Introduction
The infiltration of malignant cells into the serous membrane that lines the abdominal cavity, viscera, and coelom in amniotes is termed peritoneal surface malignancy or peritoneal cancer. This condition is categorized into primary and secondary types. Primary mesothelioma arises from the de novo development of cancer in the mesothelium of the abdomen. In contrast, secondary peritoneal cancer occurs due to the spread of tumor cells from other locations into the peritoneal cavity. Primary peritoneal cancer is further classified based on histology, with terms such as extraovarian primary peritoneal carcinoma (EOPPC), serous surface papillary carcinoma, papillary serous carcinoma of the peritoneum, extraovarian Mullerian adenocarcinoma, and normal-sized ovarian carcinoma syndrome being used to describe this type.[1]
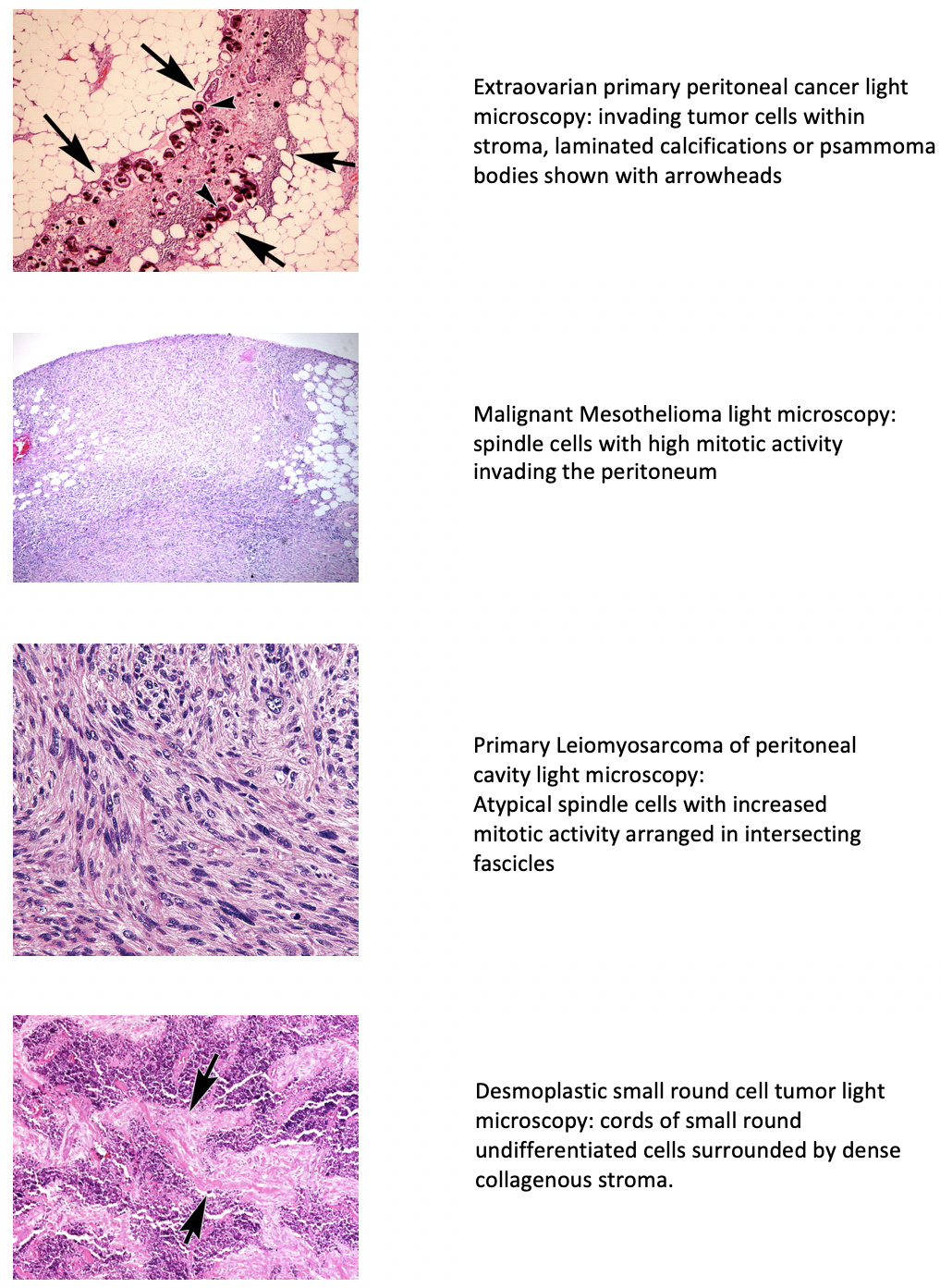
Additional types of peritoneal cancer include malignant peritoneal mesothelioma, multicystic mesothelioma, leiomyosarcomas, leiomyomatosis peritonealis disseminata, and desmoplastic small round cell tumor.[2] Swerdlow initially reported EOPPC as "mesothelioma of pelvic peritoneum" in a case study published in 1959.[3] EOPPC behaves similarly to serous ovarian cancer, often with minimal involvement of the ovaries. While these types exhibit varied histological features, they share similarities in presentation, diagnostic evaluation, and treatment approaches (see Image. Light Microscopic Features of Types of Peritoneal Cancer). Secondary or metastatic peritoneal carcinomatosis commonly originates from primitive malignancies affecting gastrointestinal and gynecological structures. Metastasis may occur through transcoelomic, vascular, or lymphatic routes, with the first description dating back to 1931, illustrating the local spread of ovarian cancer.[4]
Primary peritoneal cancer is typically classified as stage III or IV, while metastasis is categorized as stage IV. The nonspecific clinical presentation often results in delayed diagnosis, decreasing survival rates. Surgical resection and intraperitoneal chemotherapy are considered critical approaches for disease elimination. Nevertheless, advancements in understanding peritoneal physiology and tumor seeding pathways, coupled with technological progress, have facilitated the development of more effective treatment modalities. In the absence of extensive systemic disease, achieving locoregional control of the cancer holds promise in managing this late-stage condition.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Primary peritoneal cancer is an idiopathic malignancy originating from the peritoneal layers of the abdominal cavity. The subtype EOPPC shares similarities with serous ovarian carcinoma and predominantly affects women (mean age of 56-62). While rare, there are sporadic reports of EOPPC occurring in men. Germline mutations in the BRCA1 gene have been identified in approximately 17.6% of EOPPC cases.[1] Therefore, in patients with a history of familial breast cancer, the possibility of serous peritoneal cancer should be considered. Malignant peritoneal mesothelioma (MPM), on the other hand, is an aggressive tumor associated with asbestos exposure in 33% to 50% of cases. This condition primarily affects older men (60+).[5]
Disseminated peritoneal leiomyomatosis is linked to a heightened estrogenic state observed in postmenopausal women. Leiomyosarcoma, conversely, is a secondary tumor often associated with Li-Fraumeni syndrome, which also predisposes individuals to retinoblastoma. Desmoplastic small round cell tumor (DSRCT) predominantly affects adolescents, with a median age of 19, and is notably more prevalent among White individuals (85%). The condition is attributed to the [t(11;22)(p13;q12)] translocation.[6]
Secondary peritoneal carcinomatosis typically arises from invading malignant cells from tumors affecting various organs, including the stomach, colon, pancreas, gallbladder, appendix, breast, uterus, ovary, and lungs. Peritoneal involvement, specifically in appendiceal cancer, is termed pseudomyxoma peritonei; often, this is effectively managed and can result in a lifetime free from relapse. However, metastasis from ovarian, gastric, and colorectal malignancies is associated with an increased risk of recurrence and mortality. These 3 types of cancer are also the most common causes of metastatic spread to the peritoneum.
Epidemiology
Peritoneal tumors are a rare form of advanced malignancies. The age-adjusted incidence rate of primary peritoneal cancer stands at approximately 6.78 per million individuals.[7] This rate tends to be highest among White populations and lowest among Black populations. Serous carcinoma of the peritoneum is the most common histological type of primary peritoneal cancer, constituting around 10% of pelvic cancers.[8] Malignant mesothelioma, though less common, is a highly lethal malignancy. Pleural mesothelioma accounts for most cases, with MPM following closely behind. MPM occurs in approximately 6% to 20% of all mesothelioma cases in the United States, translating into approximately 600 to 800 new cases annually.[9][10][11] Notably, around 50% of leiomyosarcomas originate in the retroperitoneum.[12]
Peritoneal metastasis represents the most prevalent malignant process occurring within the peritoneal cavity. In ovarian cancers, peritoneal metastasis is detected in approximately 75% of cases at the time of diagnosis.[13] Studies indicate it occurs synchronously with the primary tumor in 55% of cases and subsequently in 45% of cases of nongynecological malignancies during follow-up.[14]
Colorectal tumors are associated with peritoneal spread at the time of diagnosis in 5% to 10% of cases, with metachronous malignant proliferation observed in 20% to 50% of cases.[15] Peritoneal dissemination from gastric cancers is present in approximately 14% of cases at initial presentation.[16] Besides the involvement of viscera within the peritoneal cavity, metastasis in the peritoneum also originates from extra-abdominal malignancies in about 9% of cases.[17] Breast (40.8%), lung (25.6%), and melanoma (9.3%) are among the most common sites contributing to this phenomenon.
Pathophysiology
The peritoneum is the serous membrane that lines the abdominal cavity, supporting the abdominal viscera and facilitating the passage of blood, lymph, and nerve impulses. The peritoneum consists of 2 layers: the parietal peritoneum lining the abdominal wall and the visceral peritoneum surrounding the organs. The space between these 2 layers is known as the abdominal cavity or coelom. Within this space lies the peritoneal fluid, which envelops abdominal organs and aids in lubricating peristaltic movements. The volume of peritoneal fluid typically ranges around 100 mL.[18]
The peritoneum is the most extensive and adaptable membrane in the body, capable of responding to various pathologies. Histologically, it comprises mesothelium and submesothelial connective tissue, separated by a thin basement membrane known as the basal lamina, which contains collagen IV and laminin. The elastic matrix consists of collagen I and III and various cell types such as fibroblasts, adipocytes, and macrophages—additionally, the peritoneum houses lymphatics and blood vessels.
The mesodermal layer, derived from mesoderm, exhibits characteristics of both epithelial and mesenchymal cells. The layer serves as the body's initial defense line due to tight junctions among its cells; it also expresses cytokeratin, fibronectin, and other markers. Moreover, the mesodermal layer is crucial in binding tumor cells to the peritoneum. Peritoneal deposits often occur at sites of immune cell aggregates, known as 'milky spots' named by Ranvier, that contain mesothelial cells and blood vessels. The submesothelial stroma promotes adhesion to cancer cells via integrins, facilitating their penetration into the peritoneum.[19]
The "seed and soil theory" proposed by Stephen Paget provides insight into the carcinogenesis and metastasis of peritoneal cancers. According to this theory, tumor cells (the "seeds") are shed from a primary tumor and disseminate throughout the body via the bloodstream or lymphatic system. These circulating tumor cells have the potential to metastasize to distant sites, but they can only thrive and proliferate in specific microenvironments that are conducive to their growth (the "soil").[20][21]
In the context of peritoneal cancers, this theory helps to elucidate why certain types of tumors (eg, colorectal, ovarian, and gastric) have a propensity for metastasizing to the peritoneum. The peritoneal cavity provides a favorable microenvironment for the survival and growth of these tumor cells, allowing them to implant and form secondary tumors within the peritoneum.
Moreover, the concept of organ-specific metastasis, elucidated by Sugarbaker in 1979, further explains the molecular interactions and compatibility between tumor cells and the host microenvironment.[22] This interaction involves specific receptors on malignant cells interacting with corresponding ligands on host cells within the peritoneum, facilitating the adhesion, invasion, and colonization of tumor cells in this anatomical site.
The spread of peritoneal cancer involves multiple mechanisms, including both primary tumor extension and secondary seeding:
- Primary spread: This occurs due to extensive intramural growth beyond the serosal layers of the primary tumor. As the tumor progresses, it invades surrounding tissues and structures within the peritoneal cavity.
- Secondary seeding: This seeding occurs during surgical tumor resections, where the manipulation of the tumor may lead to the spillage of malignant cells into the peritoneal cavity. Secondary seeding can happen inadvertently during surgical procedures and may contribute to the dissemination of cancer cells within the abdomen.
The steps of metastatic invasion:
- Local invasion: Cancer cells first invade nearby tissues or organs. They acquire the ability to penetrate the surrounding tissue barriers through a process called epithelial-mesenchymal transition.
- Intravasation: Cancer cells invade nearby blood vessels or lymphatic vessels, entering the circulatory or lymphatic system. They may travel individually or in clusters.
- Survival in circulation: Cancer cells survive in the bloodstream or lymphatic system despite shear forces and immune surveillance. They may form emboli, clumps of cells that lodge in small blood vessels.
- Extravasation: Cancer cells exit the bloodstream or lymphatic vessels at distant sites, often guided by chemotactic signals released by the target tissue.
- Micrometastasis formation: Once extravasated, cancer cells must adapt to the microenvironment of the new tissue and evade immune detection. They may remain dormant for some time before initiating growth.
- Macrometastasis formation: Dormant cancer cells eventually proliferate, forming macroscopic secondary tumors at distant sites. This process involves angiogenesis, creating new blood vessels to supply nutrients and oxygen to the growing tumor.
- Colonization and growth: Cancer cells grow and proliferate at the secondary site, establishing a new tumor mass. They may also interact with the surrounding tissue, inducing changes that support tumor growth and invasion.[19][23]
Peritoneal metastasis involves intricate interactions between tumor cells and the peritoneal microenvironment, mediated by various adhesion molecules, cytokines, and growth factors. Here is a breakdown of the key mechanisms involved:
- Adhesion molecules and cytokines
- Adhesion molecules such as intercellular adhesion molecule 1 and vascular adhesion molecule 1 on mesothelial cells interact with receptors on tumor cells, including CD44. This interaction and the release of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins expose the basement membrane, facilitating tumor cell attachment and invasion.
- CD44 and metastasis
- CD44 plays a crucial role in the metastasis of colorectal and ovarian cancers.[24] This facilitates tumor cell migration, invasion, and metastatic spread by interacting with its ligands and modulating signaling pathways involved in cell adhesion and migration.
- Translymphatic metastasis
- In some cases, tumor cells enter the lymphatic system through lymph capillaries at milky spots or lymphatic stomata. This process, known as translymphatic metastasis, is observed in conditions such as pseudomyxoma peritonei. Once in the lymphatic system, tumor cells invade the submesothelial stroma, initiating tumor growth.
- Hepatocyte growth factor and c-MET proto-oncogene
- Hepatocyte growth factor binds to its tyrosine kinase receptor, c-MET, initiating signaling pathways that promote tumor cell proliferation, survival, and invasion. This interaction plays a critical role in the growth and progression of peritoneal metastasis.
- Matrix metalloproteinases
- Fibroblasts and macrophages within the submesothelial stroma secrete matrix metalloproteinases, deleting the peritoneal-blood barrier and facilitating tumor cell invasion and dissemination.
- Growth factors
- Tumor growth is sustained by the production of growth factors such as insulin-like growth factor 1 and epidermal growth factor receptor, which promote cell proliferation and survival.
- Angiogenesis
- New blood vessels produce vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1, which promote angiogenesis in the tumor microenvironment.
Overall, the pathophysiology of peritoneal metastasis involves a complex interplay of molecular and cellular processes that facilitate tumor cell adhesion, invasion, and proliferation within the peritoneal cavity. Understanding these mechanisms is crucial for developing targeted therapies to inhibit metastatic spread and improve outcomes for patients with peritoneal malignancies.
The following are discussions regarding the development of specific types of peritoneal cancers:
EOPPC
The pathogenesis of EOPPC is multifaceted and involves a combination of genetic alterations, developmental factors, and clonal evolution. EOPPC is thought to arise from the mesothelial cells of the peritoneum undergoing a malignant transformation termed "Mullerian metaplasia."[25] This theory suggests that mesothelial cells retain the potential to differentiate into Mullerian-type epithelium, similar to that found in the ovaries. This concept explains the histological resemblance between EOPPC and ovarian tumors.
The germinal epithelium of the ovaries and the mesothelial cells of the peritoneum originate from the same embryonal mesoderm. This shared developmental origin further supports the similarity between EOPPC and ovarian tumors.
Oncogenic stimuli, such as loss of heterozygosity at the p53 or BRCA-1 loci or overexpression of HER-2/neu, contribute to the development of EOPPC.[26] These genetic alterations disrupt normal cellular processes, leading to uncontrolled proliferation and malignant transformation.
EOPPC may originate multifocally within the peritoneum. However, studies have shown that nonrandom X chromosome inactivation follows a consistent pattern, suggesting a unifocal origin for some tumors. Gu et al conducted a study on the clonality of peritoneal and ovarian cancer, revealing that 54% of patients showed nonrandom X chromosome inactivation, with most exhibiting different patterns.[27] This finding suggests that while some tumors may have a unifocal origin, others may arise independently at multiple sites within the peritoneum.
Mesothelioma
MPM primarily arises from asbestos exposure, although other risk factors such as talc, mica, erionite, volcanic ash, radiation, and chronic peritonitis have also been implicated. The pathogenesis of MPM involves a complex interplay of genetic, molecular, and environmental factors.
Asbestos exposure leads to the deposition of asbestos fibers in the peritoneal cavity, causing cellular damage and initiating carcinogenesis. These fibers produce reactive oxygen species that damage deoxyribonucleic acid, leading to genetic mutations and aberrations. Asbestos fibers can directly enter mesothelial cells, disrupting cell cycle genes and inducing genetic abnormalities. Additionally, they trigger an inflammatory response, with macrophages and mesothelial cells releasing cytokines and growth factors that promote tumorigenesis.
Specific genes such as BRCA-1-associated protein-1 and cyclin-dependent kinase inhibitor 2A/alternative reading frame play a role in mesothelial cell transformation in MPM, contributing to the initiation and progression of the disease. Furthermore, the calcium-binding protein calretinin may protect cells from asbestos cytotoxicity.
High-mobility group box 1 protein, produced by mesothelial cells, contributes to MPM necrosis, inflammation, and carcinogenesis. Tumor necrosis factor-α released by macrophages activates nuclear factor-kappa B signaling, promoting cell survival and proliferation.[28] Asbestos fibers have a large surface area, allowing them to absorb carcinogenic molecules, which further promotes malignancy by increasing exposure to carcinogens within the peritoneal cavity.
Survivin, an inhibitor of apoptosis protein, is highly expressed in MPM and contributes to increased cell growth and decreased apoptosis. Telomere maintenance mechanisms, including telomerase activity and alternative lengthening of telomeres, are prevalent in MPM and contribute to the immortalization of cancer cells. Higher expression of survivin and telomerase activity has been correlated with poor clinical outcomes in MPM, including increased risk of relapse and cancer-related death.[29]
Other Peritoneal Cancers
Leiomyosarcomas are caused by a deletion in retinoblastoma gene1 (RB1) 10q and PTEN 13q. The mutations at TP53 also add to the risk.[30] Desmoplastic small round cell tumors, DSRCT, a small round blue cell tumor, is associated with Ewing tumor family chromosomal translocation t(11;22)(p13;q12), causing the formation of EWSR1-WT1 fusion oncogene that results in tumor development.[31]
Histopathology
Peritoneal cancer encompasses a variety of histological subtypes, which can arise either as primary peritoneal malignancies or as metastases from other primary sites. Here, we will discuss the histological characteristics of the most common types:
Primary Peritoneal Cancer
- Serous carcinoma
- This is the most common histological subtype of primary peritoneal cancer.
- Serous carcinoma resembles ovarian serous carcinoma histologically and is characterized by papillary architecture, stratified epithelial cells with atypical nuclei, and psammoma bodies (calcified structures).
- Immunohistochemical staining may show positivity for WT-1, PAX8, and CK7 markers.
- Examples include EOPPC.
- Mucinous carcinoma
- Mucinous carcinoma of the peritoneum is characterized by the presence of mucin-producing epithelial cells. Tumor cells are arranged in glandular structures filled with extracellular mucin.
- Immunohistochemical staining can help differentiate mucinous carcinoma from other types of peritoneal malignancies.
- Endometrioid carcinoma
- Endometrioid carcinoma of the peritoneum histologically resembles endometrioid carcinoma of the uterus, characterized by glandular structures lined by columnar epithelial cells resembling endometrial glands.
- Immunohistochemical staining may show positivity for markers such as estrogen receptor, progesterone receptor, and CK7.
- Clear cell carcinoma
- Clear cell carcinoma of the peritoneum is characterized by clear cytoplasm due to glycogen accumulation. Histologically, it may resemble clear cell carcinoma of the ovary or kidney.
- Immunohistochemical staining can aid in distinguishing peritoneal clear cell carcinoma from other histological subtypes.
- Undifferentiated carcinoma
- Some cases of primary peritoneal cancer may present as undifferentiated carcinomas, lacking specific differentiation features. These tumors are characterized by the absence of recognizable epithelial or mesenchymal differentiation markers.
Secondary Peritoneal Cancer
- Metastatic adenocarcinoma
- Secondary peritoneal cancer often presents as metastases from primary tumors in other organs, such as the ovaries, gastrointestinal tract, pancreas, or appendix. Histologically, metastatic adenocarcinoma in the peritoneum may resemble the histology of the primary tumor, exhibiting glandular differentiation and cellular atypia characteristic of adenocarcinomas.
- Mesothelioma
- MPM is characterized by malignant proliferation of mesothelial cells lining the peritoneum. Histologically, it can present as epithelioid, sarcomatoid, or biphasic subtypes, with epithelioid mesothelioma being the most common. Epithelioid mesothelioma is characterized by cuboidal or polygonal cells forming tubular or papillary structures, while sarcomatoid mesothelioma consists of spindle-shaped cells arranged in a fascicular pattern.
- Sarcomas
- Leiomyosarcomas
- These show variable smooth muscle actin and fascicles of eosinophilic spindled cells with blunt-ended nuclei showing variable pleomorphism.
- The key histologic criteria used to diagnose leiomyosarcoma in the retroperitoneum include at least one of the following: cellular pleomorphism or atypia, coagulative tumor cell necrosis, and increased mitotic rates.
- Immunostaining is present for smooth muscle actin, muscle-specific actin, desmin, or h-caldesmon.
- Liposarcoma
- There is a presence of macroscopic fat within the primary tumor or peritoneal implants.
- Gastrointestinal stromal tumor
- This is a cellular tumor with a storiform pattern and moderate cytologic atypia.
- Positive staining for c-kit confirms the diagnosis.[32]
- Leiomyosarcomas
- Pseudomyxoma peritonei
- Signet ring cells and destructive invasion can be seen and are associated with poor outcomes.
- Degenerated mucinous cells floating in pools of mucin can also be seen. These are not considered true signet ring cells.
Other Tumors of Uncertain Origin
- DSRCT
- This consists of cords and nests of undifferentiated, uniform, small, and round malignant cells surrounded by a dense collagenous stroma. Numerous mitotic figures and single-cell necrosis are characteristic.
- Cytokeratin, desmin, and WT1 (antibody to the carboxy terminus [C terminus]) are commonly expressed.
In summary, peritoneal cancer encompasses a diverse range of histological subtypes. Accurate histological diagnosis is essential for guiding treatment decisions and predicting patient outcomes. Immunohistochemical staining and molecular profiling may further characterize peritoneal tumors and differentiate between primary and secondary malignancies.
History and Physical
Patients with peritoneal cancer often present with a variety of vague symptoms, including abdominal bloating, distension, nausea, indigestion, anorexia, weight loss, fatigue, constipation, and abdominal or back pain. Among these, abdominal distension and pain are the most commonly reported symptoms, while palpable abdominal mass and ascites are frequent signs. Approximately 85% of patients exhibit nonspecific abdominal symptoms and ascites.[33] Additionally, tumor-associated lymphadenopathy can cause local mass effects and, in some cases, superior vena cava obstruction, primarily seen in MPM. Some patients with DSRCT may present with hematemesis.[34]
The symptoms of peritoneal carcinomatosis can vary depending on the extent and location of secondary metastatic deposits. The growth of both primary and secondary tumors can exert pressure effects, leading to mechanical intestinal obstruction, potentially resulting in patients presenting with an "acute abdomen" as a medical emergency. Bowel obstructions are commonly seen in colorectal cancers, occurring in about 20% of cases.[35] Additionally, ascites may be present in various malignancies, with study results reporting its occurrence in 43% of cases of pancreatic cancer.
During physical examination, abdominal distension, palpable abdominal masses, tenderness, and percussion dullness over ascites accumulation may be noted. Depending on the extent and involvement of the cancer, signs of malnutrition, anemia, and jaundice may also be observed. However, it's important to recognize that the presentation of peritoneal cancer can be diverse and influenced by factors such as tumor location, size, and aggressiveness. Therefore, a comprehensive evaluation, including imaging studies and laboratory tests, is necessary for accurate diagnosis and staging.
Evaluation
The workup for diagnosing peritoneal cancer involves a comprehensive evaluation that includes imaging studies, laboratory tests, and possibly invasive procedures in conjunction with the history and physical examination.
Laboratory Tests
Laboratory tests, including complete blood count, comprehensive metabolic panel, amylase, lipase, coagulation studies, urinalysis, serum albumin, and blood typing, should be obtained to help assess overall health status and evaluate organ function. Additionally, tumor markers cancer antigen 125 (CA-125), carcinoembryonic antigen (CEA), CA 19-9, alpha-fetoprotein (AFP), and CA 15-3 may be elevated in patients with peritoneal cancer. However, these markers are not specific to peritoneal cancer and can also be elevated in other malignancies or benign conditions.
Imaging studies are crucial in detecting and characterizing peritoneal masses, assessing tumor extent, and evaluating metastatic disease. The following studies should be considered:
Radiological Studies
Imaging techniques are critical in diagnosing peritoneal cancer and serve various diagnostic purposes. These include identifying the primary site of metastasis and characterizing the morphology of the main tumor, which may present as solid, cystic, or mixed. Additionally, imaging helps assess the quantity of ascites present, a common feature of peritoneal cancer, and detect the presence or absence of peritoneal dissemination. Imaging also aids in distinguishing between diffuse or nodular spread of disease, evaluating lymphadenopathy, and identifying involved lymph nodes. Furthermore, imaging techniques are valuable for detecting distant sites of metastasis, if present, providing crucial information for treatment planning and management decisions in patients with peritoneal cancer.
- Ultrasound
- Abdominal ultrasound is often used as an initial imaging modality to evaluate patients with suspected peritoneal cancer.
- Although its sensitivity may be limited compared to computed tomography (CT) or magnetic resonance imaging (MRI), ultrasound can detect ascites, liver metastases, and intraabdominal masses. However, it cannot detect malignant granulations less than 2 cm.
- In peritoneal cancer, ultrasonographic features include ascites, which are echo-free or have low-level echoes, and hyperechogenic nodules representing cell deposition in the peritoneum. Adhesion of bowel loops, omental matting, and lymphadenopathy can also be seen.[36]
- Ultrasound-guided procedures, such as fine-needle aspiration or core biopsy, can be used to obtain tissue samples for histological examination.
- CT scan
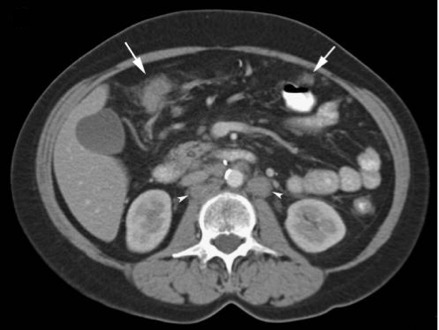
- CT scan is the primary imaging modality used in patients presenting with abdominal pain and distension, serving as the foremost diagnostic tool for peritoneal cancer. (see Image. Infiltration and Nodularity of Peritoneum).
- CT scans are highly sensitive in detecting peritoneal lesions, capable of detecting granulations as small as 5 mm. However, the sensitivity decreases as the lesion size decreases.[37] Owing to technical advances, CT has reached a sensitivity of 79% to 86% and specificity of 82% to 89% in the detection of peritoneal involvement; but, when the lesion size is less than 10 mm, the sensitivity drops to 7% to 28% for lesions 5 mm or less.[35][38] Despite its nonspecific findings, CT scans commonly reveal ascites, contrast-enhanced diffuse or nodular thickening of the peritoneum, and omental cakes, characterized by increased density of large cell masses between the bowel and anterior abdominal wall. Contrast administration aids in visualizing small peritoneal deposits but may limit the identification of calcified lesions.[39]
- In primary peritoneal cancer such as EOPPC, CT scan findings typically include ascites (82%), peritoneal nodules (73%), omental caking (64%), and pelvic masses (36%), as reported by Chiou et al.[40] Similarly, in MPM, CT scans reveal solid, heterogeneous masses with irregular margins, often accompanied by ascites in 60% to 100% of cases. Notably, MPM usually lacks lymph node involvement and distant metastasis.[41] Leiomyosarcomas display contrast-enhanced heterogeneous solid and cystic masses with septations, necrosis, and calcifications. Moreover, DSCRTs exhibit well-enhanced lobulated masses comprising necrotic, hemorrhagic, and fibrous components, typically located in the retrovesical or rectouterine space.
- Overall, CT scans serve as the primary diagnostic modality for peritoneal cancer, providing essential information for guiding interventional radiologists in biopsies and surgeons in debulking surgeries.
- MRI scan
- Gadolinium-enhanced MRI has emerged as a superior imaging modality to helical CT scans for visualizing small peritoneal carcinomatosis. MRI exhibits a higher detection sensitivity, with a sensitivity of 84% for tumors of all sizes, compared to 54% for CT scans. Moreover, MRI demonstrates an even greater sensitivity for tumors with dimensions less than 1 cm, ranging from 85% to 95%, especially located in the subphrenic a and the bowel serosa.[38][42] In MRI imaging, peritoneal tumors typically exhibit an intermediate signal on T1-weighted images, a high signal on T2-weighted images, and enhancement with gadolinium contrast.
- The utilization of MRI in peritoneal cancer diagnosis and staging has increased over time, as it offers superior contrast resolution and is considered the imaging modality of choice for identifying and staging subcentimeter lesions.[39] However, while MRI aids in visualizing peritoneal lesions, histological differentiation of tumor types typically requires biopsy due to the resemblance in radiological appearances.
- Positron emission tomography scan
- The primary imaging modalities for peritoneal cancer are typically CT scans and MRIs; however, these methods may not always detect small peritoneal implants. In such cases, fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT scan can enhance detection by identifying malignant cells based on their increased glucose metabolism. This approach aids in early detection, staging, monitoring treatment response, and long-term follow-up.[43]
- The sensitivity of fused PET-CT scans (unenhanced) and multidetector PET-CT (PET-MDCT) scans ranges from 58% to 100%. False-negative results may occur in cases where certain tumor cells do not take up 'F,' such as mucinous ovarian tumors or signet ring gastric cancer. Conversely, false-positive results can arise in benign and inflammatory conditions where cells take up 'F.' Nevertheless, the diffuse or nodular uptake of 'F' by peritoneal cells can lead to the detection of occult malignancies or metastases, significantly influencing the management of peritoneal cancer.
Invasive Techniques
The resemblance in radiological appearances between neoplastic and nonneoplastic lesions often necessitates invasive techniques for accurate diagnosis. Typically, in peritoneal cancer, establishing the histological type and subtypes of the tumor requires a biopsy. Invasive procedures commonly used in the diagnosis of peritoneal cancer include:
- Paracentesis
- This procedure involves removing ascitic fluid from the peritoneal cavity, which often accumulates in patients with peritoneal cancer. This fluid can then be analyzed, providing valuable diagnostic information, including the presence of malignant cells, protein levels, cell counts, and cytological analysis.
- This fluid removal can also provide symptomatic relief by alleviating abdominal distension and discomfort associated with ascites accumulation.
- Ascitic fluid analysis
- Cell analysis
- Frank bloody ascites are relatively rare in cases of peritoneal carcinomatosis, and they may occur in approximately 10% of cases. Similarly, ascitic fluid that appears blood-tinged, characterized by a high red blood cell count (>10,000 cells/mm), is observed in approximately 8.3% of cases.[44] These findings may be indicative of vascular invasion or hemorrhage within the peritoneal cavity secondary to the presence of malignant cells.
- Biochemical markers
- In peritoneal cancer, ascites are characterized by specific biochemical features that can aid in its diagnosis and differentiation from other causes of abdominal fluid accumulation. Typically, this ascitic fluid is exudative, exhibiting elevated levels of specific biochemical markers:
- High protein levels are typically measured at around 2.5 mg/dL. This increase in protein content reflects the leakage of proteins from blood vessels into the peritoneal cavity due to the presence of cancerous cells.
- Elevated lactate dehydrogenase values reach around 400 IU/L. Lactate dehydrogenase is an enzyme released from damaged or diseased cells, indicating cellular damage or breakdown within the peritoneal cavity.
- Low glucose levels typically measure around 40 mg/dL. This reduction may be attributed to increased glucose consumption by cancer cells or inflammatory processes within the peritoneal cavity.
- In peritoneal cancer, ascites are characterized by specific biochemical features that can aid in its diagnosis and differentiation from other causes of abdominal fluid accumulation. Typically, this ascitic fluid is exudative, exhibiting elevated levels of specific biochemical markers:
- Tumor markers
- The assessment of tumor markers in both serum and ascites has been investigated to determine their efficacy in diagnosing malignancies, particularly in cases of peritoneal cancer. Tumor markers such as CEA, CA 19-9, CA-125, and cytokeratin fragments (CYFRA) have been studied extensively, revealing a high correlation between their levels in ascites and serum. However, it's important to note that while CA-125, CEA, and CYFRA are produced by normal epithelial cells and can also be found in certain benign conditions, CYFRA has been observed to be present in higher quantities in malignant conditions.
- Initially, results from studies did not find a significant benefit in measuring tumor markers in ascites compared to serum for diagnosing malignancies.[45] However, subsequent research has shown that combining tumor marker analysis with cytology in ascitic fluid can significantly increase the diagnostic yield. Specifically, a combination of 3 tumor markers (CA 19-9, CA 15-3, CEA) has been reported to demonstrate high sensitivity (86%) and specificity (97%) in cases where cytology results are negative.[46]
- Furthermore, increased vascular endothelial growth factor (VEGF) and other tumor markers may provide additional diagnostic and prognostic information.[47] VEGF is known to promote angiogenesis, facilitating the growth and spread of tumors, and its elevation in ascitic fluid may reflect the aggressive nature of the underlying malignancy.
- Despite these findings, the significance of measuring tumor markers in ascites remains debatable due to varying results from studies using different cutoff levels for tumor markers. Consequently, it is considered an unproven and potentially unhelpful test due to its low sensitivity. Further research and standardization of protocols may be necessary to establish the clinical utility of tumor marker measurement in ascites for diagnosing peritoneal cancer and other malignancies.
- Cytology
- Cytology plays a crucial role in diagnosing malignant-related ascites, with initial specimens yielding positive results in 83% of cases, a rate that increases to 93% and 97% with the submission of 2 and 3 samples, respectively.[44] However, cytology may not provide definitive results in cases of MPM, where sensitivity ranges from 50% to 70%.[44] Subtyping the tumor based on cytology alone can be challenging, necessitating immunohistochemical (IHC) staining for accurate differentiation, grading, and treatment planning.
- Several specific IHC stains, including calretinin, cytokeratin, and BerEP4, are commonly employed in peritoneal cancer diagnosis. Calretinin, a mesothelial marker, indicates the presence of both normal and malignant mesothelial cells. Positive staining for calretinin and BerEP4 suggests an epithelial origin, aiding in diagnosing early onset primary peritoneal cancer (eg, EOPPC). MPM typically stains negative for BerEP4 but positive for cytokeratin CK5/6, calretinin, and podoplanin. DSRCT cells stain positive for cytokeratin, desmin, neuron-specific enolase, and WT1.
- Metastatic adenocarcinomas generally lack nuclear staining for calretinin and cytokeratins. Tumors originating from the upper gastrointestinal tract typically show positivity for CK-7 and varying results for CDX2/CK20, while those from the lower gastrointestinal tract are negative for CK-7 and positive for CDX2/CK20.[48][49]
- IHC staining enhances the chances of detecting malignancy and determining its pathological subtype from ascitic fluid, especially in cases where cytology results may be ambiguous. However, the definitive role of IHC staining in this context remains to be seen, and it is typically utilized as an adjunct to cytological analysis.
- Cell analysis
- Ascitic fluid analysis
- Laparoscopy
- This minimally invasive procedure offers direct visualization for targeted peritoneal lavage and identification of tumors with a remarkable sensitivity of 100%. During laparoscopy, biopsies of suspected lesions are obtained, enabling histological diagnosis and aiding in tumor staging; this is a valuable tool for differentiating between unresectable and operable tumors, helping avoid extensive procedures like open laparotomies. Laparoscopy facilitates the calculation of the peritoneal carcinomatosis index (PCI), which evaluates tumor spread across 13 abdominal regions, assigning a score of 0 to 3 for each region. The total PCI score ranges from 0 to 39, with higher scores indicating a worse prognosis. Additionally, PCI helps predict the response to surgery in patients with peritoneal cancer.[50][51]
- Laparotomy
- Compared to laparoscopy, abdominal exploration via open laparotomy allows the detection of even small lesions measuring 1 to 2 mm in size.
These laboratory tests, imaging studies, and clinical evaluations are essential components in diagnosing peritoneal cancer, aiding in assessing its extent and guiding treatment decisions. However, it is imperative to interpret these results within the context of the patient's clinical presentation and other diagnostic findings. The often concealed nature of malignant tumors within the peritoneum underscores the importance of early and timely diagnosis to prevent unnecessary surgeries for unresectable tumors and avoid the potential harm from unnecessary chemotherapy drugs. Hence, practical methods for early detection are crucial in improving patient outcomes and reducing fatality rates associated with peritoneal cancer.
Treatment / Management
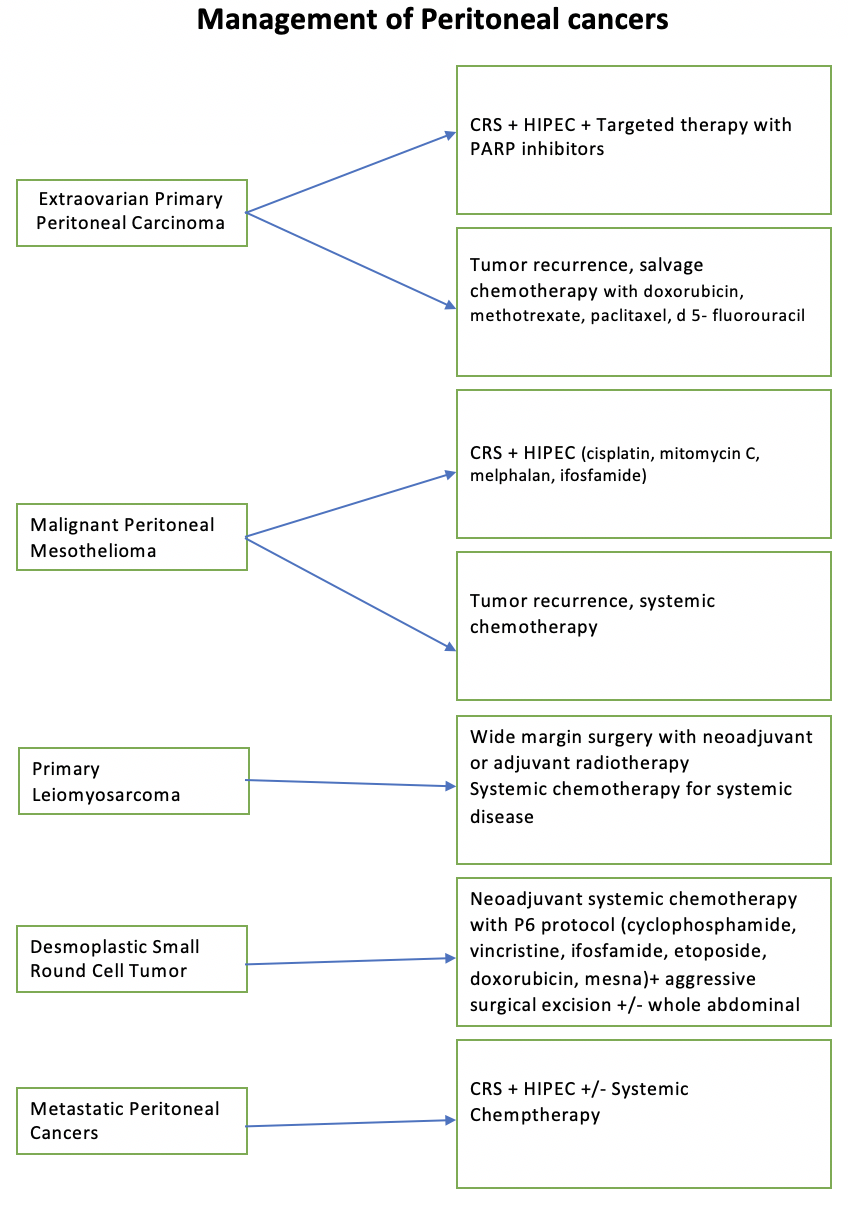
The optimal therapeutic approach for peritoneal cancer involves adopting multimodal therapy, which combines surgery, chemotherapy, and targeted therapy. This comprehensive treatment strategy, pioneered by Dr. Sugarbaker, has been supported by numerous clinical trials and systematic reviews. Compared to the traditional palliative approach, multimodal therapy has demonstrated significant survival benefits, with some studies reporting survival durations of up to 60 months compared to 4 to 12 months (see Image. Management of Peritoneal Cancer).[52] This approach underscores the importance of integrating various treatment modalities to improve patient outcomes and extend survival in peritoneal cancer cases.
The following are treatments for peritoneal cancer:
Cytoreductive Surgery
Cytoreductive surgery (CRS) is a procedure aimed at surgically removing all visible tumors from both the parietal and visceral peritoneal layers. This comprehensive approach involves en-bloc resections of affected organs or tissues and peritonectomy, ensuring no tumor nodule larger than 2.5 mm is left behind. Electrosurgery may be utilized for visceral implants to facilitate excision and control bleeding from the visceral peritoneum. The primary goal of CRS is eradicating macroscopically visible disease, although patient selection for this procedure is critical due to its associated enhanced morbidity.
Patient selection for CRS involves several considerations, including the peritoneal cancer index, PCI, which quantifies the extent of peritoneal involvement, and the histological tumor grade. Additionally, a good patient performance status is essential. Contraindications for CRS include a PCI score >17 in colorectal-associated peritoneal cancer and PCI >12 in gastric cancer. Tumor involvement of critical anatomic sites in the abdomen and multiple extra-abdominal metastatic lesions also preclude CRS.[53]
The effectiveness of CRS is evaluated using a 'completeness of cytoreduction' score (CCR), which categorizes the extent of residual disease. A CCR 0 indicates no residual disease, CCR 1 signifies minimal disease <2.5 mm, CCR 2 indicates tumor nodules between 2.5 mm and 2.5 cm, and CCR 3 indicates residual disease >2.5 cm.[53] However, CRS is associated with postoperative complications that contribute to long-term morbidity. These complications may include venous-thrombotic events, operative site abscesses, anastomotic leaks, fistula formation, and prolonged intensive care stay. Therefore, careful patient selection and meticulous surgical technique are essential for optimizing outcomes and minimizing complications associated with CRS.
Hypothermic Intraperitoneal Chemotherapy
Hypothermic intraperitoneal chemotherapy (HIPEC) involves delivering potent chemotherapy drugs into the peritoneal cavity at a temperature higher than normal body temperature (typically around 108 °F or 41-43 °C) for approximately 2 hours immediately following surgery. The elevated temperature of the chemotherapy solution induces hyperthermia, which impairs deoxyribonucleic acid repair in cancer cells, promotes apoptosis, inhibits angiogenesis, and denatures proteins. This cytotoxic effect causes cancer cells to perish at temperatures as low as 104 °F, while healthy cells remain viable up to 111 °F. The procedure enhances drug absorption and ensures homogeneous distribution within the peritoneal cavity, minimizing systemic exposure and maximizing local action against residual disease. Additionally, fluid introduced into the peritoneal cavity during HIPEC is drained by the portal venous system; therefore, the chemotherapeutic agent reaches a high concentration in the liver and may destroy parenchymal micrometastases.[54] The primary objective of HIPEC is to eliminate microscopic residual disease effectively. Because penetration of the chemotherapeutic agent is limited to 2 to 5 mm, optimal cytoreduction (R0 and R1 resections) is crucial.[55]
Common chemotherapy agents utilized in HIPEC include mitomycin C, oxaliplatin, cisplatin, and doxorubicin.[56] The duration of the HIPEC procedure typically ranges from 60 to 100 minutes. Common side effects of HIPEC may include neutropenia, spontaneous bowel perforations, electrolyte imbalances, acute renal failure, and bleeding diathesis.[57](A1)
In addition to the traditional open surgery approach, HIPEC can also be administered laparoscopically. Laparoscopic HIPEC has demonstrated effectiveness, particularly in cases of refractory malignant ascites, with a success rate of 95% reported in a systematic literature review.[58] This minimally invasive approach can be employed as adjuvant, neoadjuvant, or palliative therapy, with palliation being the primary indication. Laparoscopic HIPEC offers several advantages, including increased intraabdominal pressure, which enhances the penetration of chemotherapy drugs. Furthermore, this modality is associated with significantly lower morbidity and mortality rates compared to open surgery. However, 1 drawback of laparoscopic HIPEC is higher recurrence rates.[59] (A1)
Despite the potential risks, HIPEC remains a valuable therapeutic option for selected patients with peritoneal cancer, offering the potential for improved outcomes and quality of life.
Early Postoperative Intraperitoneal Chemotherapy
Early postoperative intraperitoneal chemotherapy (EPIC) is a regimen for intraperitoneal delivery of chemotherapy drugs following surgery. This therapy is initiated on the first postoperative day and then administered continuously for 5 to 7 days. During the procedure, a solution containing chemotherapy drugs is introduced into the peritoneal cavity, where it bathes the mesothelium for a duration between 4 and 24 hours. Subsequently, the solution is drained over an hour and then readministered.[60] Before starting EPIC therapy, it is essential to ensure the patient's stable postoperative status, including a normal white blood cell count and tolerance to treatment. Depending on the patient's condition, the therapy may be delayed until the second postoperative day. A catheter is secured with sutures to facilitate drug delivery, and multiple closed suction drains are placed for drainage. The chemotherapy drugs employed in EPIC are typically cell-specific, in contrast to the cell cycle nonspecific drugs used in other intraperitoneal chemotherapy regimens such as HIPEC. Commonly used agents include 5-fluorouracil, taxanes, and leucovorin.[61] EPIC offers a targeted approach to delivering chemotherapy directly to the peritoneal cavity, potentially enhancing therapeutic efficacy while minimizing systemic side effects.
EPIC vs HIPEC
EPIC offers several advantages over HIPEC, primarily due to increased drug contact time with the peritoneal surface. Unlike HIPEC, where the instilled solution covers only 30% to 40% of the peritoneal surface if allowed for a short period, EPIC allows for prolonged contact with the peritoneum, resulting in more uniform drug distribution and enhanced cytotoxic effects. Additionally, the formation of postoperative adhesions, which can entrap cancer cells, does not hinder the effectiveness of chemotherapy drugs over the 5 days allowed in EPIC. Moreover, EPIC simplifies the closed abdominal technique and eliminates the need to heat the chemotherapy drugs, making it a more convenient option. Studies conducted in mice have indicated the superiority of EPIC over HIPEC in terms of overall survival.[62] However, clinical studies comparing the 2 intraperitoneal regimens have yielded mixed results.(B3)
While some studies have shown favorable outcomes with EPIC, including improved overall survival, others have reported higher incidences of complications. For instance, results in one study found a higher rate of digestive fistulas and peritoneal carcinomatosis recurrence in the EPIC group compared to HIPEC, with overall survival statistically insignificant.[63] Similarly, another study assessing the adjunctive use of EPIC after HIPEC and CRS reported increased morbidity and longer hospitalization without a significant improvement in overall survival.[64] These findings suggest that while EPIC may offer certain advantages, carefully considering patient-specific factors and potential risks is necessary when determining the most appropriate treatment approach for peritoneal cancer.(B2)
Pressurized Intraperitoneal Aerosol Chemotherapy
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) represents a novel approach to delivering intraperitoneal chemotherapy minimally invasively, particularly for patients who are not candidates for CRS + HIPEC. PIPAC is often used as a palliative measure in patients with a significant tumor burden or persistent ascites, aiming to alleviate symptoms and improve quality of life. The procedure involves using a device called Capnopen, which is connected to an injector and inserted into the peritoneum through a trocar. Chemotherapy drugs, such as cisplatin and doxorubicin, are then pressurized into aerosols using nebulizer technology and administered into the peritoneal cavity over about 1 hour.
PIPAC offers several advantages, including the ability to deliver chemotherapy in a repeated and safe manner without significant systemic side effects. The pressurized delivery allows for smaller doses, resulting in higher drug concentrations, deeper penetration, and more effective distribution throughout the peritoneal cavity.[65] Moreover, PIPAC has been associated with decreased chemical bowel perforations compared to HIPEC and minimal to no renal and hepatic toxicity post-procedure.[66] Studies investigating PIPAC in peritoneal cancer arising from intestinal, appendiceal, gastric, and ovarian origins have underscored its safety profile, improved tolerability, and efficacy in controlling ascites production.[67][68][69] Median survival after PIPAC administration has been reported to be approximately 15.7 months.[70] These findings suggest that PIPAC holds promise as a treatment option for patients with peritoneal cancer, offering potential benefits in terms of disease control and patient outcomes.(A1)
However, there are limitations to its utilization. The aerosols may not reach certain anatomical locations within the peritoneal cavity, and adhesions from previous surgeries can impede aerosol diffusion. As such, PIPAC may be unsuitable for patients in the early stages of the disease or those experiencing recurrence after CRS.[71] PIPAC is contraindicated in cases of biliary or small bowel obstructions, as well as in the presence of extra-abdominal metastasis. Additionally, tumor response following PIPAC alone is often insufficient, necessitating the addition of systemic chemotherapy to improve clinical outcomes and quality of life. This combined approach, known as a bidirectional treatment, has shown promise in improving patient outcomes, including PCI improvement from 50% to 88%.[72] While studies on PIPAC have demonstrated its safety, tolerability, and potential benefits in controlling ascites production, further clinical trials are needed to fully evaluate its efficacy and optimal use in managing peritoneal cancer.(A1)
Bidirectional/Neoadjuvant Intraperitoneal and Systemic Chemotherapy
The bidirectional/neoadjuvant intraperitoneal and systemic chemotherapy (BIPSC/NIPS) approach is a novel treatment strategy developed in Japan, primarily aimed at optimizing outcomes for patients with gastric cancer. This comprehensive procedure involves several sequential steps to address different aspects of the disease. Initially, patients undergo NIPS to reduce the tumor burden. This neoadjuvant chemotherapy is administered both systemically and directly into the peritoneal cavity, aiming to target cancer cells both within the stomach and any potential peritoneal spread. Following NIPS, patients undergo CRS to remove any remaining macroscopic tumor lesions surgically. This extensive surgical procedure aims to eliminate visible tumors and achieve complete cytoreduction. Subsequently, patients receive HIPEC, which involves delivering heated chemotherapy directly into the abdominal cavity to destroy any remaining cancer cells and prevent disease recurrence.
The final component of the BIPSC/NIPS approach is EPIC. EPIC involves administering chemotherapy directly into the peritoneal cavity in the immediate postoperative period, aiming to target and eradicate residual microscopic peritoneal deposits. By combining systemic chemotherapy, intraperitoneal chemotherapy, CRS, HIPEC, and EPIC, the BIPSC/NIPS approach aims to manage gastric cancer (and potentially other peritoneal cancers) comprehensively, addressing both localized and peritoneal disease spread while minimizing the risk of recurrence and improving patient outcomes.[38][73](B3)
Treatment by Cancer Type
- EOPPC
- This is managed similarly to serous ovarian carcinomas, with a multifaceted treatment approach aimed at optimizing patient outcomes. The standard treatment protocol involves surgical intervention followed by chemotherapy and targeted therapy. Surgical procedures typically include hysterectomy with bilateral salpingo-oophorectomy and omentectomy, which are performed in all cases to remove the primary tumor and any involved tissues.
- Following surgery, patients receive chemotherapy, often incorporating platinum-based agents as part of a neoadjuvant or adjuvant treatment strategy. However, the emergence of platinum-resistant tumors has necessitated the exploration of alternative treatment modalities. Targeted therapy with poly (ADP-ribose) polymerase (PARP) inhibitors has shown promising results in blocking deoxyribonucleic acid repair mechanisms and enhancing treatment efficacy. PARP inhibitors such as olaparib, rucaparib, olaparib, or veliparib are combined with chemotherapy to target specific molecular pathways in cancer growth and progression.
- Additionally, intraperitoneal chemotherapy has demonstrated superior outcomes to intravenous administration, as evidenced by a phase III clinical trial showing improved overall survival rates.[74] Debulking surgery, CRS, is crucial in achieving optimal outcomes, excising as much tumor tissue as possible, leaving <2 cm residual nodules. CRS is performed with chemotherapy, producing optimal results in 33% to 69% of patients.[1]
- In cases of tumor recurrence, salvage chemotherapy regimens are employed, which may include agents such as doxorubicin, methotrexate, paclitaxel, and 5-fluorouracil. The combination of surgery, chemotherapy, and targeted therapy offers a comprehensive approach to managing EOPPC, with the potential to improve patient prognosis and quality of life.
- MPM
- CRS coupled with intraperitoneal chemotherapy is the primary treatment approach in managing MPM. HIPEC or EPIC are commonly employed. Among these modalities, HIPEC has garnered preference based on collected data.[41] The chemotherapeutic agents utilized in these procedures often include cisplatin in combination with mitomycin C, melphalan, or ifosfamide, administered directly into the peritoneal cavity to target residual disease and micrometastases.
- For patients deemed at high surgical risk or those experiencing recurrent tumors, systemic chemotherapy may be considered an adjunctive or alternative treatment option. Furthermore, recent advancements in immunotherapy, particularly involving checkpoint inhibitors, have shown promising activity in managing MPM. These immunotherapeutic agents work by modulating the immune response to target cancer cells, offering a potential avenue for improved treatment outcomes and patient prognosis in MPM.
- DSRCT
- A neoadjuvant chemotherapy regimen is the cornerstone of treatment in managing DSRCT. This systemic chemotherapy typically involves a combination of cyclophosphamide, ifosfamide, vincristine, etoposide, doxorubicin, and mesna, often called the P6 protocol. Following neoadjuvant chemotherapy, aggressive surgical excision of the tumor is pursued to remove residual disease and optimize treatment outcomes.
- While the role of HIPEC as an adjunct therapy in DSRCT treatment is still being investigated, studies are ongoing to evaluate its potential benefits in conjunction with other therapeutic modalities. Additionally, consolidative whole abdominal radiotherapy is considered integral, particularly in the pediatric population and adults, to enhance treatment efficacy and improve overall patient outcomes.[75]
- Primary leiomyosarcoma of the peritoneal cavity
- These present as aggressive tumors requiring comprehensive management strategies. Resectable tumors are typically addressed with extensive wide-margin surgeries aimed at complete tumor removal. For metastatic disease, systemic chemotherapy plays a crucial role in controlling tumor progression and improving patient outcomes. Both preoperative and postoperative radiotherapy are considered essential components of treatment to enhance local tumor control and reduce the risk of recurrence.
- In recent years, CRS and HIPEC have emerged as significant therapeutic approaches for managing peritoneal leiomyosarcomas. This combined modality treatment has shown promising results in improving patient survival and disease control by delivering chemotherapy directly to the peritoneal cavity. Intraperitoneal chemotherapy achieves higher local drug concentrations by bypassing hepatic metabolism and the first-pass effect, leading to more effective tumor eradication and better clinical outcomes.
(A1)
Differential Diagnosis
The following conditions should be considered in the differential diagnosis when peritoneal cancer is suspected:
- Peritoneal lymphomatosis
- Peritoneal tuberculosis
- Granulomatous peritonitis from histoplasmosis
- Peritoneal melanosis
- Gliomatosis peritonei
- Desmoid tumor
- Solitary fibrous tumor
- Carcinoid tumor
- Actinomycosis
- Splenosis implants
- Endometriosis
- Foreign body reaction [38]
Staging
Primary Peritoneal Cancer Staging
Primary peritoneal cancer is always classed as stages III and IV. Thus, high-grade adenocarcinomas that develop from the mesothelium of the peritoneal cavity are extrauterine adenocarcinomas of Mullerian epithelial origin and are staged like ovarian carcinomas.[76] The staging is as follows:
- Stage III
- This stage is defined as the confinement of the tumor within the peritoneal cavity and has further subdivisions as follows:
- IIIA: Cancer is found in pelvic organs and lymph nodes within the abdominal cavity.
- IIIB: Cancer has spread to the peritoneum outside the pelvis, and the tumor size in the peritoneum is 2 cm or less. Also, lymph nodes outside the peritoneum will be involved.
- IIIC: Cancer involves the peritoneum outside the pelvis, and the tumor size in the peritoneum is greater than 2 cm. There is also spread to lymph nodes outside the peritoneum.
- This stage is defined as the confinement of the tumor within the peritoneal cavity and has further subdivisions as follows:
- Stage IV
- This stage is defined as cancer that has metastasized to other organs of the body; this is further subdivided into:
- IVA: Malignant pleural effusions develop
- IVB: Cancer has spread to organs and tissues outside the peritoneal cavity, such as the liver, lungs, or groin lymph nodes
- This stage is defined as cancer that has metastasized to other organs of the body; this is further subdivided into:
Secondary Peritoneal Cancer Staging
Secondary peritoneal cancer was described by Gilly et al and is as follows:
- Stage 0
- No macroscopic disease.
- Stage I
- Malignant lesion of size <5 mm localized to 1 part of the abdomen.
- Stage II
- The lesion is <5 mm but diffuses to the whole abdomen.
- Stage III
- Malignant granulations are >5 mm but <2 cm.
- Stage IV
- Large malignant cakes >2 cm.[77]
Prognosis
Peritoneal cancer represents a terminal illness with a challenging prognosis, whether originating in the peritoneum or metastasizing from elsewhere. Primary peritoneal cancer typically presents as advanced stage IV disease, with a survival rate ranging from 11 to 17 months.[78] Secondary peritoneal cancer, characterized by cancer spreading to the peritoneum from other sites, demonstrates a median survival of around 6 months, varying according to the stage of cancer progression. Factors such as the primary tumor's location significantly influence survival rates, with pancreatic origin associated with the poorest prognosis (2.9 months), followed by gastric (6.5 months) and colorectal origin (6.9 months).[72] The presence of ascites and hepatic metastasis further negatively impacts survival outcomes.
In the case of EOPPC, prognostic factors include patient age, stage, performance status, and residual tumor size after CRS. In multivariate analysis, performance status and residual tumor size have been identified as significant predictors of prognosis.[79] MPM prognosis is influenced by the histological subtype, with the epithelioid subtype having a more favorable prognosis than the biphasic subtype. In 1 study, results showed that median survival in epithelioid MPM was 55 months as opposed to 13 months in the biphasic subtype. Factors such as sarcomatoid features, depth of invasion, CCR score, and inflammatory stroma contribute to prognosis.[80] DSRCT prognosis is notably poor, with a 5-year survival rate ranging from 15% to 30%. Factors such as the presence of extra-abdominal tumors and the efficacy of surgical resection and radiotherapy influence survival outcomes.[81]
Leiomyosarcoma prognosis is influenced by factors such as the stage and size of the tumor, with an overall 5-year survival rate of 64%.[12] Prognostic factors in secondary peritoneal cancer originating from gastrointestinal tumors include tumor histopathology, stage, PCI score, and CCR score.[82] A study by Vaira et al showed that CCR is strictly related to overall survival, and PCI has no statistically significant effect.[83] In peritoneal cancer originating from ovarian cancer, low-grade, small-volume tumors, adjuvant chemotherapy in treatment, and complete CCR were associated with favorable outcomes, with PCI being the best indicator for survival.[84] Understanding these prognostic factors is crucial for guiding treatment decisions and providing patients with realistic expectations regarding their prognosis and survival outcomes.
Deterrence and Patient Education
While peritoneal cancer often arises from metastasis of other primary tumors, efforts to prevent primary cancers, such as those of the ovaries, gastrointestinal tract, or mesothelium, can indirectly reduce the incidence of peritoneal cancer. Primary prevention strategies may include promoting healthy lifestyle behaviors, such as maintaining a balanced diet rich in fruits and vegetables, regular exercise, avoiding tobacco use, limiting alcohol consumption, and reducing exposure to environmental carcinogens like asbestos.
Educating individuals about the risk factors associated with peritoneal cancer can help in its early detection and management. Risk factors may include a personal or family history of cancer, genetic predispositions (eg, BRCA mutations for ovarian cancer), exposure to carcinogens, chronic inflammation (eg, inflammatory bowel diseases for gastrointestinal cancers), and specific hereditary syndromes (eg, Lynch syndrome). Patient education should emphasize recognizing early warning signs and symptoms of peritoneal cancer. These may include abdominal pain or discomfort, bloating, changes in bowel habits, unexplained weight loss, loss of appetite, fatigue, and ascites. Encouraging individuals to promptly report any persistent or concerning symptoms to their healthcare providers can facilitate timely diagnosis and intervention.
While no specific screening tests exist for peritoneal cancer in the general population, individuals at increased risk due to genetic predispositions or prior cancer history may benefit from tailored surveillance protocols. Patient education should stress the importance of adhering to recommended screening guidelines and attending regular follow-up appointments with healthcare providers for early detection of any suspicious changes. In individuals with a family history suggestive of hereditary cancer syndromes, such as BRCA mutations associated with ovarian cancer, genetic counseling, and testing can provide valuable information about their cancer risk. Patient education efforts should raise awareness about the availability and benefits of genetic counseling and testing, empowering individuals to make informed decisions about their healthcare and risk-reduction strategies.
Patient education should also involve connecting individuals diagnosed with peritoneal cancer and their caregivers to advocacy organizations and support groups. These resources can offer valuable information, emotional support, and practical assistance throughout the cancer journey, helping patients cope with the challenges associated with diagnosis, treatment, and survivorship. In summary, deterrence and patient education on peritoneal cancer encompasses a comprehensive approach that spans primary prevention, early detection, risk awareness, symptom recognition, screening, genetic counseling, and psychosocial support. By empowering individuals with knowledge and resources, healthcare providers can contribute to reducing the burden of peritoneal cancer and improving patient outcomes.
Enhancing Healthcare Team Outcomes
Effective management of peritoneal cancer requires a multidisciplinary approach involving physicians, advanced practitioners, nurses, pharmacists, and other health professionals to optimize patient-centered care, outcomes, patient safety, and team performance. Physicians and advanced practitioners play a crucial role in developing treatment plans, conducting cytoreductive surgery, administering chemotherapy, and monitoring patient progress. Nurses provide comprehensive patient care, including symptom management, wound care, and emotional support, while pharmacists ensure safe medication management, monitor for drug interactions, and educate patients on medication adherence.
Interprofessional communication is vital for sharing patient information, discussing treatment options, and coordinating care transitions between healthcare settings. Collaboration among team members facilitates comprehensive assessment, individualized care planning, and timely interventions to address patient needs and preferences. Additionally, regular team meetings, case conferences, and quality improvement initiatives promote continuous learning, shared decision-making, and optimal utilization of resources, ultimately improving patient outcomes and enhancing team performance in managing peritoneal cancer.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Eltabbakh GH, Piver MS. Extraovarian primary peritoneal carcinoma. Oncology (Williston Park, N.Y.). 1998 Jun:12(6):813-9; discussion 820, 825-6 [PubMed PMID: 9644683]
Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2008 Mar-Apr:28(2):583-607; quiz 621-2. doi: 10.1148/rg.282075175. Epub [PubMed PMID: 18349460]
SWERDLOW M. Mesothelioma of the pelvic peritoneum resembling papillary cystadenocarcinoma of the ovary; case report. American journal of obstetrics and gynecology. 1959 Jan:77(1):197-200 [PubMed PMID: 13606191]
Level 3 (low-level) evidenceSampson JA. Implantation Peritoneal Carcinomatosis of Ovarian Origin. The American journal of pathology. 1931 Sep:7(5):423-444.39 [PubMed PMID: 19969977]
Boussios S, Moschetta M, Karathanasi A, Tsiouris AK, Kanellos FS, Tatsi K, Katsanos KH, Christodoulou DK. Malignant peritoneal mesothelioma: clinical aspects, and therapeutic perspectives. Annals of gastroenterology. 2018 Nov-Dec:31(6):659-669. doi: 10.20524/aog.2018.0305. Epub 2018 Sep 14 [PubMed PMID: 30386115]
Level 3 (low-level) evidenceSawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. The American journal of surgical pathology. 1992 Apr:16(4):411-6 [PubMed PMID: 1314522]
Level 3 (low-level) evidenceGoodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Jan:18(1):132-9. doi: 10.1158/1055-9965.EPI-08-0771. Epub [PubMed PMID: 19124490]
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L, Catena F. Peritoneal carcinomatosis. World journal of gastroenterology. 2013 Nov 7:19(41):6979-94. doi: 10.3748/wjg.v19.i41.6979. Epub [PubMed PMID: 24222942]
Rodríguez D, Cheung MC, Housri N, Koniaris LG. Malignant abdominal mesothelioma: defining the role of surgery. Journal of surgical oncology. 2009 Jan 1:99(1):51-7. doi: 10.1002/jso.21167. Epub [PubMed PMID: 18942074]
Level 2 (mid-level) evidenceChun CP, Song LX, Zhang HP, Guo DD, Xu GX, Li Y, Xin X, Cao J, Li F. Malignant peritoneal mesothelioma. The American journal of the medical sciences. 2023 Jan:365(1):99-103. doi: 10.1016/j.amjms.2022.07.008. Epub 2022 Aug 6 [PubMed PMID: 35940275]
Foster JM, Zhang C, Rehman S, Sharma P, Alexander HR. The contemporary management of peritoneal metastasis: A journey from the cold past of treatment futility to a warm present and a bright future. CA: a cancer journal for clinicians. 2023 Jan:73(1):49-71. doi: 10.3322/caac.21749. Epub 2022 Aug 15 [PubMed PMID: 35969103]
Bharti JN, Dey B, Desai P, Gupta R, Khurana N, Gandhi G. Primary leiomyosarcoma of peritoneal cavity. Rare tumors. 2014 Jan 23:6(1):5165. doi: 10.4081/rt.2014.5165. Epub 2014 Mar 26 [PubMed PMID: 24711906]
Level 3 (low-level) evidenceLengyel E. Ovarian cancer development and metastasis. The American journal of pathology. 2010 Sep:177(3):1053-64. doi: 10.2353/ajpath.2010.100105. Epub 2010 Jul 22 [PubMed PMID: 20651229]
Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009 Mar-Apr:29(2):347-73. doi: 10.1148/rg.292085189. Epub [PubMed PMID: 19325052]
Nadler A, McCart JA, Govindarajan A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clinics in colon and rectal surgery. 2015 Dec:28(4):234-46. doi: 10.1055/s-0035-1564431. Epub [PubMed PMID: 26648794]
Level 1 (high-level) evidenceThomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. International journal of cancer. 2014 Feb 1:134(3):622-8. doi: 10.1002/ijc.28373. Epub 2013 Aug 5 [PubMed PMID: 23832847]
Flanagan M, Solon J, Chang KH, Deady S, Moran B, Cahill R, Shields C, Mulsow J. Peritoneal metastases from extra-abdominal cancer - A population-based study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018 Nov:44(11):1811-1817. doi: 10.1016/j.ejso.2018.07.049. Epub 2018 Jul 26 [PubMed PMID: 30139510]
Pannu HK, Oliphant M. The subperitoneal space and peritoneal cavity: basic concepts. Abdominal imaging. 2015 Oct:40(7):2710-22. doi: 10.1007/s00261-015-0429-5. Epub [PubMed PMID: 26006061]
Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016 May:37(5):5715-26. doi: 10.1007/s13277-016-4887-3. Epub 2016 Jan 29 [PubMed PMID: 26831659]
Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International journal of cancer. 2011 Jun 1:128(11):2527-35. doi: 10.1002/ijc.26031. Epub 2011 Mar 25 [PubMed PMID: 21365651]
Level 3 (low-level) evidenceMikuła-Pietrasik J, Uruski P, Tykarski A, Książek K. The peritoneal "soil" for a cancerous "seed": a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cellular and molecular life sciences : CMLS. 2018 Feb:75(3):509-525. doi: 10.1007/s00018-017-2663-1. Epub 2017 Sep 27 [PubMed PMID: 28956065]
Sugarbaker EV. Cancer metastasis: a product of tumor-host interactions. Current problems in cancer. 1979 Jan:3(7):1-59 [PubMed PMID: 371913]
Level 3 (low-level) evidenceCortés-Guiral D, Hübner M, Alyami M, Bhatt A, Ceelen W, Glehen O, Lordick F, Ramsay R, Sgarbura O, Van Der Speeten K, Turaga KK, Chand M. Primary and metastatic peritoneal surface malignancies. Nature reviews. Disease primers. 2021 Dec 16:7(1):91. doi: 10.1038/s41572-021-00326-6. Epub 2021 Dec 16 [PubMed PMID: 34916522]
Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World journal of gastroenterology. 2016 Sep 14:22(34):7692-707. doi: 10.3748/wjg.v22.i34.7692. Epub [PubMed PMID: 27678351]
Kannerstein M, Churg J, McCaughey WT, Hill DP. Papillary tumors of the peritoneum in women: mesothelioma or papillary carcinoma. American journal of obstetrics and gynecology. 1977 Feb 1:127(3):306-14 [PubMed PMID: 835626]
Schorge JO, Muto MG, Lee SJ, Huang LW, Welch WR, Bell DA, Keung EZ, Berkowitz RS, Mok SC. BRCA1-related papillary serous carcinoma of the peritoneum has a unique molecular pathogenesis. Cancer research. 2000 Mar 1:60(5):1361-4 [PubMed PMID: 10728699]
Gu J, Roth LM, Younger C, Michael H, Abdul-Karim FW, Zhang S, Ulbright TM, Eble JN, Cheng L. Molecular evidence for the independent origin of extra-ovarian papillary serous tumors of low malignant potential. Journal of the National Cancer Institute. 2001 Aug 1:93(15):1147-52 [PubMed PMID: 11481386]
Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013 Jul:34(7):1413-9. doi: 10.1093/carcin/bgt166. Epub 2013 May 14 [PubMed PMID: 23677068]
Kusamura S, Baratti D, Zaffaroni N, Villa R, Laterza B, Balestra MR, Deraco M. Pathophysiology and biology of peritoneal carcinomatosis. World journal of gastrointestinal oncology. 2010 Jan 15:2(1):12-8. doi: 10.4251/wjgo.v2.i1.12. Epub [PubMed PMID: 21160812]
George S, Serrano C, Hensley ML, Ray-Coquard I. Soft Tissue and Uterine Leiomyosarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Jan 10:36(2):144-150. doi: 10.1200/JCO.2017.75.9845. Epub 2017 Dec 8 [PubMed PMID: 29220301]
Thway K, Noujaim J, Zaidi S, Miah AB, Benson C, Messiou C, Jones RL, Fisher C. Desmoplastic Small Round Cell Tumor: Pathology, Genetics, and Potential Therapeutic Strategies. International journal of surgical pathology. 2016 Dec:24(8):672-684 [PubMed PMID: 27621277]
Miguez González J, Calaf Forn F, Pelegrí Martínez L, Lozano Arranz P, Oliveira Caiafa R, Català Forteza J, Palacio Arteaga LM, Losa Gaspà F, Ramos Bernadó I, Barrios Sánchez P, Ayuso Colella JR. Primary and secondary tumors of the peritoneum: key imaging features and differential diagnosis with surgical and pathological correlation. Insights into imaging. 2023 Jul 3:14(1):115. doi: 10.1186/s13244-023-01417-6. Epub 2023 Jul 3 [PubMed PMID: 37395913]
Bhuyan P, Mahapatra S, Mahapatra S, Sethy S, Parida P, Satpathy S. Extraovarian primary peritoneal papillary serous carcinoma. Archives of gynecology and obstetrics. 2010 Mar:281(3):561-4. doi: 10.1007/s00404-009-1201-2. Epub 2009 Aug 20 [PubMed PMID: 19693524]
Level 3 (low-level) evidenceWatanabe T, Miyamoto S, Kitagori K, Horimatsu T, Morita S, Mashimo Y, Ezoe Y, Muto M, Chiba T. A case of long-term survival of metastatic desmoplastic small round cell tumor treated with multimodal therapy. Oncology letters. 2012 Jan:3(1):30-34 [PubMed PMID: 22740851]
Level 3 (low-level) evidenceSadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000 Jan 15:88(2):358-63 [PubMed PMID: 10640968]
Goerg C, Schwerk WB. Malignant ascites: sonographic signs of peritoneal carcinomatosis. European journal of cancer (Oxford, England : 1990). 1991:27(6):720-3 [PubMed PMID: 1829911]
Level 2 (mid-level) evidenceWeill FS, Costaz R, Guetarni S, Maltoni I, Rohmer P. [Echographic diagnosis of peritoneal metastases in patients with ascites]. Journal de radiologie. 1990 May:71(5):365-8 [PubMed PMID: 2213700]
Szadkowska MA, Pałucki J, Cieszanowski A. Diagnosis and treatment of peritoneal carcinomatosis - a comprehensive overview. Polish journal of radiology. 2023:88():e89-e97. doi: 10.5114/pjr.2023.125027. Epub 2023 Feb 9 [PubMed PMID: 36910885]
Level 3 (low-level) evidencePatel CM, Sahdev A, Reznek RH. CT, MRI and PET imaging in peritoneal malignancy. Cancer imaging : the official publication of the International Cancer Imaging Society. 2011 Aug 24:11(1):123-39. doi: 10.1102/1470-7330.2011.0016. Epub 2011 Aug 24 [PubMed PMID: 21865109]
Chiou SY, Sheu MH, Wang JH, Chang CY. Peritoneal serous papillary carcinoma: a reappraisal of CT imaging features and literature review. Abdominal imaging. 2003 Nov-Dec:28(6):815-9 [PubMed PMID: 14753596]
Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Annals of translational medicine. 2017 Jun:5(11):236. doi: 10.21037/atm.2017.03.96. Epub [PubMed PMID: 28706904]
Low RN. MR imaging of the peritoneal spread of malignancy. Abdominal imaging. 2007 May-Jun:32(3):267-83 [PubMed PMID: 17334873]
De Gaetano AM, Calcagni ML, Rufini V, Valenza V, Giordano A, Bonomo L. Imaging of peritoneal carcinomatosis with FDG PET-CT: diagnostic patterns, case examples and pitfalls. Abdominal imaging. 2009 May-Jun:34(3):391-402. doi: 10.1007/s00261-008-9405-7. Epub [PubMed PMID: 18446399]
Level 3 (low-level) evidenceRunyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology (Baltimore, Md.). 1988 Sep-Oct:8(5):1104-9 [PubMed PMID: 3417231]
Tuzun Y, Celik Y, Bayan K, Yilmaz S, Dursun M, Canoruc F. Correlation of tumour markers in ascitic fluid and serum: are measurements of ascitic tumour markers a futile attempt? The Journal of international medical research. 2009 Jan-Feb:37(1):79-86 [PubMed PMID: 19215676]
Liu F, Kong X, Dou Q, Ye J, Xu D, Shang H, Xu K, Song Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Annals of hepatology. 2014 May-Jun:13(3):357-63 [PubMed PMID: 24756011]
Level 2 (mid-level) evidenceHuang LL, Xia HH, Zhu SL. Ascitic Fluid Analysis in the Differential Diagnosis of Ascites: Focus on Cirrhotic Ascites. Journal of clinical and translational hepatology. 2014 Mar:2(1):58-64. doi: 10.14218/JCTH.2013.00010. Epub 2014 Mar 15 [PubMed PMID: 26357618]
Rodriguez EF, Monaco SE, Khalbuss W, Austin RM, Pantanowitz L. Abdominopelvic washings: A comprehensive review. CytoJournal. 2013:10():7. doi: 10.4103/1742-6413.111080. Epub 2013 Apr 24 [PubMed PMID: 23858317]
Maseki Z, Kajiyama H, Nishikawa E, Satake T, Misawa T, Kikkawa F. Is cell block technique useful to predict histological type in patients with ovarian mass and/or body cavity fluids? Nagoya journal of medical science. 2020 May:82(2):225-235. doi: 10.18999/nagjms.82.2.225. Epub [PubMed PMID: 32581403]
Elzarkaa AA, Shaalan W, Elemam D, Mansour H, Melis M, Malik E, Soliman AA. Peritoneal cancer index as a predictor of survival in advanced stage serous epithelial ovarian cancer: a prospective study. Journal of gynecologic oncology. 2018 Jul:29(4):e47. doi: 10.3802/jgo.2018.29.e47. Epub 2018 Mar 8 [PubMed PMID: 29770618]
Burnett A, Lecompte MA, Trabulsi N, Dubé P, Gervais MK, Trilling B, Cloutier AS, Sideris L. Peritoneal carcinomatosis index predicts survival in colorectal patients undergoing HIPEC using oxaliplatin: a retrospective single-arm cohort study. World journal of surgical oncology. 2019 May 15:17(1):83. doi: 10.1186/s12957-019-1618-4. Epub 2019 May 15 [PubMed PMID: 31092250]
Level 2 (mid-level) evidenceLungoci C, Mironiuc AI, Muntean V, Oniu T, Leebmann H, Mayr M, Piso P. Multimodality treatment strategies have changed prognosis of peritoneal metastases. World journal of gastrointestinal oncology. 2016 Jan 15:8(1):67-82. doi: 10.4251/wjgo.v8.i1.67. Epub [PubMed PMID: 26798438]
Mehta SS, Bhatt A, Glehen O. Cytoreductive Surgery and Peritonectomy Procedures. Indian journal of surgical oncology. 2016 Jun:7(2):139-51. doi: 10.1007/s13193-016-0505-5. Epub 2016 Feb 3 [PubMed PMID: 27065704]
Behrenbruch C, Hollande F, Thomson B, Michael M, Warrier SK, Lynch C, Heriot A. Treatment of peritoneal carcinomatosis with hyperthermic intraperitoneal chemotherapy in colorectal cancer. ANZ journal of surgery. 2017 Sep:87(9):665-670. doi: 10.1111/ans.14077. Epub 2017 Jun 30 [PubMed PMID: 28664645]
Aherne EA, Fenlon HM, Shields CJ, Mulsow JJ, Cronin CG. What the Radiologist Should Know About Treatment of Peritoneal Malignancy. AJR. American journal of roentgenology. 2017 Mar:208(3):531-543. doi: 10.2214/AJR.16.16646. Epub 2017 Jan 11 [PubMed PMID: 28075611]
Shaligram A. Management of peritoneal surface malignancies in laparoscopic era: a concise review. International journal of surgery. Oncology. 2016 Dec:1(2):e05. doi: 10.1097/IJ9.0000000000000005. Epub 2016 Nov 11 [PubMed PMID: 29177208]
Yurttas C, Hoffmann G, Tolios A, Haen SP, Schwab M, Königsrainer I, Königsrainer A, Beckert S, Löffler MW. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. Journal of clinical medicine. 2018 Dec 19:7(12):. doi: 10.3390/jcm7120567. Epub 2018 Dec 19 [PubMed PMID: 30572653]
Level 1 (high-level) evidenceFacchiano E, Risio D, Kianmanesh R, Msika S. Laparoscopic hyperthermic intraperitoneal chemotherapy: indications, aims, and results: a systematic review of the literature. Annals of surgical oncology. 2012 Sep:19(9):2946-50. doi: 10.1245/s10434-012-2360-0. Epub 2012 Apr 24 [PubMed PMID: 22526907]
Level 1 (high-level) evidenceJayakrishnan TT, Zacharias AJ, Sharma A, Pappas SG, Gamblin TC, Turaga KK. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World journal of surgical oncology. 2014 Aug 21:12():270. doi: 10.1186/1477-7819-12-270. Epub 2014 Aug 21 [PubMed PMID: 25145962]
Level 2 (mid-level) evidenceSugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, Oliff L, Schlag P. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer research. 1990 Sep 15:50(18):5790-4 [PubMed PMID: 2118420]
Goodman MD, McPartland S, Detelich D, Saif MW. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. Journal of gastrointestinal oncology. 2016 Feb:7(1):45-57. doi: 10.3978/j.issn.2078-6891.2015.111. Epub [PubMed PMID: 26941983]
Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Intraoperative versus early postoperative intraperitoneal chemotherapy after cytoreduction for colorectal peritoneal carcinomatosis: an experimental study. Annals of surgical oncology. 2012 Jul:19 Suppl 3():S475-82. doi: 10.1245/s10434-011-1984-9. Epub 2011 Aug 12 [PubMed PMID: 21837528]
Level 3 (low-level) evidenceElias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Annals of surgical oncology. 2007 Feb:14(2):509-14 [PubMed PMID: 17096054]
Level 2 (mid-level) evidenceTan GH, Ong WS, Chia CS, Tham CK, Soo KC, Teo MC. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2016 May:32(3):281-8. doi: 10.3109/02656736.2015.1135485. Epub 2016 Feb 10 [PubMed PMID: 26862667]
Kurtz F, Struller F, Horvath P, Solass W, Bösmüller H, Königsrainer A, Reymond MA. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterology research and practice. 2018:2018():2743985. doi: 10.1155/2018/2743985. Epub 2018 Oct 24 [PubMed PMID: 30473706]
Level 2 (mid-level) evidenceRobella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World journal of surgical oncology. 2016 Apr 29:14():128. doi: 10.1186/s12957-016-0892-7. Epub 2016 Apr 29 [PubMed PMID: 27125996]
Level 2 (mid-level) evidenceGockel I, Jansen-Winkeln B, Haase L, Niebisch S, Moulla Y, Lyros O, Lordick F, Schierle K, Wittekind C, Thieme R. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in patients with peritoneal metastasized colorectal, appendiceal and small bowel cancer. Tumori. 2020 Feb:106(1):70-78. doi: 10.1177/0300891619868013. Epub 2019 Aug 30 [PubMed PMID: 31469058]
Gockel I, Jansen-Winkeln B, Haase L, Rhode P, Mehdorn M, Niebisch S, Moulla Y, Lyros O, Lordick F, Schierle K, Wittekind C, Thieme R. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Gastric Cancer Patients with Peritoneal Metastasis (PM): Results of a Single-Center Experience and Register Study. Journal of gastric cancer. 2018 Dec:18(4):379-391. doi: 10.5230/jgc.2018.18.e37. Epub 2018 Dec 14 [PubMed PMID: 30607301]
Tempfer C, Giger-Pabst U, Hilal Z, Dogan A, Rezniczek GA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Archives of gynecology and obstetrics. 2018 Aug:298(2):243-257. doi: 10.1007/s00404-018-4784-7. Epub 2018 Jun 4 [PubMed PMID: 29869089]
Level 1 (high-level) evidenceKoumpa FS, Xylas D, Konopka M, Galea D, Veselkov K, Antoniou A, Mehta A, Mirnezami R. Colorectal Peritoneal Metastases: A Systematic Review of Current and Emerging Trends in Clinical and Translational Research. Gastroenterology research and practice. 2019:2019():5180895. doi: 10.1155/2019/5180895. Epub 2019 Apr 1 [PubMed PMID: 31065262]
Level 1 (high-level) evidenceNadiradze G, Horvath P, Sautkin Y, Archid R, Weinreich FJ, Königsrainer A, Reymond MA. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers. 2019 Dec 20:12(1):. doi: 10.3390/cancers12010034. Epub 2019 Dec 20 [PubMed PMID: 31877647]
Ploug M, Graversen M, Pfeiffer P, Mortensen MB. Bidirectional treatment of peritoneal metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC cancer. 2020 Feb 10:20(1):105. doi: 10.1186/s12885-020-6572-6. Epub 2020 Feb 10 [PubMed PMID: 32041558]
Level 1 (high-level) evidenceSeshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World journal of gastroenterology. 2016 Jan 21:22(3):1114-30. doi: 10.3748/wjg.v22.i3.1114. Epub [PubMed PMID: 26811651]
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. The New England journal of medicine. 2006 Jan 5:354(1):34-43 [PubMed PMID: 16394300]
Level 1 (high-level) evidenceHayes-Jordan A, LaQuaglia MP, Modak S. Management of desmoplastic small round cell tumor. Seminars in pediatric surgery. 2016 Oct:25(5):299-304. doi: 10.1053/j.sempedsurg.2016.09.005. Epub 2016 Sep 14 [PubMed PMID: 27955733]
Piura B, Meirovitz M, Bartfeld M, Yanai-Inbar I, Cohen Y. Peritoneal papillary serous carcinoma: study of 15 cases and comparison with stage III-IV ovarian papillary serous carcinoma. Journal of surgical oncology. 1998 Jul:68(3):173-8 [PubMed PMID: 9701210]
Level 3 (low-level) evidenceGilly FN, Carry PY, Sayag AC, Brachet A, Panteix G, Salle B, Bienvenu J, Burgard G, Guibert B, Banssillon V. Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis. Hepato-gastroenterology. 1994 Apr:41(2):124-9 [PubMed PMID: 8056398]
Yun WS, Bae JM. Primary peritoneal serous carcinoma, an extremely rare malignancy: A case report and review of the literature. Oncology letters. 2016 Jun:11(6):4063-4065 [PubMed PMID: 27313741]
Level 3 (low-level) evidenceEltabbakh GH, Werness BA, Piver S, Blumenson LE. Prognostic factors in extraovarian primary peritoneal carcinoma. Gynecologic oncology. 1998 Nov:71(2):230-9 [PubMed PMID: 9826465]
Level 2 (mid-level) evidenceLiu S, Staats P, Lee M, Alexander HR, Burke AP. Diffuse mesothelioma of the peritoneum: correlation between histological and clinical parameters and survival in 73 patients. Pathology. 2014 Dec:46(7):604-9. doi: 10.1097/PAT.0000000000000181. Epub [PubMed PMID: 25393250]
Level 2 (mid-level) evidenceWong HH, Hatcher HM, Benson C, Al-Muderis O, Horan G, Fisher C, Earl HM, Judson I. Desmoplastic small round cell tumour: characteristics and prognostic factors of 41 patients and review of the literature. Clinical sarcoma research. 2013 Nov 26:3(1):14. doi: 10.1186/2045-3329-3-14. Epub 2013 Nov 26 [PubMed PMID: 24280007]
Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. International seminars in surgical oncology : ISSO. 2005 Feb 8:2(1):3 [PubMed PMID: 15701175]
Vaira M, Cioppa T, D'Amico S, de Marco G, D'Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In vivo (Athens, Greece). 2010 Jan-Feb:24(1):79-84 [PubMed PMID: 20133981]
Tentes AA, Tripsiannis G, Markakidis SK, Karanikiotis CN, Tzegas G, Georgiadis G, Avgidou K. Peritoneal cancer index: a prognostic indicator of survival in advanced ovarian cancer. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2003 Feb:29(1):69-73 [PubMed PMID: 12559080]