Introduction
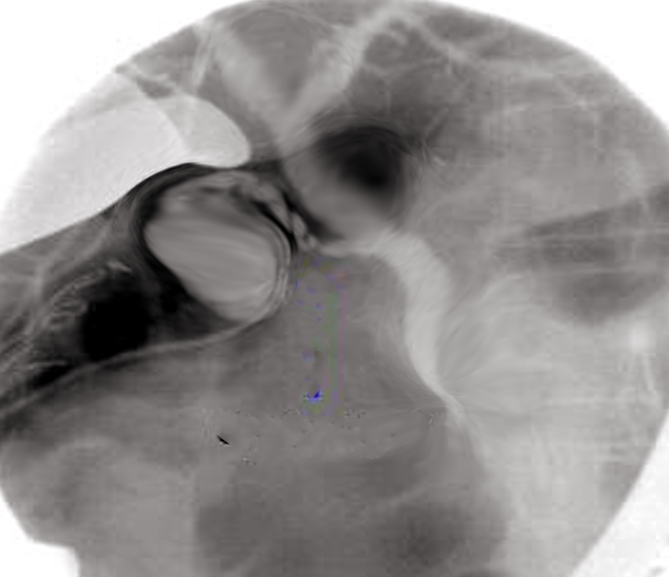
Initially reported in 1937 and popularized in the 1950s, percutaneous transhepatic cholangiography (PTC) is an invasive procedure utilized for both diagnostic and therapeutic purposes, involving the insertion of a needle into the biliary tree followed by catheter placement for percutaneous transhepatic biliary drainage (PTBD). Contrast is injected into 1 or more bile ducts (cholangiography) and possibly the duodenum. PTC may be performed with fluoroscopic or ultrasound guidance (see Image. Percutaneous Transhepatic Cholangiography).[1][2][3]
PTC is crucial in managing obstructive jaundice in malignancies, as it helps reduce bilirubin levels, improve liver function, and enable other treatments such as drainage and diagnostics. In cases of suspected malignant biliary strictures, relying solely on imaging for diagnosis may not yield precise results, necessitating additional diagnostic procedures. Fine needle aspiration, brush cytology, and forceps biopsy are commonly employed during percutaneous biliary catheterization as part of PTC. This approach, utilized since the 1980s, remains an integral part of the diagnostic toolkit for suspected malignant biliary strictures.
PTBD via PTC, introduced in 1981, is considered safe and effective for drainage. This is particularly beneficial for patients with multiple comorbidities who cannot tolerate surgical intervention and for those for whom endoscopic retrograde cholangiopancreatography (ERCP) is not an option. However, it primarily focuses on symptom relief rather than providing a cure.[4] The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) has established practice guidelines for PTC.[5]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Successful PTC requires a comprehensive understanding of the extrahepatic and intrahepatic components of the biliary system.
The extrahepatic biliary system consists of the common hepatic duct, which emerges from the liver and travels inferiorly, carrying bile toward the duodenum. At the porta hepatis, the common hepatic duct joins with the cystic duct, originating from the gallbladder, to form the common bile duct. The common bile duct descends behind the first part of the duodenum, where it may receive the pancreatic duct before entering the duodenal wall at the major duodenal papilla. This junction, regulated by the sphincter of Oddi under neurologic and endocrine stimulation, serves as the controlling valve for releasing bile into the duodenum or retaining bile within the ducts, causing backflow into the gallbladder via the cystic duct. The gallbladder acts as a reservoir for bile. This extrahepatic anatomic configuration is almost invariable, whereas the intrahepatic system configuration is variable.
The intrahepatic biliary system comprises a network of small bile ducts within the liver parenchyma, which arise from the bile canaliculi between hepatocytes. These ducts are part of the hepatic lobules' portal triad, which includes a biliary duct, hepatic artery, and portal vein. Typically, they are not easily visualized in traditional imaging studies until pathologically enlarged. The intrahepatic ducts progressively converge to form larger ducts, eventually becoming the right and left hepatic ducts. The right hepatic duct drains bile from the right lobe of the liver, while the left hepatic duct drains bile from the left lobe. These two ducts unite to form the common hepatic duct.
The most common configuration, seen in about 50% of people, consists of a single duct draining the left lobe and a single duct formed by the union of an anterior and posterior duct draining the right lobe. The most common variant of this configuration features a right anterior segmental branch that drains directly into the common bile duct and a right posterior segmental branch that drains elsewhere. The right anterior duct, which drains segments V and VIII, usually courses in a somewhat craniocaudal orientation, whereas the right posterior duct, which drains segments VI and VII, typically courses more lateromedially.
Understanding potential congenital anomalies is essential when performing cholangiography. Pertinent anomalies include aberrant biliary ducts, aberrant cystic duct insertion, choledochal cysts, diverticula, and the anomalous junction of the biliary and pancreatic ducts.
Indications
The role of PTC is multifaceted. By providing direct access to the biliary system, PTC plays a crucial role in diagnosing, treating, and managing various biliary disorders. PTC includes the following indications as listed below.
- Evaluation of obstructive jaundice: PTC helps diagnose the cause of obstructive jaundice, such as biliary strictures, stones, or tumors.
- Biliary drainage:
- PTC can be used to place internal or external biliary drainage catheters (eg, PTBD) to relieve obstructive jaundice.
- The 2 most common interventions for treating painless obstructive jaundice from cancer are ERCP and PTBD.[6][7]
- For patients with low-level obstruction, the surgical success rates of both procedures are similar. However, PTBD has a higher overall surgical success rate and is more successful in patients with high-level obstruction.[8]
- Conventional peroral methods (eg, ERCP) are not feasible in some patients with altered anatomy or biliary obstruction. In cases of ERCP failure, PTBD is considered the preferred rescue strategy.[9][10]
- PTBD is also an option for those patients with biliary atresia who underwent a Kasai portoenterostomy and now have biliary complications, including recurrent cholangitis and biliary stricture.[11][12]
- Assessment and treatment of biliary leaks:
- PTC can identify and characterize biliary leaks, particularly following hepatobiliary surgery or trauma.
- Traditionally, postoperative bile leakages are treated with an endoscopic approach via ERCP and percutaneous transhepatic approaches such as PTC and PTBD.[13] ERCP with biliary stent insertion or PTBD is the primary and effective treatment for bile leakage.[13]
- Biliary stenting: Biliary access gained through PTC allows for the placement of stents to maintain bile duct patency and alleviate biliary obstruction.
- Preoperative assessment: PTC may be performed before surgical interventions involving the biliary tract to aid in preoperative planning and decision-making.
- Therapeutic interventions: PTC can facilitate multiple interventions, as mentioned below.
- Stone removal and lithotripsy.[14]
- Tissue collection through biopsies, aspirations, and brushings.
- Dilation of biliary strictures.
- Evaluation of biliary anatomy: PTC provides detailed imaging of the biliary system, aiding in assessing anatomical variations or anomalies.
- Retrieval of foreign bodies: Case reports have documented the successful interventional removal of various foreign objects. For instance, a report describes the successful removal of a common bile duct–impacted Dormia basket using a parallel approach involving PTC with PTBD alongside simultaneous endoscopy, enabling the complete removal of the basket.[15]
Contraindications
PTC is contraindicated in certain situations due to potential risks and complications. Contraindications may include the indications mentioned below.
- The possibility of performing ERCP:
- ERCP should be considered as potential first-line therapy for stenoses or obstructions that are reachable via this method, given decreased adverse events, greater survival, and improved quality of life.[6]
- The National Comprehensive Cancer Network 2017 guidelines recommend ERCP as the first-line intervention for extrahepatic ductal lesions. Current medical society guidelines do not differentiate which patients should have ERCP attempted first for intrahepatic ductal lesions.
- Coagulopathy or bleeding disorders:
- PTC involves the insertion of a needle into the liver to access the bile ducts, which can lead to bleeding. Patients with coagulopathies or other bleeding disorders are at increased risk of hemorrhage during the procedure.
- The Society of Interventional Radiology (SIR) categorizes PTC as a level 2 procedure in terms of hemorrhage risk. For patients undergoing PTC, the SIR recommends maintaining an international normalized ratio (INR) of no greater than 1.5 and ensuring a platelet count of at least 50,000 cells/μL.
- Irreversible coagulopathy (eg, stopping or correcting anticoagulation puts the patient at more risk than the benefit of the PTC) is an absolute contraindication.
- Nondilated ductal system: Attempting PTC on a nondilated ductal system, such as when the goal is to divert bile away from a common bile duct or cystic duct leak, can significantly increase the chance of failed access. PTC may be deferred temporarily if there is a possibility that the ducts will increase in size with a short delay.
- Unstable hemodynamics: Hemodynamically unstable patients, such as those in shock or with severe cardiovascular compromise, may not be suitable candidates for PTC due to the risks associated with the procedure and anesthesia.
- Uncontrolled infections: Active infection, particularly in the area surrounding the liver or biliary tract, can increase the risk of complications such as sepsis or abscess formation. PTC should be deferred until the infection is adequately controlled.
- Allergy to contrast material: Contrast agents are often used during PTC to visualize the bile ducts and diagnose biliary tract disorders. Patients with a known allergy to contrast material may experience an allergic reaction during the procedure.
- Uncooperative patient: PTC requires patient cooperation and the ability to lie still during the procedure. Patients who are unable to follow instructions or remain still may not be suitable candidates for PTC.
- Anatomical limitations: Certain anatomical variations or abnormalities may make PTC technically challenging or impossible to perform safely. These limitations should be carefully evaluated before proceeding with the procedure.
- Pregnancy: PTC involves radiation exposure, which can pose risks to the developing fetus. Whenever possible, the procedure should be deferred until after pregnancy unless it is deemed medically necessary and the benefits outweigh the potential risks to the fetus.
The healthcare team should consider and evaluate these contraindications before proceeding with PTC. Alternative diagnostic or therapeutic options may be explored for patients who are unsuitable candidates for PTC. Additional issues that could be considered relative contraindications are discussed below in the section on preparation.
Equipment
Performing PTC requires specialized equipment to ensure accuracy and safety. The essential equipment includes the following:
- Fluoroscopy machine: A fluoroscopy machine provides real-time x-ray images that guide the needle's insertion into the liver and bile ducts during the procedure.
- Local anesthetic medications: These medications are necessary to numb the skin and tissues overlying the liver at the insertion site, thereby minimizing the patient's discomfort.
- Needles: Specialized needles, such as Chiba needles or modified Seldinger needles, are used to puncture the skin and access the bile ducts within the liver.
- Contrast media: Contrast dye is injected into the bile ducts to visualize them on x-ray images. Water-soluble iodinated contrast agents are commonly used for PTC.
- Syringes and injection devices: These devices administer the contrast dye through the needle into the bile ducts.
- Sterile drapes, gowns, and gloves: These items maintain aseptic conditions during the procedure to reduce the risk of infection.
- Dressing materials: After the procedure, the insertion site is covered with dressing materials, such as sterile gauze and adhesive bandages, to prevent bleeding and infection.
- Monitoring equipment: This is used to monitor the patient's vital signs during the procedure.
- Sedation equipment and sedatives (optional): Depending on the patient's condition and the complexity of the procedure, sedation equipment may be used to help keep the patient comfortable and relaxed.
- Accessory equipment: Additional equipment, such as ultrasound machines or guidance devices, may assist with needle placement, especially in cases where the bile ducts are difficult to visualize with fluoroscopy alone.
Personnel
Performing PTC requires a multidisciplinary team to ensure the procedure is conducted safely and effectively. The personnel typically involved are as follows:
- An interventional radiologist
- A radiology technologist
- An anesthesiologist or a nurse anesthetist (optional)
- A circulating nurse
- A support staff, such as medical assistants or surgical technicians
Preparation
Preparation for PTC involves several key steps to ensure the safety and success of the procedure. While cross-sectional imaging is not mandatory, it plays a crucial role in preprocedural planning by providing valuable insights into the patient's ductal drainage and biliary dilatation patterns, the level of obstruction (ie, high or low), and possibly the obstructive cause. This information can guide decisions regarding the approach, such as whether a right- or left-lobe approach should be considered initially. Additionally, radionuclide scintigraphy, such as a hepatobiliary iminodiacetic acid scan, may be utilized to confirm the presence or absence of a bile duct leak.[16]
The literature continues to debate the optimal antibiotic prophylaxis regimen for biliary procedures such as PTC. Generally, most physicians agree that all patients receive antibiotics to cover gram-negative bacteria regardless of infectious symptoms. This is because studies show reduced rates of sepsis with prophylactic gram-negative bacterial therapy despite no evidence of retrograde contamination of the biliary tree with enteric flora. Some physicians also cover gram-positive and anaerobic bacteria, although this is unnecessary based on antibiotic guidelines from the SIR. Along with some studies, the SIR recommends intravenous administration of 1 g of ceftriaxone, second-generation cephalosporins, or 1.5 g of ampicillin-sulbactam. Alternatively, single-dose prophylactic antibiotics, typically third-generation cephalosporins, are advocated for diagnostic imaging cases. Antibiotic therapy is tailored in consultation with clinicians to ensure appropriate coverage and minimize risks for patients with comorbidities.[3]
Patients referred for biliary drainage due to malignancy are often dehydrated, posing an increased risk of hepatorenal failure. Hence, intravenous fluid administration should be initiated upon referral to mitigate dehydration effects. Coagulation parameters are closely monitored, and any deviations from SIR-recommended parameters are corrected accordingly. In cases of elevated INR, fresh frozen plasma delivery may be considered before, during, and after the procedure to maintain hemostasis.
Lastly, considering the potential hemodynamic instability and sedation-related risks, it is advisable to consult and have an anesthesiologist available during the procedure, especially for patients with preexisting conditions or advanced airway management requirements. This proactive approach ensures optimal patient safety and management throughout the PTC procedure.
Technique or Treatment
PTC is a diagnostic procedure for visualizing the biliary tract. A basic outline of how the procedure is typically performed is mentioned below.
Patient Positioning and Preparation
The patient should be in a comfortable supine position, ensuring appropriate access to the liver. The upper abdomen should be prepped and draped in a sterile manner.
Anesthesia
The procedure involves painful steps, including a percutaneous catheter traversing the skin, intercostal muscles, and the liver capsule. Therefore, it should be performed using local anesthesia and moderate sedation. Deeper sedation becomes particularly important when additional steps such as biopsies or stenting are necessary.
Access and Cannulation
The most preferred approach to limit complications is accessing the right bile duct from a slightly anterior approach at the midaxillary line, below the tenth rib. Other techniques include a left-sided epigastric approach or a transgallbladder approach. The method utilized should be determined based on the expected pathology. Ultrasound guidance should be used when the bile duct will be accessed percutaneously.[17]
A sequence of wire and catheter exchanges is performed until bile is aspirated, confirming biliary access. Once the needle is in the desired position within a bile duct, under fluoroscopy, a contrast dye is injected through the needle into the bile duct system.[12] The contrast dye fills the bile ducts and outlines them, allowing the radiologist to identify any abnormalities, such as blockages or strictures. The cholangiographic findings will then direct the management of the defined pathology, including biliary drainage placement, biliary dilation, and stent placement. Once all other procedures are completed, the needle is removed, and pressure is applied to the skin to prevent bleeding. A sterile dressing may be applied to the insertion site.
Postprocedure Care
The patient is monitored for a brief period to ensure there are no immediate complications. For a short time after the procedure, they may be advised to avoid strenuous activities.
Complications
PTC is generally safe but carries risks and potential complications like any invasive procedure. The SIR has reported complication rates for PTC and PTBD to be between 2% and 10%. Complications commonly associated with PTC and PTBD include biliary tract infection, bleeding, bile leakage, duct obstruction, and pneumothorax.[1][4][18] Those and other complications associated with PTC are listed below.
- Bleeding: Bleeding may occur in the form of hemothorax, hemoperitoneum, hemobilia, subcapsular hepatic hematoma, melena, pseudoaneurysm, and catheter/drain site bleeding, happening in roughly 2% to 3% of patients undergoing PTBD.[19]
- The leading cause of bleeding is the obscuration of the puncture path of PTC/PTBD and the inadvertent puncture of the patient's blood vessels during the puncture.[20] Hemorrhage following PTC/PTBD may result from arterial, portal, or hepatic venous injury.[21]
- The SIR has published a guideline indicating a major bleeding complication rate of 2.5% following PTBD, recommending a practice review should the cutoff rate of 5% be exceeded.[19]
- Bleeding may range from minor to severe and potentially require blood transfusion or additional interventions to control.
- Hepatic artery bleeding following PTBD is marked by bright red and occasionally pulsatile blood in the tube, sometimes along with hemodynamic instability.
- If arterial bleeding is suspected, drain injection and/or pullback cholangiogram should be avoided, as the injected contrast will obscure the subsequent angiogram and delay definitive treatment. Hepatic artery arteriography in different obliquities should be performed to locate the injury.
- Treatment options include stent graft placement and embolization.
- Stent graft placement across the injury site is feasible only with a central injury and favorable anatomy. The stent graft should extend 5 to 10 mm proximal and distal to the site of injury.
- Embolization, typically performed with coils, is more common. Coils are preferred over liquid embolics, as the latter may lead to excessively distal embolization with a risk of abscess formation. Additionally, liquid embolics may extend into the biliary tree when arterial-biliary communication is present. Ideally, coil embolization should start just beyond the arterial injury site, across and proximal to the site of injury. Following that, the assessment of other potentially masked injuries entailed by prior procedures should be conducted using global arteriography.[19]
- Treatment options include stent graft placement and embolization.
- Portal vein bleeding is suspected when dark blood is present without significant hemodynamic instability or a large drop in hematocrit levels. A pullback cholangiogram is performed to confirm the diagnosis and assess the size and location of the portal vein branch that has been traversed.
- Central portal vein injury requires definitive management. Treatment options include stent graft placement across the injury site or coiling. The best choice depends on the site of injury and the patient's condition.
- If the traversed vessel is a small peripheral portal vein branch, typically seen, treatment involves tube upsizing with central side holes to the portal vein branch. This temporary measure addresses the issue and also fixes the underlying abnormality by allowing epithelialization of the hepatic parenchymal tract and/or thrombosis of the portal vein branch. Alternatively, if new PTBD access is gained, coils can be placed across the entire transhepatic tract.
- Bile leak:
- Accidental perforation of the bile ducts during the procedure can lead to bile leakage into the abdominal cavity, resulting in peritonitis or abscess formation. Alternatively, it may leak into the chest, resulting in pleuritis or empyema.
- If bile leaks from or around the catheter, it should be repositioned, unclogged, or upsized to stem the leak. Biloma typically resolves on its own as the body can absorb the fluid. However, percutaneous drainage may be necessary if it becomes painful or infected.
- Infections:
- Despite antibiotic prophylaxis, PTC increases susceptibility to infection; PTC +/- PTBD provides a conduit for bacteria to enter the biliary tract from an external source.[22]
- The most common complications are bloodstream (ie, sepsis) and biliary tract infections (eg, cholangitis or liver abscess) secondary to bacteremia. Different studies have reported varying infection rates: 67 of 343 patients (19.5%), 46 of 152 patients (30%), and 83 of 193 patients (43%).[3]
- A risk of infection also exists at the puncture site.
- Antibiotics are the mainstay of treatment.
- Pain: Patients may experience discomfort or pain at the puncture site or in the abdomen during or after the procedure, which is usually temporary but may require medication for management.
- Pancreatitis: The manipulation of the catheter within the biliary system can irritate the pancreatic duct, potentially leading to inflammation and pancreatitis.
- Pneumothorax:
- Risk of pneumothorax exists, especially in cases where the abdominal puncture site is close to the diaphragm, or the puncture is transpleural, particularly in patients with underlying lung disease or emphysema.
- Transpleural punctures led to pneumothorax in 8.6% to 22% of cases in a review.[19]
- Allergic reactions: Some patients may experience allergic reactions to the contrast dye used during the procedure, with symptoms ranging from mild skin reactions to severe anaphylaxis.
- Electrolyte depletion: High-output external drainage can lead to electrolyte imbalances. Treatment involves electrolyte replacement, and transitioning the catheter to internal drainage should be considered.
- Damage to adjacent organs: In rare cases, the needle or catheter used in PTC may inadvertently puncture adjacent organs such as the lung, gallbladder, or bowel, leading to complications requiring additional interventions.
- Catheter dislodgement:
- A safety stitch at the skin surface and a catheter with a self-retaining mechanism are usually used to prevent dislodgement.
- As the initial drainage catheter requires 4 to 6 weeks to establish a stable patent tract, avoiding catheter dislodgement during this period is crucial.
- If the catheter becomes partially dislodged, replacing the catheter over a guidewire is relatively straightforward. However, complete retraction out of the liver presents greater challenges. If the catheter tract can be opacified with contrast, an angiographic-type catheter and a guidewire can often negotiate the transhepatic tract and reinsert a new drainage catheter through the original tract.
- Death: An increased mortality risk associated with the PTC approach has been reported in multiple studies.[23]
Clinical Significance
PTC is clinically significant in diagnosing and managing various biliary tract disorders. This technique allows for the visualization of both intrahepatic and extrahepatic biliary anatomy, aiding in the diagnosis of conditions such as bile duct strictures, stones, tumors, and congenital abnormalities. Additionally, PTC serves as a therapeutic modality by enabling interventions such as PTBD, stent placement, and stone extraction. This procedure is crucial in guiding treatment decisions, facilitating minimally invasive interventions, and ultimately improving patient outcomes in hepatobiliary disorders.
Enhancing Healthcare Team Outcomes
Performing PTC requires a collaborative effort among various healthcare professionals to ensure optimal patient-centered care, outcomes, safety, and team performance. Physicians, including interventional radiologists and hepatobiliary surgeons, are critical in planning and executing PTC procedures, as well as utilizing their expertise in image-guided interventions and hepatobiliary anatomy. Advanced care practitioners, such as nurse practitioners and physician assistants, contribute by assisting in patient assessment, obtaining informed consent, and providing periprocedural care. Nurses are integral members of the healthcare team and are responsible for patient monitoring, administering medications, and educating patients about the procedure and postprocedural care. Pharmacists are crucial in medication management, ensuring appropriate prophylactic antibiotics, and managing potential drug interactions or adverse effects.
Effective interprofessional communication among healthcare providers is essential throughout the PTC process—from preprocedural planning to postprocedural follow-up—to ensure that all team members are aligned in their roles and responsibilities, thereby minimizing errors and optimizing patient safety and outcomes. Care coordination involves seamless collaboration among healthcare team members, ensuring that the patient's care pathway is well-coordinated and that any issues or concerns are addressed promptly to enhance the overall quality of care and patient experience. Additionally, continuous quality improvement initiatives, such as regular team meetings, case reviews, and performance feedback sessions, help identify areas for improvement and implement strategies to enhance team performance, patient-centered care, and safety. This multidisciplinary approach fosters collaboration, shared decision-making, and continuous learning, ultimately improving patient outcomes, satisfaction, and healthcare delivery efficiency in treating patients with hepatobiliary disorders requiring PTC.
Media
References
Mastier C, Valette PJ, Adham M, Mabrut JY, Glehen O, Ponchon T, Rousset P, Rode A. Complex Biliary Leaks: Effectiveness of Percutaneous Radiological Treatment Compared to Simple Leaks in 101 Patients. Cardiovascular and interventional radiology. 2018 Oct:41(10):1566-1572. doi: 10.1007/s00270-018-2005-1. Epub 2018 Jun 5 [PubMed PMID: 29872897]
Azeemuddin M, Turab N Al Qamari, Chaudhry MBH, Hamid S, Hasan M, Sayani R. Percutaneous Management of Biliary Enteric Anastomotic Strictures: An Institutional Review. Cureus. 2018 Feb 26:10(2):e2228. doi: 10.7759/cureus.2228. Epub 2018 Feb 26 [PubMed PMID: 29713573]
Araz H, Eren T, Kocagül-Çelikbaş A, Özdemir N. Evaluation of Blood Stream and Biliary Tract Infections Related to Percutaneous Transhepatic Cholangiography and Prophylaxis Given in Patients with Malignancy. Infectious diseases & clinical microbiology. 2022 Dec:4(4):274-279. doi: 10.36519/idcm.2022.176. Epub 2022 Dec 21 [PubMed PMID: 38633711]
Chen L, Wu Z, Guo C, Wang G, Tu K, Jiang J. Evaluation of Clinical Indications of Three Treatments for Choledocholithiasis with Acute Cholangitis. International journal of general medicine. 2023:16():4669-4680. doi: 10.2147/IJGM.S429781. Epub 2023 Oct 16 [PubMed PMID: 37868815]
Das M, van der Leij C, Katoh M, Benten D, Hendriks BMF, Hatzidakis A. CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography, Biliary Drainage and Stenting. Cardiovascular and interventional radiology. 2021 Oct:44(10):1499-1509. doi: 10.1007/s00270-021-02903-4. Epub 2021 Jul 29 [PubMed PMID: 34327586]
Tavakkoli A, Beauchamp A, Prasad T, Zhu H, Singal AG, Elmunzer BJ, Kubiliun NM, Kwon RS, Hughes AE, Pruitt SL. Accessibility to ERCP-performing hospitals among patients with pancreatic cancer living in SEER regions. Cancer medicine. 2024 Feb:13(3):e7020. doi: 10.1002/cam4.7020. Epub [PubMed PMID: 38400670]
Mocan T, Horhat A, Mois E, Graur F, Tefas C, Craciun R, Nenu I, Spârchez M, Sparchez Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World journal of gastrointestinal oncology. 2021 Dec 15:13(12):2050-2063. doi: 10.4251/wjgo.v13.i12.2050. Epub [PubMed PMID: 35070041]
Wang Y, Zhao X, She Y, Kang Q, Chen X. The clinical efficacy and safety of different biliary drainage in malignant obstructive jaundice: a meta-analysis. Frontiers in oncology. 2024:14():1370383. doi: 10.3389/fonc.2024.1370383. Epub 2024 Apr 9 [PubMed PMID: 38655140]
Level 1 (high-level) evidenceSpadaccini M, Binda C, Fugazza A, Repici A, Tarantino I, Fabbri C, Cugia L, Anderloni A, On Behalf Of The Interventional Endoscopy Amp Ultra Sound I-Eus Group. Informed Consent for Endoscopic Biliary Drainage: Time for a New Paradigm. Medicina (Kaunas, Lithuania). 2022 Feb 22:58(3):. doi: 10.3390/medicina58030331. Epub 2022 Feb 22 [PubMed PMID: 35334507]
Ulvund Solstad T, Thorsteinsson M, Schultz N, Larsen PN, Taudorf M, Achiam M. Cholangioscopy with Spyglass DS using percutaneous transhepatic cholangiography access: a retrospective cohort study. Annals of medicine and surgery (2012). 2024 Apr:86(4):1867-1872. doi: 10.1097/MS9.0000000000001840. Epub 2024 Feb 16 [PubMed PMID: 38576952]
Level 2 (mid-level) evidenceAndo H, Inomata Y, Iwanaka T, Kuroda T, Nio M, Matsui A, Yoshida M, Japanese Biliary Atresia Society. Clinical practice guidelines for biliary atresia in Japan: A secondary publication of the abbreviated version translated into English. Journal of hepato-biliary-pancreatic sciences. 2021 Jan:28(1):55-61. doi: 10.1002/jhbp.816. Epub 2020 Oct 4 [PubMed PMID: 32780928]
Level 1 (high-level) evidenceOnishi Y, Shimizu H, Ohno T, Furuta A, Isoda H, Okamoto T, Okajima H, Nakamoto Y. Percutaneous Transhepatic Biliary Intervention in Adult Biliary Atresia Patients After Kasai Portoenterostomy. JPGN reports. 2022 May:3(2):e206. doi: 10.1097/PG9.0000000000000206. Epub 2022 May 9 [PubMed PMID: 37168905]
Jeong CY, Choi JW, Kim JR, Jang JY, Cho JK. Successful treatment through staged laparoscopic transgastric endoscopic retrograde cholangiopancreatography for postoperative bile leakage: A case report. Medicine. 2022 Sep 2:101(35):e30312. doi: 10.1097/MD.0000000000030312. Epub [PubMed PMID: 36107600]
Level 3 (low-level) evidenceAlabraba E, Travis S, Beckingham I. Percutaneous transhepatic cholangioscopy and lithotripsy in treating difficult biliary ductal stones: Two case reports. World journal of gastrointestinal endoscopy. 2019 Apr 16:11(4):298-307. doi: 10.4253/wjge.v11.i4.298. Epub [PubMed PMID: 31040891]
Level 3 (low-level) evidenceMisbahuddin-Leis M, Ankolvi M, Mishra M, Dubasz K, Marinov A, Müller T, Graeb C, Radeleff B. Unlocking the enigma: Combined percutaneous-transhepatic and endoscopic strategies for retrieval of severed Dormia basket in choledocholithiasis. A case report and literature review. Radiology case reports. 2024 Jul:19(7):2745-2750. doi: 10.1016/j.radcr.2024.03.074. Epub 2024 Apr 19 [PubMed PMID: 38680740]
Level 3 (low-level) evidenceGawlik C, Carneval M. A Review of the Management of Bile Leaks. Cureus. 2021 May 10:13(5):e14937. doi: 10.7759/cureus.14937. Epub 2021 May 10 [PubMed PMID: 34123634]
Schmitz D, Valiente CT, Dollhopf M, Perez-Miranda M, Küllmer A, Gornals J, Vila J, Weigt J, Voigtländer T, Redondo-Cerezo E, von Hahn T, Albert J, Vom Dahl S, Beyna T, Hartmann D, Franck F, García-Alonso FJ, Schmidt A, Garcia-Sumalla A, Arrubla A, Joerdens M, Kleemann T, Tomo JRA, Grassmann F, Rudi J. Percutaneous transhepatic or endoscopic ultrasound-guided biliary drainage in malignant distal bile duct obstruction using a self-expanding metal stent: Study protocol for a prospective European multicenter trial (PUMa trial). PloS one. 2022:17(10):e0275029. doi: 10.1371/journal.pone.0275029. Epub 2022 Oct 27 [PubMed PMID: 36302047]
Level 1 (high-level) evidencePedersoli F, Schröder A, Zimmermann M, Schulze-Hagen M, Keil S, Ulmer TF, Neumann UP, Kuhl CK, Bruners P, Isfort P. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts: technical considerations and complications. European radiology. 2021 May:31(5):3035-3041. doi: 10.1007/s00330-020-07368-6. Epub 2020 Oct 13 [PubMed PMID: 33051733]
Widyaningtiyas I, Sarastika HY, Utama HW. Bleeding after percutaneous transhepatic biliary drainage due to arterial injury: A case study in patient with stable hemodynamic. Radiology case reports. 2022 Dec:17(12):4868-4873. doi: 10.1016/j.radcr.2022.09.061. Epub 2022 Oct 12 [PubMed PMID: 36263331]
Level 3 (low-level) evidenceShabunin AV, Tavobilov MM, Lebedev SS, Karpov AA. [Mechanisms and prevention of biliary stent occlusion]. Khirurgiia. 2020:(5):70-75. doi: 10.17116/hirurgia202005170. Epub [PubMed PMID: 32500692]
Patel RK, Alagappan A, Tripathy T, Nayak HK, Pattnaik B, Dutta T, Gupta S, Mohakud S, Naik S, Deep Bag N. Bloody Bile and Rescue Intervention-A Case Series of Post-PTBD Hemorrhagic Complications With a Review of the Literature. Journal of clinical and experimental hepatology. 2024 Jul-Aug:14(4):101392. doi: 10.1016/j.jceh.2024.101392. Epub 2024 Mar 5 [PubMed PMID: 38558862]
Level 2 (mid-level) evidenceTokdemir Sisman S, Boynukara C, Sisman G. Infected percutaneous transhepatic cholangiography catheter and rescue hepaticogastrostomy in patient with gallbladder carcinoma. Endoscopy. 2024 Dec:56(S 01):E129. doi: 10.1055/a-2241-1656. Epub 2024 Feb 7 [PubMed PMID: 38325418]
Lubbe J, Lindemann J, Gondo W, Kolev N, Aclavio P, Hofmeyr S, Jonas E. Endoscopic versus percutaneous intervention for palliation in malignant hilar bile duct obstruction - A comparative cohort study. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2022 Dec:24(12):2145-2156. doi: 10.1016/j.hpb.2022.09.005. Epub 2022 Sep 16 [PubMed PMID: 36253268]
Level 2 (mid-level) evidence