Introduction
Pemphigoid gestationis (PG), formerly herpes gestationis, is a rare, self-limiting, autoimmune subepidermal bullous dermatosis of pregnancy. PG, first described in 1872 by Laws Milton, was previously called herpes gestationis due to the herpetiform appearance of the blisters. However, the condition was not associated with the herpes virus, and the name was subsequently changed to pemphigoid gestationis.[1][2] The condition typically presents during the third trimester, though it may occur during any trimester or the postpartum period, with inflammatory skin lesions and severe pruritus. The skin lesions commonly begin in the umbilical area and spread across the abdomen to the extremities; the face and mucous membranes are often spared. PG recurs in 30% to 50% of subsequent pregnancies, although typically with an earlier onset and a more severe course.[3] Characteristic clinical features and direct immunofluorescence are typically utilized to diagnose pemphigoid gestationis; other diagnostic studies may be performed to exclude other dermatologic conditions. Treatment is comprised of supportive therapies and the prevention of new skin lesions. Corticosteroids are frequently used to treat moderate to severe cases, though intravenous immunoglobulins have also been effective in some case studies.[2] Although PG spontaneously resolves in most patients, there is evidence that the condition is associated with preterm labor, small-for-gestational-age infants, and maternal Graves' disease.[2] Due to the adverse effects associated with PG, clinicians should have a good comprehension of the appropriate diagnostic tests and treatments involved with pemphigoid gestationis and the ability to effectively collaborate with an interprofessional team to improve maternal and fetal outcomes.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In PG, the skin lesions are caused by a maternal autoimmune process involving the hemidesmosomal proteins bullous pemphigoid 180 (BP 180) and bullous pemphigoid 230 (BP 230) that are located in the dermal basement membrane zone and placental tissue. The maternal immune system identifies BP 180 and BP 230, also known as BPAG2 and collagen, XVII, as foreign proteins. However, the precise trigger for forming these autoantibodies is not well understood.[2] PG is strongly associated with the maternal HLA-DRB1*0301 (HLA-DR3) and HLA-DRB1*0401/040X (HLA-DR4), which indicates the vital role of major histocompatibility complex (MHC) class II in the pathogenesis of the disease. There is also a strong association with human leukocyte antigens PG3 and PG4.[4]
Epidemiology
The incidence of PG is variably estimated, ranging from 1 out of 2,000 to 60,000 pregnancies, with most cases occurring in the second and third trimesters.[3] The condition is more prevalent in multiparous than primiparous women, commonly recurring in subsequent pregnancies. However, recurrent PG typically has an earlier onset and more severe symptoms. Some studies have also reported a rare association of PG with hydatidiform moles and choriocarcinomas.[3]
Pathophysiology
PG is thought to be caused by the loss of maternal immunotolerance to the placenta and fetus.[5] Though the precise provocation is not well understood, maternal IgG autoantibodies are produced against bullous pemphigoid protein antigens, BP180 and BP 230, which are expressed in the skin, placental cytotrophoblastic and syncytiotrophoblastic cells, and amniotic membrane epithelial cells. Autoimmunization may occur due to intolerance to this BP 180 placental antigen, resulting in a local allogeneic reaction against the fetoplacental unit. This process, promoted by abnormal expression of placental HLA class II antigens, is accompanied by alterations in the placental basement membrane. Subsequently, an immune response in the dermoepidermal junction of the skin occurs, causing disruption of the basement membrane and the characteristic bullous skin lesions.[6][7][8][9]
Histopathology
Routine histopathologic studies are helpful to exclude other skin disease processes, but the findings depend on the phase and severity of the disease. In the pre-bullous stage, papillary edema and infiltration of the dermis are characteristic, consisting of lymphocytes, histiocytes, and a variable number of eosinophils. Subepidermal blistering is usually observed in the bullous stage. However, histologic examination is not typically sufficient for the diagnosis because these findings are not specific to PG.[3]
History and Physical
Clinical Course of Pemphigoid Gestationis
Being knowledgeable of the characteristic clinical features of pemphigoid gestationis (PG) and the findings that differentiate it from similar skin conditions significantly assist clinicians with the diagnosis of this rare disease. Obtaining a comprehensive history, including a past and current obstetrical history, is essential since the natural clinical course of PG can involve exacerbations and remissions, with flares being common during the parturition and postpartum periods.[3] PG usually resolves spontaneously within 1 to 2 months after delivery. However, symptoms may persist or even exacerbate following pregnancy due to a sudden increase in antibodies. Furthermore, recurrence during any subsequent pregnancy (eg, miscarriage and abortion), or less commonly with menstrual cycles or combination oral contraceptive use, is well established. Fetal risks have also been observed in pregnancies with PG, including fetal growth restriction, preterm labor, and temporary neonatal skin lesions, which usually resolve several weeks after birth. This risk to the pregnancy appears to be correlated with disease severity; however, no increased risk of stillbirth or miscarriage has been observed. Therefore, obtaining a complete history helps to guide clinicians when considering differential diagnoses.[3]
Clinical Features of Pemphigoid Gestationis
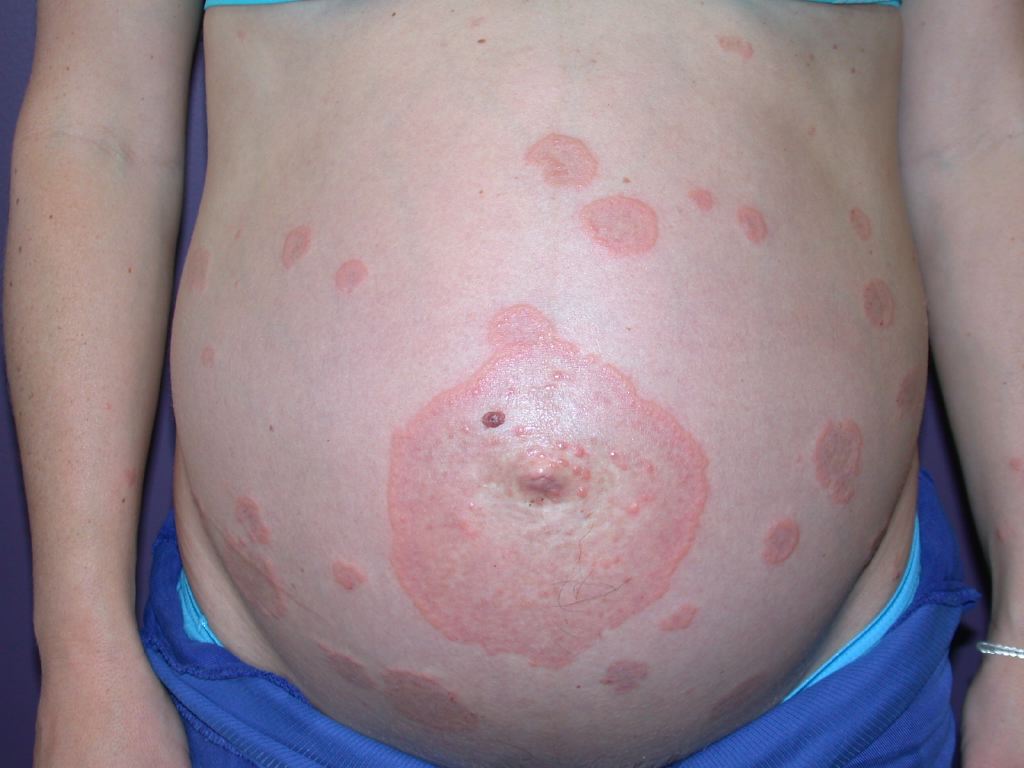
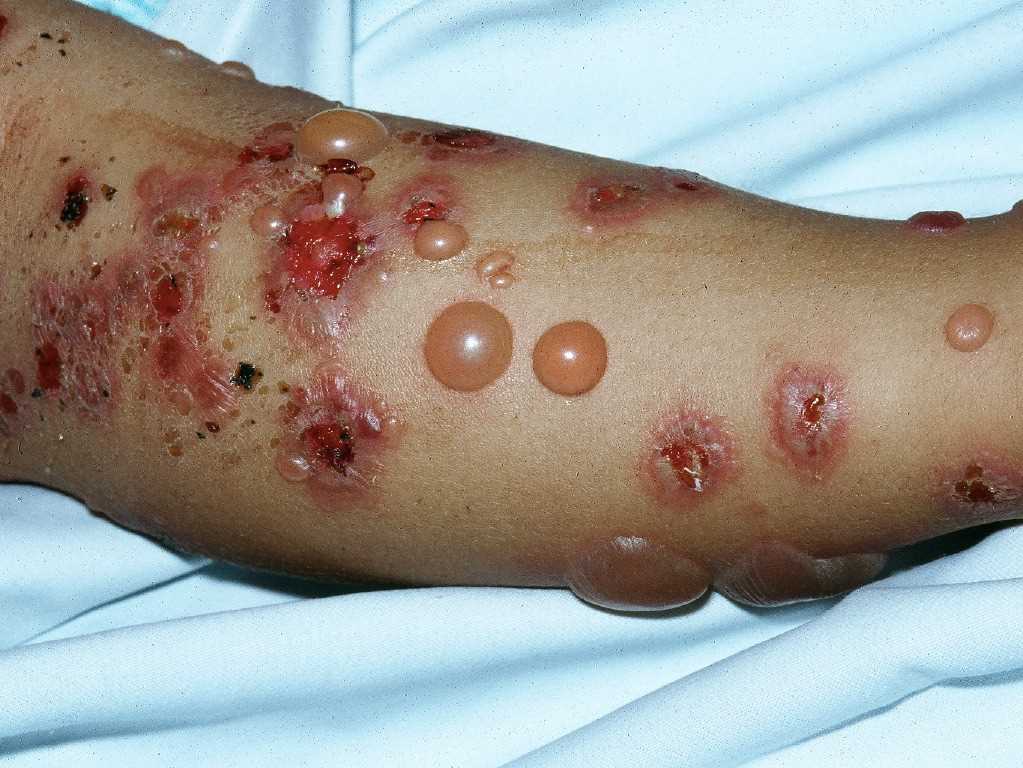
Pruritus is the primary clinical symptom, which may precede skin manifestations in some patients. Because the lesions go through different stages, the clinical features can vary. The polymorphic eruptions can include eczematous or erythema multiforme-like lesions, erythematous urticarial plaques, and papules, which progress to vesicles, tense blisters, and bullae in over 65% of cases (see Image. Pemphigoid Gestationis). The lesions can vary in severity, having a significant psychological impact on the patient. Initially, the lesions erupt in the umbilical area and subsequently spread to other parts of the abdominal region and the extremities, including the thighs, palms, and soles (see Image. Abdominal Pemphigoid Gestationis Lesions). The face and mucosal membranes are often spared.[2] The physical examination findings and distribution of the skin lesions are frequently nonspecific during the initial phase; clinicians may have difficulty distinguishing PG from other pruriginous dermatoses of pregnancy, especially the polymorphic eruption of pregnancy (PEP), also known as polymorphic urticarial papules and plaques of pregnancy (PUPPP). PEP can also develop in the third trimester of pregnancy and exhibit similar clinical features as PG. Because the differential diagnoses are challenging to differentiate symptomatically and histopathologically in the pre-bullous stage, direct immunofluorescence is typically used to confirm diagnosis.[2][3]
Evaluation
Because clinical features alone are nonspecific, diagnostic studies, including histopathology, direct immunofluorescence, and enzyme-linked immunosorbent assay (ELISA), are frequently used to diagnose PG.[10] However, direct immunofluorescence (DIF) staining on skin biopsy specimens is considered the most sensitive and specific diagnostic test to confirm PG.[3] Characteristic findings of PG on DIF include linear C3 deposits and IgG autoantibodies at the basement membrane zone. C3 deposits are considered diagnostic of PG, as this has been observed in all DIF stains of patients with PG.[2] Interestingly, DIF sometimes remains positive for 6 months to 4 years after clinical remission.
In addition to DIF, several other studies can be used to diagnose PG. Routine histopathologic studies are helpful to exclude other skin disease processes, but the findings depend on the phase and severity of the disease. In the pre-bullous stage, papillary edema and infiltration of the dermis are characteristic, consisting of lymphocytes, histiocytes, and a variable number of eosinophils.[3] Immunochemistry studies in patients with PG show C4d deposits along the dermal-epidermal junction. Indirect immunofluorescence microscopy detects circulating IgG antibodies in the serum of approximately 30% to 100% of patients with PG.[11][12][13][14] ELISA testing distinguishes PG from other pregnancy dermatoses by identifying BP 180 IgG antibodies in serum specimens. ELISA has a reportedly high sensitivity and specificity of approximately 95% that may potentially render direct immunofluorescence, currently the reference standard, obsolete. Limitations of both DIF and indirect immunofluorescence include the need for non-formalin tissue fixation and appropriately equipped laboratory facilities with an increased cost, which may be difficult in developing countries.[15][10].
Treatment / Management
Treatment of PG depends on the severity and the stage of the skin lesions. The main goal is to relieve itching and prevent the formation of new blisters. The mainstay of treatment is topical high-potency corticosteroids and antihistamines. However, additional therapies may be needed for patients unresponsive to initial therapy. More recent treatment regimens have been utilized to reduce the use of high-dose steroids and prevent potential complications in pregnancy.[3][2](B3)
Although most PG treatment recommendations currently come from case studies, topical corticosteroids and oral antihistamines have typically been preferred treatments, especially when the disease is localized with minimal blistering.[16][17] Patients resistant to topical treatment or with a severe form involving >10% of the body surface area may require systemic corticosteroids. Treatment includes oral prednisolone 0.5 mg/kg daily, with a recommended total daily dose of <20 mg. Prednisone and prednisolone are preferred over other agents due to the reduced concentration crossing the placenta. If there are no new blisters within 2 weeks, treatment is considered successful, and clinicians should taper patients off of corticosteroid therapy.[3] For nonresponsive patients, immunosuppressant agents such as cyclosporine, dapsone, or azathioprine are considered for disease control; however, clinicians should be aware of potential adverse effects with these medications (eg, bone marrow suppression, preterm birth, and liver impairment).[4][3][2] Intravenous immunoglobulins (IVIG) have more recently been utilized successfully alone or in addition to other agents and have a better safety profile than immunosuppressants.[4] (B3)
Dupilumab, a monoclonal antibody that targets the alpha subunit of the interleukin 4 receptor, has most recently been used as biotherapy, especially in refractory and corticosteroid-dependent bullous pemphigoid. This seems to be a new and promising therapy, especially in women with co-morbidities, like diabetes, where systemic steroid therapy may be contraindicated. Dupilumab use was associated with rapid remission of symptoms and the ability to wean from high-dose systemic steroid use.[5] Additionally, rituximab has reportedly been effective in preventing recurrent PG when given preconceptually. Patients should avoid a subsequent pregnancy for 12 months following the last infusion.[3](B3)
Differential Diagnosis
Other conditions that should be considered when evaluating patients for PG include:[3][2]
- Urticaria
- Allergic contact dermatitis
- Bullous pemphigoid
- Cicatricial pemphigoid
- Dermatitis herpetiformis
- Erythema multiforme
- Impetigo
- Intrahepatic cholestasis of pregnancy
- Linear IgA dermatosis
- Bullous drug eruption
- Atopic eruption of pregnancy
Prognosis
The overall prognosis of PG is typically good. There is an increased risk of PG recurrence in subsequent pregnancies. Mothers who have had a pregnancy affected by PG are also at risk of developing other autoimmune diseases, specifically Grave's disease, in the future. In approximately 10% of affected pregnancies, neonatal pemphigoid will occur due to the transplacental passage of maternal antibodies.[10] Spontaneous resolution of these neonatal lesions is usual.
The most significant fetal risks include preterm labor and intrauterine growth retardation.[3][2] Minimizing and preventing the risk of adverse effects for the fetus depends on the timely clinical and histopathological diagnosis of pemphigoid gestationis. Screening for intrauterine fetal growth restriction during pregnancy and monitoring the pregnancy for side effects of high-dose steroid treatment is imperative to minimize fetal and maternal complications. A multidisciplinary approach to care involving neonatology, pediatrics, obstetrics, and dermatology is crucial for the benefit of the pregnant woman and her infant during and after the pregnancy, as well as in subsequent pregnancies.[3][2]
Complications
Although PG has limited maternal and fetal complications as noted above, the itching that it causes can be debilitating. Additionally, the long-term use of corticosteroids in pregnancy may be associated with an increased risk of preterm birth, preeclampsia, preterm premature rupture of membranes, venous thromboembolism, possible congenital anomalies, pyelonephritis, and potentially intrauterine fetal death.[18] Although small for gestational age babies are more commonly seen with pregnancies where long-term corticosteroids are used, it is unclear if steroidal treatment is the direct cause or if the underlying condition being treated is the causative factor. Some animal studies have shown that steroid use may be associated with abnormal placentation, which in turn causes fetal growth restriction.[18] The exact mechanism and association are still poorly understood. Risks and benefits must be factored into treatment strategies that involve long-term steroid use in pregnancy. Further research is needed to discern if there are any long-term effects on the children born to mothers who required treatment with corticosteroids during pregnancy, as well.
Enhancing Healthcare Team Outcomes
PG is a rare disease that typically does not pose a significant health burden. However, this condition frequently causes significant maternal distress and poses a diagnostic dilemma until appropriate diagnostic testing is performed. When patients present with clinical symptoms, clinicians should immediately begin evaluation and initiate treatment as soon as the diagnosis is established, as the disease usually responds well to treatment with topical steroids and antihistamines. An interprofessional approach between obstetric and dermatologic clinicians and ancillary health professionals is required to ensure coordinated monitoring, including assessing signs of prematurity and abnormal fetal growth. Furthermore, health team members such as pharmacists should assist in guiding adverse effects treatment and encourage medication compliance to help ease intense pruritus.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Podolec-Rubiś M, Wołek M, Brzewski P, Wojas-Pelc A. Suspicion of pulmonary embolism during treatment of pemphigoid gestationis. Postepy dermatologii i alergologii. 2013 Feb:30(1):59-61. doi: 10.5114/pdia.2013.33381. Epub 2013 Feb 20 [PubMed PMID: 24278048]
Level 3 (low-level) evidenceSävervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clinical, cosmetic and investigational dermatology. 2017:10():441-449. doi: 10.2147/CCID.S128144. Epub 2017 Nov 8 [PubMed PMID: 29184427]
Level 3 (low-level) evidenceStefaniak AA, Pereira MP, Zeidler C, Ständer S. Pruritus in Pregnancy. American journal of clinical dermatology. 2022 Mar:23(2):231-246. doi: 10.1007/s40257-021-00668-7. Epub 2022 Feb 21 [PubMed PMID: 35191007]
Almeida FT, Sarabando R, Pardal J, Brito C. Pemphigoid gestationis successfully treated with intravenous immunoglobulin. BMJ case reports. 2018 Apr 7:2018():. pii: bcr-2018-224346. doi: 10.1136/bcr-2018-224346. Epub 2018 Apr 7 [PubMed PMID: 29627782]
Level 3 (low-level) evidenceAlvarez Martinez D, Russo G, Fontao L, Laffitte E. Successful therapy of pemphigoid gestationis with dupilumab-A new case. Journal of the European Academy of Dermatology and Venereology : JEADV. 2023 Jun:37(6):e752-e753. doi: 10.1111/jdv.18911. Epub 2023 Jan 31 [PubMed PMID: 36688319]
Level 3 (low-level) evidenceDaniel BS, Murrell DF. Review of autoimmune blistering diseases: the Pemphigoid diseases. Journal of the European Academy of Dermatology and Venereology : JEADV. 2019 Sep:33(9):1685-1694. doi: 10.1111/jdv.15679. Epub 2019 Jul 11 [PubMed PMID: 31087464]
Kukkamalla RM, Bayless P. Pemphigoid Gestationis. Clinical practice and cases in emergency medicine. 2019 Feb:3(1):79-80. doi: 10.5811/cpcem.2018.11.39258. Epub 2019 Jan 7 [PubMed PMID: 30775676]
Level 3 (low-level) evidenceLobato-Berezo A, Fernández Figueras MT, Moreno Romero JA, Pujol RM. Pemphigoid Gestationis Mimicking Erythema Multiforme With Mucosal Involvement. Actas dermo-sifiliograficas. 2019 Oct:110(8):696-697. doi: 10.1016/j.ad.2018.02.038. Epub 2019 Feb 4 [PubMed PMID: 30732852]
Maglie R, Quintarelli L, Verdelli A, Fabbri P, Antiga E, Caproni M. Specific dermatoses of pregnancy other than pemphigoid gestationis. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2019 Jun:154(3):286-298. doi: 10.23736/S0392-0488.18.06159-X. Epub 2018 Oct 29 [PubMed PMID: 30375214]
Schauer F, Mai S, Hofmann SC, Mai Y, Izumi K, Kern JS, Kiritsi D. Autoreactivity to BP180 Neoepitopes in Patients With Pemphigoid Gestationis. JAMA dermatology. 2022 Feb 1:158(2):212-214. doi: 10.1001/jamadermatol.2021.5294. Epub [PubMed PMID: 34985500]
Feliciani C, Genovese G, D'astolto R, Pontini P, Marzano AV. Autoimmune bullous diseases during pregnancy: insight into pathogenetic mechanisms and clinical features. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2019 Jun:154(3):256-262. doi: 10.23736/S0392-0488.18.06153-9. Epub 2018 Oct 29 [PubMed PMID: 30375213]
Papapanagiotou IK, Tsagouri S, Liakou CG, Besharat A, Vogiatzis N, Ntzeros K, Petrakis E, Koutroumanis P, Thomakos N, Loutradis D. Pemphigoid gestationis. Clinical case reports. 2018 Jul:6(7):1364-1365. doi: 10.1002/ccr3.1545. Epub 2018 May 7 [PubMed PMID: 29988643]
Level 3 (low-level) evidenceBechtel MA. Pruritus in Pregnancy and Its Management. Dermatologic clinics. 2018 Jul:36(3):259-265. doi: 10.1016/j.det.2018.02.012. Epub 2018 Apr 26 [PubMed PMID: 29929597]
Yang A, Uhlenhake E, Murrell DF. Pemphigoid gestationis and intravenous immunoglobulin therapy. International journal of women's dermatology. 2018 Sep:4(3):166-169. doi: 10.1016/j.ijwd.2018.03.007. Epub 2018 May 3 [PubMed PMID: 30175219]
Al-Shenawy HA. Can immunohistochemistry replace immunofluorescence in diagnosis of skin bullous diseases? APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2017 Feb:125(2):114-121. doi: 10.1111/apm.12643. Epub 2017 Jan 3 [PubMed PMID: 28052410]
Cabral R, Teixeira V, Brinca A, Fernandes B, Reis JP. Case for diagnosis. Pemphigoid gestationis. Anais brasileiros de dermatologia. 2014 Jan-Feb:89(1):167-8. doi: 10.1590/abd1806-4841.20142456. Epub [PubMed PMID: 24626668]
Level 3 (low-level) evidenceGenovese G, Derlino F, Berti E, Marzano AV. Treatment of Autoimmune Bullous Diseases During Pregnancy and Lactation: A Review Focusing on Pemphigus and Pemphigoid Gestationis. Frontiers in pharmacology. 2020:11():583354. doi: 10.3389/fphar.2020.583354. Epub 2020 Oct 2 [PubMed PMID: 33117178]
Cai E, Czuzoj-Shulman N, Abenhaim HA. Maternal and fetal outcomes in pregnancies with long-term corticosteroid use. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2021 Jun:34(11):1797-1804. doi: 10.1080/14767058.2019.1649392. Epub 2019 Aug 20 [PubMed PMID: 31429349]