub 2008 Feb 21 [PubMed PMID: 18371491]
Level 2 (mid-level) evidenceIribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000 Jun 7:283(21):2810-5 [PubMed PMID: 10838649]
Level 2 (mid-level) evidencePascual I, Avanzas P, Muñoz-García AJ, López-Otero D, Jimenez-Navarro MF, Cid-Alvarez B, del Valle R, Alonso-Briales JH, Ocaranza-Sanchez R, Alfonso F, Hernández JM, Trillo-Nouche R, Morís C. Percutaneous implantation of the CoreValve® self-expanding valve prosthesis in patients with severe aortic stenosis and porcelain aorta: medium-term follow-up. Revista espanola de cardiologia (English ed.). 2013 Oct:66(10):775-81. doi: 10.1016/j.rec.2013.03.001. Epub 2013 Jun 2 [PubMed PMID: 24773857]
Level 2 (mid-level) evidenceLev-Ran O, Ben-Gal Y, Matsa M, Paz Y, Kramer A, Pevni D, Locker C, Uretzky G, Mohr R. 'No touch' techniques for porcelain ascending aorta: comparison between cardiopulmonary bypass with femoral artery cannulation and off-pump myocardial revascularization. Journal of cardiac surgery. 2002 Sep-Oct:17(5):370-6 [PubMed PMID: 12630532]
Van Mieghem NM, Van Der Boon RM. Porcelain aorta and severe aortic stenosis: is transcatheter aortic valve implantation the new standard? Revista espanola de cardiologia (English ed.). 2013 Oct:66(10):765-7. doi: 10.1016/j.rec.2013.05.008. Epub 2013 Jul 23 [PubMed PMID: 24773854]
Sirin G, Sarkislali K, Konakci M, Demirsoy E. Extraanatomical coronary artery bypass grafting in patients with severely atherosclerotic (Porcelain) aorta. Journal of cardiothoracic surgery. 2013 Apr 15:8():86. doi: 10.1186/1749-8090-8-86. Epub 2013 Apr 15 [PubMed PMID: 23587129]
Level 2 (mid-level) evidenceSnow T, Semple T, Duncan A, Barker S, Rubens M, DiMario C, Davies S, Moat N, Nicol ED. 'Porcelain aorta': a proposed definition and classification of ascending aortic calcification. Open heart. 2018:5(1):e000703. doi: 10.1136/openhrt-2017-000703. Epub 2018 Jan 26 [PubMed PMID: 29387428]
Bapat VN, Attia RQ, Thomas M. Distribution of calcium in the ascending aorta in patients undergoing transcatheter aortic valve implantation and its relevance to the transaortic approach. JACC. Cardiovascular interventions. 2012 May:5(5):470-476. doi: 10.1016/j.jcin.2012.03.006. Epub [PubMed PMID: 22625183]
Dohmen G, Hatam N, Goetzenich A, Mahnken A, Autschbach R, Spillner J. PAS-Port® clampless proximal anastomotic device for coronary bypass surgery in porcelain aorta. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Jan:39(1):49-52. doi: 10.1016/j.ejcts.2010.04.010. Epub [PubMed PMID: 20537548]
Level 2 (mid-level) evidenceLev-Ran O, Braunstein R, Sharony R, Kramer A, Paz Y, Mohr R, Uretzky G. No-touch aorta off-pump coronary surgery: the effect on stroke. The Journal of thoracic and cardiovascular surgery. 2005 Feb:129(2):307-13 [PubMed PMID: 15678040]
Salenger R, Rodriquez E, Efird JT, Gouge CA, Trubiano P, Lundy EF. Clampless technique during coronary artery bypass grafting for proximal anastomoses in the hostile aorta. The Journal of thoracic and cardiovascular surgery. 2013 Jun:145(6):1584-8. doi: 10.1016/j.jtcvs.2012.05.045. Epub 2012 Jun 15 [PubMed PMID: 22704289]
Svensson LG, Blackstone EH, Rajeswaran J, Sabik JF 3rd, Lytle BW, Gonzalez-Stawinski G, Varvitsiotis P, Banbury MK, McCarthy PM, Pettersson GB, Cosgrove DM. Does the arterial cannulation site for circulatory arrest influence stroke risk? The Annals of thoracic surgery. 2004 Oct:78(4):1274-84; discussion 1274-84 [PubMed PMID: 15464485]
Osaka S, Tanaka M. Strategy for Porcelain Ascending Aorta in Cardiac Surgery. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2018 Apr 20:24(2):57-64. doi: 10.5761/atcs.ra.17-00181. Epub 2018 Mar 1 [PubMed PMID: 29491196]
Reddy DD, Floten HS, Gately HL. CABG in calcified aorta under circulatory arrest. The Annals of thoracic surgery. 1995 Jun:59(6):1571-3 [PubMed PMID: 7771847]
Takami Y, Tajima K, Terazawa S, Okada N, Fujii K, Sakai Y. Safer aortic crossclamping during short-term moderate hypothermic circulatory arrest for cardiac surgery in patients with a bad ascending aorta. The Journal of thoracic and cardiovascular surgery. 2009 Apr:137(4):875-80. doi: 10.1016/j.jtcvs.2008.09.022. Epub [PubMed PMID: 19327511]
Level 2 (mid-level) evidenceLoulmet DF, Patel NC, Jennings JM, Subramanian VA. Less invasive intracardiac surgery performed without aortic clamping. The Annals of thoracic surgery. 2008 May:85(5):1551-5. doi: 10.1016/j.athoracsur.2008.01.071. Epub [PubMed PMID: 18442536]
Eisen A, Tenenbaum A, Koren-Morag N, Tanne D, Shemesh J, Imazio M, Fisman EZ, Motro M, Schwammenthal E, Adler Y. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008 Sep 23:118(13):1328-34. doi: 10.1161/CIRCULATIONAHA.107.712141. Epub 2008 Sep 8 [PubMed PMID: 18779448]
Jacobs PC, Gondrie MJ, Mali WP, Oen AL, Prokop M, Grobbee DE, van der Graaf Y. Unrequested information from routine diagnostic chest CT predicts future cardiovascular events. European radiology. 2011 Aug:21(8):1577-85. doi: 10.1007/s00330-011-2112-8. Epub 2011 May 21 [PubMed PMID: 21603881]
Level 2 (mid-level) evidencevan der Linden J, Hadjinikolaou L, Bergman P, Lindblom D. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. Journal of the American College of Cardiology. 2001 Jul:38(1):131-5 [PubMed PMID: 11451262]
Hilker M, Arlt M, Keyser A, Schopka S, Klose A, Diez C, Schmid C. Minimizing the risk of perioperative stroke by clampless off-pump bypass surgery: a retrospective observational analysis. Journal of cardiothoracic surgery. 2010 Mar 25:5():14. doi: 10.1186/1749-8090-5-14. Epub 2010 Mar 25 [PubMed PMID: 20334704]
Level 2 (mid-level) evidenceDesai MY, Cremer PC, Schoenhagen P. Thoracic Aortic Calcification: Diagnostic, Prognostic, and Management Considerations. JACC. Cardiovascular imaging. 2018 Jul:11(7):1012-1026. doi: 10.1016/j.jcmg.2018.03.023. Epub [PubMed PMID: 29976300]