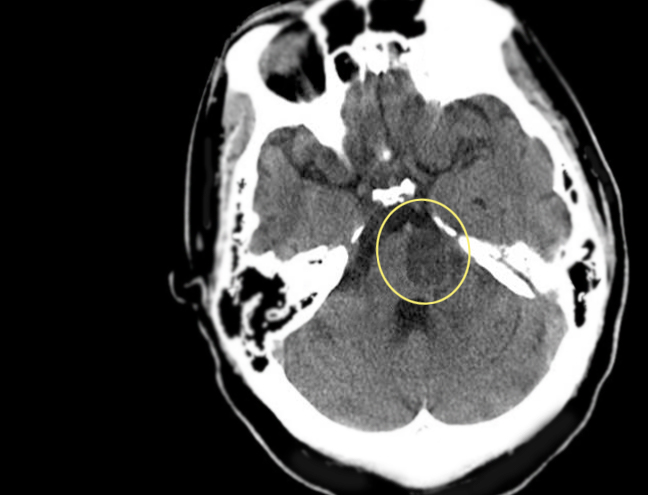
Introduction
Pons is the largest component of the brainstem located distal to the midbrain and proximal to the medulla oblongata. Any obstruction of blood supply to the pons, whether acute or chronic, causes pontine infarction, a type of ischemic stroke. Clinical presentation of a pontine infarction can vary, ranging from the classical crossed syndrome (ipsilateral cranial nerve palsy and contralateral motor and/or sensory impairment) to the less common pure motor hemiparesis or hemiplegia or pure sensory stroke. Clinical presentation is primarily determined by the anatomical boundaries of the infarcted region within the pons and the blood vessels involved.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The most common causes of pontine infarction include small artery disease, large artery atherosclerosis, and cardiogenic emboli, with the latter two being less frequent causes.[2][3][4][5] The majority of the blood supply of pons is from the paramedian perforating arteries and the short circumferential arteries which arise from the basilar artery of the posterior circulation. Other sources of supply include the anterior inferior cerebellar artery and the superior cerebellar artery. The primary lesion can be unilateral or bilateral, anterior or posterior, medial, or lateral or, more commonly, a combination of these regions. The established risk factors for ischemic stroke, hypertension, and diabetes are also the leading risk factors for pontine infarction.[1][3][6][7][8] Long-standing hypertension or diabetes can lead to lipohyalinosis of the small perforating arteries of the pons, leading to chronic ischemia and eventual infarction.[9] Infarction can also result from atheromatous plaques in the larger arteries (vertebral or basilar artery), which in turn can obstruct blood flow to the smaller perforating arteries of pons (microatheromas).[9] Other risk factors postulated include smoking, hypercholesterolemia, history of ischemic heart disease, hypercoagulable states, and vasculitis.[8][10] However, the risk factors that increase the risk of infarction, specifically in the pons, are unclear.[9]
Epidemiology
Epidemiological studies investigating particularly pontine infarctions are lacking. While pontine infarctions are relatively common, they generally occur as a part of larger posterior circulation stroke. Within pons, presentation further varies depending on the arterial territory involved:
- The anteromedial and anterolateral region supplied by the basilar artery is the most commonly affected
- Lateral region supplied by the basilar artery and anterior inferior cerebellar artery
- Posterior/dorsal region supplied by the superior cerebellar region.
Ischemic stroke incidence overall is higher among men than women and Black and Hispanic adults compared to their White counterparts, with some geographic variability.[11][12][13] However, studies examining pontine infarctions exclusively are lacking.
History and Physical
Ischemic stroke risk increases with age, and hence a typical presentation of a pontine infarction would be an elderly individual with a history of chronic conditions like hypertension, diabetes, dyslipidemia, or history of heart disease. However, pontine infarction can also occur in younger individuals with vascular disorders like CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), hypercoagulable states, vertebral artery dissections et cetera.[14][15][16] Establishing the time of onset of stroke symptoms heavily determines the treatment strategy (thrombolytics vs. thrombectomy). The clinical presentation of a pontine infarct depends on the anatomical/arterial territories involved and is clinically classified as below:[17]
- Ventro-caudal pontine infarction is caused due to decreased blood flow in the paramedian perforating arteries arising from the basilar artery. Affected individuals have contralateral motor hemiparesis or hemiplegia due to the large infarcts of the unilateral corticospinal tract. Ipsilateral abducens and/or facial nerve palsy can occur secondary to the involvement of the nerve fibers and nucleus, respectively. Lateral spinothalamic tract infarction leads to decreased pain and temperature sensation on the opposite side of the body. Clinically, the presenting symptoms can be grouped into Millard-Gubler syndrome (contralateral hemiparesis, ipsilateral abducens, and facial nerve palsy), Foville syndrome (conjugate gaze palsy in addition to the features of Millard-Gubler syndrome), and Raymond syndrome (contralateral body hemiparesis, ipsilateral abducens palsy without facial nerve palsy).
- Mid-pontine base infarction is also due to decreased blood flow in the paramedian arteries or the short circumferential arteries arising from the basilar artery. Presenting symptoms depend on the extent of involvement of various structures within the pons. For instance, ipsilateral ataxia is due to the infarction of pontine nuclei; sensorimotor weakness of the ipsilateral face is due to the trigeminal nerve fibers' involvement and contralateral hemiparesis due to the corticospinal tract. Various combination of these symptoms results in distinct syndromes like pure motor hemiparesis (lacunar infarcts of the corticospinal tract), ataxic hemiparesis (lacunar infarcts of the pontine nuclei), dysarthria-clumsy hand syndrome (dysarthria, dysphagia, impaired dexterity and weakness of hand), and rare presentations like dysarthria-dysmetria and dysarthria-facial paresis.
- Tegmental pontine syndrome can affect various structures, including cranial nerve (trigeminal, abducens, facial, and vestibulocochlear) nuclei, medial lemniscus, medial longitudinal fasciculus, respiratory centers, and the pontine reticular formation. Obstruction of blood flow in the anterior inferior or superior cerebellar arteries causes the rostral pontine syndrome. Symptoms of this syndrome include ipsilateral facial sensory disturbance and masticator paralysis (trigeminal nuclei), impaired blinking (tectospinal tract); contralateral hemisensory loss (lateral spinothalamic tract and medial lemniscus); and ipsilateral hemiataxia (superior cerebellar peduncle). The caudal pontine syndrome is caused due to decreased blood flow in the short circumferential or the anterior inferior cerebellar artery. Clinically, they can present with ipsilateral conjugate gaze palsy and nystagmus (medial longitudinal fasciculus), ipsilateral impaired eye abduction (abducens nucleus), ipsilateral hemiataxia (middle cerebellar peduncle), and contralateral hemisensory loss (lateral spinothalamic tract and medial lemniscus).
- Multiple pontine infarcts primarily affecting the perforating arteries' territories result in pseudobulbar palsy due to the involvement of the corticobulbar fibers. A pseudobulbar palsy presentation includes severe dysphagia and dysarthria. Some individuals may present with pathological crying or laughing.[18]
- Bilateral pontine infarcts happen secondary to impeded blood flow in the larger basilar artery. Being bilateral, both left and right, upper, and lower limbs can be involved (tetraplegia) and also impair the consciousness. Large infarcts affecting the corticospinal, corticobulbar, and corticopontine tracts result in the locked-in syndrome. Locked-in syndrome presents with tetraplegia, bilateral facial paralysis, pharyngeal, and horizontal gaze palsy with retained consciousness and cognitive abilities. Recurrent stereotypical episodes of dysarthria, ophthalmoplegia, motor, or sensory disturbances are more recently referred to as the "pontine warning syndrome." It is hypothesized that this syndrome can progress into a bilateral pontine infarct.[19]
Few distinguishing features that can be used to identify the anatomical location of the pontine infarction include:
- Contralateral hemiparesis, internuclear ophthalmoplegia, and conjugate horizontal gaze paresis for medial infarction.
- Contralateral hemisensory loss and ataxia for lateral infarction.
- Ipsilateral lower motor neuron palsy of the facial nerve, abducens palsy, sensorineural hearing loss, and vertigo for caudal infarction.
Evaluation
Initial assessment of an individual presenting with stroke symptoms should include blood pressure and heart rate measurement along with respiratory function assessment (respiratory rate and pulse oximetry). As pons houses the respiratory centers, large pontine infarctions can compromise respiratory function and may warrant intubation and mechanical ventilation. If hemodynamically stable, a complete neurological examination in addition to routine history taking and physical examination is conducted. While the neurological exam assists in localizing the anatomical region affected and the type of stroke (ischemic vs. hemorrhagic), relevant history and vitals can point to the predisposing conditions (e.g., hypertension). After proper stabilization, a complete neurological examination including an assessment of consciousness, cognition, gait, coordination, reflexes, cranial nerve examination, and sensory and motor system should be performed. Followed by physical examination, neuroimaging is urgently indicated in all suspected stroke cases, and the timing of the imaging determines the treatment modality and its success. Computed tomography (CT) scan due to its availability and ability to detect hemorrhages is used to triage a stroke patient. However, magnetic resonance imaging (MRI) remains the mainstay of diagnosis for ischemic strokes due to its ability to detect acute ischemia within minutes of onset (diffusion-weighted MRI).[20] Neurovascular imaging (CT or MR angiography) is performed in those with large artery obstruction (e.g., basilar artery) as they might have to undergo mechanical thrombectomy. In cases where an embolus is suspected, imaging studies like carotid doppler or transthoracic echocardiography may be required to identify the degree of stenosis or the source of embolus. Basic investigations like complete blood count, serum glucose, serum electrolytes, renal and liver function tests, lipid profile, and a 12-lead electrocardiogram are routinely ordered.[21] Various other biomarkers (e.g., c-Fn, MMP-9) are being investigated as possible predictors of stroke outcomes and can very well be recommended as routine tests for all stroke patients in the future.[22]
Treatment / Management
Pontine infarction treatment is akin to other types of ischemic stroke with some variability considering the lack of strong clinical evidence in posterior circulation stroke treatment compared to anterior circulation. This is especially true in patients with a large vessel occlusion, causing a pontine stroke. In general, as with any patient with ischemic stroke, immediately after identifying the subtype of stroke, treatment should be targeted to:
- Reverse the extent of ischemic penumbra tissue adjacent to the infarcted region
- Prevent complications
- Facilitate early improvement in functional status
However, this comes secondary to the initial stabilization of the airway, breathing, and circulation. Intravenous alteplase is the thrombolytic of choice for those presenting within 4.5 hours of symptom onset and without any absolute contraindications to thrombolytic therapy. Mechanical thrombectomy is the procedure of choice for those with large artery occlusion presenting within 24 hours of symptoms onset.[23][24][25][26][27] Blood pressure is generally elevated in acute stroke patients, and this works in favor as the cerebral autoregulation of blood pressures is impaired, especially in patients with large vessel occlusion. In patients receiving thrombolytics, it is recommended that the pressure is maintained below 180/105 mmHg for at least 24 hours following thrombolytic administration to limit reperfusion injury.[23] In patients not receiving thrombolytic therapy, hypertension is not treated unless severe (>220/120 mmHg) to facilitate cerebral perfusion in the setting of ischemia. In addition to the above steps, aspirin, clopidogrel, or similar antiplatelet agents or anticoagulants like warfarin, apixaban, dabigatran, etc. are used in secondary prevention based on the etiology of the ischemic stroke. Lipid-lowering therapy is also initiated to prevent recurrence with high-intensity statins. Antihypertensives are added to the treatment regimen at discharge for those with elevated blood pressure. Lastly, but most importantly, lifestyle modifications such as exercise, smoking cessation, dietary changes, and weight loss are strongly recommended.(A1)
Differential Diagnosis
Isolated pontine infarction is a less frequent occurrence than a larger brainstem infarction.
A few of the many predisposing conditions are hypertension, diabetes, basilar artery atherosclerosis, and vertebrobasilar insufficiency. Clinical presentation may include many signs and symptoms due to the underlying condition making it even more essential to rule out a multitude of diseases like:
- Todd paralysis
- Intracranial hemorrhage
- Intracranial mass (tumor or abscess)
- Spinal cord compression
- Hypoglycemia
- Wernicke encephalopathy
- Conversion disorder
- Complex migraine
Initial neuroimaging would aid in evaluating any other intracranial pathologies. CT imaging delineates hemorrhagic lesions well, while MRI imaging is better suited for mass lesions, abscesses, infectious issues, and other etiologies. High index suspicion should be used for the less common differential diagnoses.
Prognosis
The overall prognosis of those with unilateral pontine infarction is good.[1][6][7] Infarctions, especially lacunar in nature, involving the lateral or rostral to mid pons, are associated with more favorable outcomes.[28][6] On the other hand, bilateral and caudal pontine infarctions have a worse prognosis.[29][1] Progressive neurological deficit following isolated pontine infarction has also been reported.[5] The long-term prognosis is considered good based on a single study with a 4 to 9 year follow-up time.[30] However, Kumral et al. observed the long-term risk of recurrent stroke to be high in those with pontine infarction.[1] The prognosis of isolated pontine infarction in comparison to the other posterior circulation stroke syndromes or any non-pontine infarctions is yet to be determined.
Complications
The common medical complications of pontine infarction include:
- Aspiration pneumonia secondary to pseudobulbar palsy or locked-in syndrome is the most severe one.
- Dysphagia warranting tube feeding
- Urinary incontinence requiring catheterization which in turn increases the risk of urinary tract infections.
- Remnant motor weakness resulting in falls
- Post-stroke depression
- Complications due to prolonged immobilization (e.g., pressure ulcers, deep venous thrombosis, pulmonary embolism).[31][32]
- Long-term disability is also common following pontine infarction and is assessed using various scales like the Barthel Index, the Glasgow Outcome Scale, and the modified Rankin Scale (mRS).[17]
- Neurological complications of pontine infarction include cerebral edema, hemorrhagic transformation of the infarcted region, palatal myoclonus, and neurological deterioration despite treatment.
Postoperative and Rehabilitation Care
Rehabilitation for pontine infarction can include sensory reeducation, physical therapy, speech therapy, and occupational therapy.
Deterrence and Patient Education
Primary prevention of stroke calls for aggressive risk factor control in individuals with high risk. The most important modifiable risk factors are high blood pressure (not necessarily a diagnosis of hypertension), diabetes, dyslipidemia, and smoking. However, non-modifiable risk factors like age, race, sex, and family history of stroke should also be considered in the prevention efforts. The most recent American Heart Association/American Stroke Association guidelines for primary prevention of stroke suggested for the general population includes:[23]
- Blood pressure control (regular screening, pharmacological treatment with a goal of 140/90 mmHg, self-monitoring of blood pressure)
- Dyslipidemia treatment (lifestyle modification and statins for those with elevated stroke risk)
- Among those with diabetes, statin initiation and blood pressure below 140/90 mmHg
- Smoking cessation (counseling and pharmacological interventions, if needed)
- Dietary modifications (DASH diet, a diet low in sodium, or a Mediterranean diet)
- Regular exercise (moderate to severe exercise for 40 mins/day for 3 to 4 days/week)
- Weigh reduction for those who are obese or overweight
- Prophylactic anticoagulants (aspirin or warfarin) for individuals with atrial fibrillation
Educational programs about stroke, its traditional risk factors, and behavioral modifications to reduce stroke risk, have shown to be effective in increasing the knowledge and risk perception among individuals receiving them.[33][34] Though individuals prefer to know their risk of stroke, the tools used to assess the risk may not have any additional effect on the individual’s behavior than the routine risk factor education.[35]
Enhancing Healthcare Team Outcomes
Proper identification of stroke symptoms by the patients themselves or by individuals witnessing the patient’s symptoms is vital for timely initiation of the emergency medical system (EMS). Stroke education campaigns targeting communities could influence the timely recognition of symptom onset. Once the EMS is triggered, healthcare professionals managing the patient should be well trained to identify stroke and manage accordingly. Stroke care, when strategically organized, can decrease the stroke burden considerably.[36][37] Such organized stroke units can include, stroke units (separate wards), stroke teams (groups of readily available stroke specialists), stroke rehabilitation units (for after stroke care), and comprehensive units for both acute care and rehabilitation services.[38] In addition to recanalization of the affected pontine artery, proper care in the acute stroke unit to prevent complications and appropriate measures in the rehabilitation units are much needed for optimum stroke outcomes.
Media
(Click Image to Enlarge)
References
Kumral E, Bayülkem G, Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. Journal of neurology. 2002 Dec:249(12):1659-70 [PubMed PMID: 12529787]
Fisher CM,Caplan LR, Basilar artery branch occlusion: a cause of pontine infarction. Neurology. 1971 Sep; [PubMed PMID: 5106254]
Bassetti C,Bogousslavsky J,Barth A,Regli F, Isolated infarcts of the pons. Neurology. 1996 Jan; [PubMed PMID: 8559368]
Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989 Sep:39(9):1246-50 [PubMed PMID: 2671793]
Gökçal E, Niftaliyev E, Baran G, Deniz Ç, Asil T. Progressive deficit in isolated pontine infarction: the association with etiological subtype, lesion topography and outcome. Acta neurologica Belgica. 2017 Sep:117(3):649-654. doi: 10.1007/s13760-017-0827-2. Epub 2017 Aug 3 [PubMed PMID: 28776182]
Kataoka S, Hori A, Shirakawa T, Hirose G. Paramedian pontine infarction. Neurological/topographical correlation. Stroke. 1997 Apr:28(4):809-15 [PubMed PMID: 9099201]
Kim JS, Lee JH, Im JH, Lee MC. Syndromes of pontine base infarction. A clinical-radiological correlation study. Stroke. 1995 Jun:26(6):950-5 [PubMed PMID: 7762044]
Lin Y, Zhang L, Bao J, Zhang B, Li H, Chen S, Sun S, Men X, Lu Z. Risk factors and etiological subtype analysis of brainstem infarctions. Journal of the neurological sciences. 2014 Mar 15:338(1-2):118-21. doi: 10.1016/j.jns.2013.12.028. Epub 2013 Dec 27 [PubMed PMID: 24411406]
Level 2 (mid-level) evidenceCaplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. Journal of stroke. 2015 Jan:17(1):2-6. doi: 10.5853/jos.2015.17.1.2. Epub 2015 Jan 30 [PubMed PMID: 25692102]
Baran G, Gultekin TO, Baran O, Deniz C, Katar S, Yildiz GB, Asil T. Association between etiology and lesion site in ischemic brainstem infarcts: a retrospective observational study. Neuropsychiatric disease and treatment. 2018:14():757-766. doi: 10.2147/NDT.S154224. Epub 2018 Mar 13 [PubMed PMID: 29559783]
Level 2 (mid-level) evidenceBarker-Collo S, Bennett DA, Krishnamurthi RV, Parmar P, Feigin VL, Naghavi M, Forouzanfar MH, Johnson CO, Nguyen G, Mensah GA, Vos T, Murray CJ, Roth GA, GBD 2013 Writing Group, GBD 2013 Stroke Panel Experts Group. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology. 2015:45(3):203-14. doi: 10.1159/000441103. Epub 2015 Oct 28 [PubMed PMID: 26505984]
Lackland DT, Bachman DL, Carter TD, Barker DL, Timms S, Kohli H. The geographic variation in stroke incidence in two areas of the southeastern stroke belt: the Anderson and Pee Dee Stroke Study. Stroke. 1998 Oct:29(10):2061-8 [PubMed PMID: 9756582]
Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997 Aug:28(8):1639-47 [PubMed PMID: 9259762]
Moon K, Albuquerque FC, Cole T, Gross BA, McDougall CG. Stroke prevention by endovascular treatment of carotid and vertebral artery dissections. Journal of neurointerventional surgery. 2017 Oct:9(10):952-957. doi: 10.1136/neurintsurg-2016-012565. Epub 2016 Sep 23 [PubMed PMID: 27663558]
Stojanov D, Vojinovic S, Aracki-Trenkic A, Tasic A, Benedeto-Stojanov D, Ljubisavljevic S, Vujnovic S. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosnian journal of basic medical sciences. 2015 Feb 9:15(1):1-8. doi: 10.17305/bjbms.2015.247. Epub 2015 Feb 9 [PubMed PMID: 25725137]
Janardhan V, Wolf PA, Kase CS, Massaro JM, D'Agostino RB, Franzblau C, Wilson PW. Anticardiolipin antibodies and risk of ischemic stroke and transient ischemic attack: the Framingham cohort and offspring study. Stroke. 2004 Mar:35(3):736-41 [PubMed PMID: 14764933]
Level 2 (mid-level) evidencePiechowski-Jóźwiak B, Bogousslavsky J. Posterior circulation strokes. Handbook of clinical neurology. 2009:93():537-58. doi: 10.1016/S0072-9752(08)93026-8. Epub [PubMed PMID: 18804667]
Elyas AE, Bulters DO, Sparrow OC. Pathological laughter and crying in patients with pontine lesions. Journal of neurosurgery. Pediatrics. 2011 Dec:8(6):544-7. doi: 10.3171/2011.8.PEDS11265. Epub [PubMed PMID: 22132910]
Level 3 (low-level) evidenceSaposnik G, Noel de Tilly L, Caplan LR. Pontine warning syndrome. Archives of neurology. 2008 Oct:65(10):1375-7. doi: 10.1001/archneur.65.10.1375. Epub [PubMed PMID: 18852355]
Level 3 (low-level) evidenceWintermark M, Fiebach J. Imaging of brain parenchyma in stroke. Handbook of clinical neurology. 2009:94():1011-9. doi: 10.1016/S0072-9752(08)94049-5. Epub [PubMed PMID: 18793886]
Castellanos M, Castillo J, Dávalos A. Laboratory studies in the investigation of stroke. Handbook of clinical neurology. 2009:94():1081-95. doi: 10.1016/S0072-9752(08)94053-7. Epub [PubMed PMID: 18793890]
Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015 Mar:46(3):915-20. doi: 10.1161/STROKEAHA.114.005604. Epub 2015 Feb 5 [PubMed PMID: 25657186]
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. The New England journal of medicine. 2018 Feb 22:378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24 [PubMed PMID: 29364767]
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11 [PubMed PMID: 29129157]
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008 Sep 25:359(13):1317-29. doi: 10.1056/NEJMoa0804656. Epub [PubMed PMID: 18815396]
Level 1 (high-level) evidenceNational Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The New England journal of medicine. 1995 Dec 14:333(24):1581-7 [PubMed PMID: 7477192]
Level 1 (high-level) evidenceErro ME, Gállego J, Herrera M, Bermejo B. Isolated pontine infarcts: etiopathogenic mechanisms. European journal of neurology. 2005 Dec:12(12):984-8 [PubMed PMID: 16324092]
Level 2 (mid-level) evidenceOh S, Bang OY, Chung CS, Lee KH, Chang WH, Kim GM. Topographic location of acute pontine infarction is associated with the development of progressive motor deficits. Stroke. 2012 Mar:43(3):708-13. doi: 10.1161/STROKEAHA.111.632307. Epub 2012 Feb 16 [PubMed PMID: 22343639]
Kunz S, Griese H, Busse O. Etiology and long-term prognosis of unilateral paramedian pontine infarction with progressive symptoms. European neurology. 2003:50(3):136-40 [PubMed PMID: 14530618]
Level 2 (mid-level) evidenceLanghorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000 Jun:31(6):1223-9 [PubMed PMID: 10835436]
Level 2 (mid-level) evidenceChua KS, Kong KH. Functional outcome in brain stem stroke patients after rehabilitation. Archives of physical medicine and rehabilitation. 1996 Feb:77(2):194-7 [PubMed PMID: 8607746]
Yvonne Chan YF, Nagurka R, Richardson LD, Zaets SB, Brimacombe MB, Levine SR. Effectiveness of stroke education in the emergency department waiting room. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2010 May:19(3):209-215. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.009. Epub [PubMed PMID: 20434048]
Level 3 (low-level) evidenceChaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, Morton SC, Shekelle PG. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Annals of internal medicine. 2006 May 16:144(10):742-52 [PubMed PMID: 16702590]
Level 1 (high-level) evidencePowers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. American heart journal. 2011 Apr:161(4):673-80. doi: 10.1016/j.ahj.2010.12.021. Epub [PubMed PMID: 21473965]
Level 1 (high-level) evidenceStroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. The Cochrane database of systematic reviews. 2013 Sep 11:2013(9):CD000197. doi: 10.1002/14651858.CD000197.pub3. Epub 2013 Sep 11 [PubMed PMID: 24026639]
Level 1 (high-level) evidenceGorelick AR, Gorelick PB, Sloan EP. Emergency department evaluation and management of stroke: acute assessment, stroke teams and care pathways. Neurologic clinics. 2008 Nov:26(4):923-42, viii. doi: 10.1016/j.ncl.2008.05.008. Epub [PubMed PMID: 19026897]
Lyrer PA. Acute stroke units and teams. Handbook of clinical neurology. 2009:94():1195-203. doi: 10.1016/S0072-9752(08)94058-6. Epub [PubMed PMID: 18793895]