Introduction
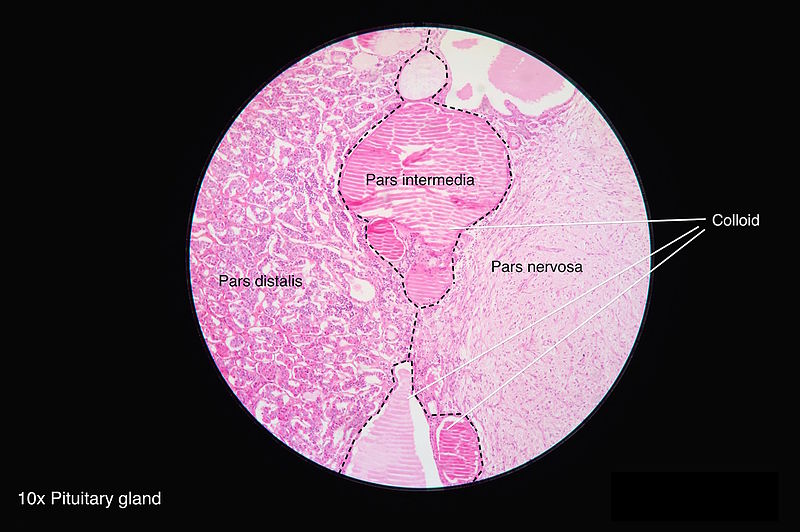
The pars nervosa is a neuroendocrine structure that, along with the anterior lobe, intermediate lobe, and infundibular stalk, makes up the pituitary gland. This structure lies within the sella turcica, a saddle-shaped indentation in the sphenoid bone that lies posterior to the nasopharynx. The pars nervosa is responsible for the secretion of the neurohypophysial hormones oxytocin and arginine vasopressin into the systemic circulation. Because these hormones play important roles in the regulation of blood pressure and osmolarity as well as parturition and lactation, their regulated secretion is typically considered the primary physiologic function of the pars nervosa.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
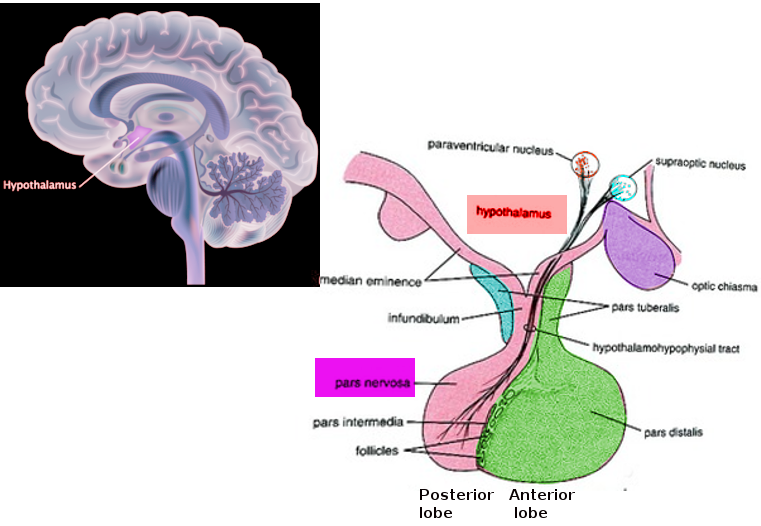
The pars nervosa, in conjunction with the infundibular stalk, makes up the posterior pituitary gland. The infundibular stalk contains unmyelinated axonal bodies of magnocellular neurosecretory cells originating in the hypothalamus. These neurons project to the pars nervosa, where they release the neuroendocrine hormones oxytocin and arginine vasopressin (AVP) into the systemic circulation via fenestrated sinusoidal capillaries. Because these hormones get synthesized by neuronal cell bodies within the paraventricular and supraoptic nuclei, the pars nervosa is often considered an extension of the hypothalamus.
The two hormones released in the pars nervosa have distinct physiologic roles. Oxytocin generates smooth muscle and myoepithelial cell contraction, facilitating uterine contraction during parturition and milk letdown in lactation. AVP, also known as antidiuretic hormone (ADH), prevents consequences of volume depletion through a variety of mechanisms, each associated with a unique receptor subtype. The V1 receptor is expressed primarily on vascular smooth muscles and causes arteriolar contraction when stimulated by AVP. Expression of the V2 receptor occurs on the renal collecting ducts and tubular epithelium where AVP triggers the synthesis and expression of water transporter Aquaporin-2, increasing solute-free water reuptake and inducing a hypotonic shift in blood osmolarity. In concert, these receptors allow AVP to regulate blood tonicity and support blood pressure during volume loss. The V3 (also known as V1b) receptor is present in a variety of organs, including the anterior pituitary, where it stimulates the release of adrenocorticotropin (ACTH), beta-endorphin, and prolactin.[2]
Embryology
The pars nervosa originates from neuroectoderm similarly to the rest of the hypothalamus; this is in contrast to the anterior and intermediate lobes of the pituitary gland, which originate from migratory oral ectoderm called the Rathke pouch. Between weeks four and eight of fetal development, a region of the ventral diencephalon projects downwards to meet the superiorly migrating involution of oral ectoderm, forming the neuro- and adenohypophysis respectively.[3][4]
Blood Supply and Lymphatics
The pars nervosa is supplied primarily by the inferior and middle hypophyseal arteries with some contribution of the superior hypophyseal artery, all of which branches of the internal carotid artery. While the hypophyseal veins serve as primary drainage of the pars nervosa, portal venous branches also carry some blood to the anterior pituitary, permitting the direct effects of AVP on secretory activity within the anterior pituitary. Both of these venous systems drain through the intercavernous sinus and eventually into the internal jugular vein.[5][6]
Physiologic Variants
Most variations in pituitary anatomy result in pathology, so a few physiologic variants exist. Empty sella syndrome describes the absence of an identifiable hypophysis within the sella turcica, a bony feature that typically surrounds the pituitary gland. While secondary causes usually correlate with pathologic pituitary insufficiency, one primary etiology of empty sella syndrome involves an anatomic defect affecting cerebrospinal fluid (CSF) drainage that results in elevated CSF pressures within sella turcica. This increased pressure remodels the pituitary superiorly into the hypothalamus, thus, while imaging characteristically demonstrates an empty sella turcica, the tissue itself (as well as its function) are often preserved.[7][8]
Surgical Considerations
The most common indications for pituitary surgery are space-occupying sellar lesions of various etiology. Tumors of the pars nervosa itself occur but are rare relative to those of the anterior pituitary.[9] However, the close proximity of these two structures, surgical access, risks, and considerations are nearly identical regardless of whether the anterior or posterior pituitary itself has warranted resection.[10] In general, surgical access to the pituitary is achieved using a transsphenoidal approach, though transcranial approaches are also an option.[11] Microsurgical and endoscopic techniques are at the discretion of the surgeon. Common complications of pituitary surgery include syndrome of inappropriate antidiuretic hormone, diabetes insipidus and other syndromes of pituitary insufficiency, electrolyte imbalances, hemorrhage, post-operative fever, and bacterial meningitis, among others.[12]
Clinical Significance
Because the pars nervosa's primary purpose concerns the regulated secretion of oxytocin and AVP, the physiologic roles that these hormones play and the circumstances surrounding their secretion serve as a relatively comprehensive depiction of its clinical importance.
Oxytocin plays an integral role in inducing uterine contractions that contribute to parturition directly and prevent excessive blood loss thereafter. During childbirth, signals caused by cervical stretching reach the hypothalamus and trigger the release of oxytocin into the systemic circulation. In turn, oxytocin acts on uterine smooth muscle inducing and strengthening contraction. Because of this phenomenon, pharmacologic forms of oxytocin are often used to induce labor. In lactation, suckling creates afferent nerve impulses that travel to the hypothalamus and trigger the release of oxytocin, which stimulates receptors on myoepithelial cells of the lactiferous ducts resulting in milk letdown. While abnormal oxytocin levels correlate in the context of various psychiatric disorders, it is not currently a treatment in this context.[13]
AVP secretion occurs in response to changes in both blood osmolarity and pressure through two independent neurologic circuits. Small changes in blood osmolarity sensed by osmoreceptors in the hypothalamus and lamina terminalis drive adjustments in pituitary vasopressin secretion, which in turn impact renal reabsorption of solute free water in the collecting tubules and ducts. Increased reabsorption of water reduces blood osmolarity, feeding back against vasopressin release. This circuit provides precise governance of overall blood osmolarity.[13]
In tandem, significant reductions in blood pressure generate sympathetic signals that travel to the hypothalamus to trigger vasopressin release. High levels of circulating AVP cause arteriolar smooth muscle contraction to elevate blood pressure, increase water reabsorption via the aforementioned renal mechanism, and promote hemostasis through stimulation of platelet aggregation and von Willibrand’s factor release. These mechanisms work in unison to adapt and respond to a fluid-deficient state.
Pathology of the pars nervosa, regardless of etiology, relates to either inadequate or excessive secretion of neurohypophyseal hormones. While oxytocin plays a variety of physiologic roles, derangements in its secretion are rarely implicated in disease pathophysiology or treatment. With that said, abnormal oxytocin levels have been the target of research in a variety of psychiatric disorders. Disordered AVP secretion causes diabetes insipidus (DI) if inadequate, and the syndrome of inappropriate antidiuretic hormone (SIADH) if excessive.
Diabetes Insipidus (DI) occurs when inadequate or ineffective AVP secretion results in an inability of the kidney to concentrate urine adequately. Typically, DI presents as excessive thirst, dehydration, and hypernatremia in a patient excreting abnormally large volumes of dilute urine. It can result from pituitary insufficiency, renal malfunction, or excessive consumption of water. Of these, only pituitary insufficiency is a disorder of the pars nervosa.[14]
Pituitary insufficiency often subdivides into three etiologic categories- traumatic, infectious/inflammatory, and oncologic. Traumatic causes of pituitary insufficiency involve sequelae of hemorrhagic/ischemic infarcts and direct damage to the pituitary associated with blunt or penetrating brain injuries. Infectious/inflammatory etiologies include autoimmune hypophysitis, sarcoidosis, meningitis, encephalitis, and brain abscess. Oncologic pituitary injury results from direct mass effects of a nearby malignancy or injury during tumor resection or radiation therapy. Empty sella syndrome serves as the general diagnosis for the physical absence of a pituitary gland. Primary empty sella syndrome is often asymptomatic and typically stems anatomical abnormalities that cause elevated intracranial pressure within the sella turcica, consequently flattening the pituitary gland due to mass effect. Secondary empty sella syndrome typically presents due to the above etiologies.[15]
SIADH occurs when vasopressin levels become abnormally/persistently elevated due to hypothalamic dysregulation or ectopic production. It presents as a hyponatremic patient with excessively concentrated urine because excess vasopressin drives the kidney to reabsorb maximal amounts of solute free water. One noteworthy consequence of hyponatremia is increased intracellular volume and consequent swelling, which has particularly profound effects on cells of the central nervous system (CNS) and can lead to neurologic dysfunction, coma, and death.[16]
Excess vasopressin associated with SIADH can be due to a wide variety of causes that are grossly separable into those that derange the hypothalamohypophyseal pathway and those that circumvent it entirely. Pituitary dysregulation can occur secondary to central nervous system (CNS) infections of multiple etiology, hydrocephalus, trauma, and CNS diseases like multiple sclerosis, among other causes. A wide array of medications, including selective serotonin reuptake inhibitors (SSRIs), chlorpropamide, carbamazepine, valproic acid, and morphine, can also cause SIADH via a variety of mechanisms involving the kidney and CNS. Numerous cancers have demonstrated the capacity to produce vasopressin, including small cell lung cancer, mesothelioma, gastrointestinal/genitourinary cancers, lymphomas, etc. Additionally, severe pulmonary infections and sarcoidosis can trigger supraphysiologic levels sufficient to cause clinically significant SIADH. Etiology aside, the hyponatremia associated with SIADH warrants immediate treatment to prevent complications and death.[17]
Media
(Click Image to Enlarge)
References
Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurgery clinics of North America. 2003 Jan:14(1):11-23, v [PubMed PMID: 12690976]
Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997 Oct:138(10):4109-22 [PubMed PMID: 9322919]
Level 3 (low-level) evidenceMusumeci G, Castorina S, Castrogiovanni P, Loreto C, Leonardi R, Aiello FC, Magro G, Imbesi R. A journey through the pituitary gland: Development, structure and function, with emphasis on embryo-foetal and later development. Acta histochemica. 2015 May-Jun:117(4-5):355-66. doi: 10.1016/j.acthis.2015.02.008. Epub 2015 Apr 6 [PubMed PMID: 25858531]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Larkin S, Ansorge O. Development And Microscopic Anatomy Of The Pituitary Gland. Endotext. 2000:(): [PubMed PMID: 28402619]
Liu W, Xu ZM, Liu XM, Kong L, Yin WN, Wang GH. Microsurgical anatomy of perforated arteries in the hypothalamic area. Turkish neurosurgery. 2015:25(1):63-8. doi: 10.5137/1019-5149.JTN.9840-13.1. Epub [PubMed PMID: 25640547]
Truong HQ, Najera E, Zanabria-Ortiz R, Celtikci E, Sun X, Borghei-Razavi H, Gardner PA, Fernandez-Miranda JC. Surgical anatomy of the superior hypophyseal artery and its relevance for endoscopic endonasal surgery. Journal of neurosurgery. 2018 Jul 13:131(1):154-162. doi: 10.3171/2018.2.JNS172959. Epub [PubMed PMID: 30004277]
Auer MK, Stieg MR, Crispin A, Sievers C, Stalla GK, Kopczak A. Primary Empty Sella Syndrome and the Prevalence of Hormonal Dysregulation. Deutsches Arzteblatt international. 2018 Feb 16:115(7):99-105. doi: 10.3238/arztebl.2018.0099. Epub [PubMed PMID: 29510819]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Evanson J. Radiology of the Pituitary. Endotext. 2000:(): [PubMed PMID: 25905384]
Guerrero-Pérez F, Vidal N, Marengo AP, Pozo CD, Blanco C, Rivero-Celada D, Díez JJ, Iglesias P, Picó A, Villabona C. Posterior pituitary tumours: the spectrum of a unique entity. A clinical and histological study of a large case series. Endocrine. 2019 Jan:63(1):36-43. doi: 10.1007/s12020-018-1774-2. Epub 2018 Oct 1 [PubMed PMID: 30276594]
Level 2 (mid-level) evidenceBuchfelder M. Treatment of pituitary tumors: surgery. Endocrine. 2005 Oct:28(1):67-75 [PubMed PMID: 16311412]
Miller BA, Ioachimescu AG, Oyesiku NM. Contemporary indications for transsphenoidal pituitary surgery. World neurosurgery. 2014 Dec:82(6 Suppl):S147-51. doi: 10.1016/j.wneu.2014.07.037. Epub [PubMed PMID: 25496626]
Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of Complications, Trends, and Costs in Endoscopic vs Microscopic Pituitary Surgery: Analysis From a US Health Claims Database. Neurosurgery. 2017 Sep 1:81(3):458-472. doi: 10.1093/neuros/nyx350. Epub [PubMed PMID: 28859453]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Christ-Crain M, Ball S. The Neurohypophysis: Endocrinology of Vasopressin and Oxytocin. Endotext. 2000:(): [PubMed PMID: 25905380]
Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, Rossi A, Maghnie M. Diabetes insipidus--diagnosis and management. Hormone research in paediatrics. 2012:77(2):69-84. doi: 10.1159/000336333. Epub 2012 Mar 16 [PubMed PMID: 22433947]
Level 3 (low-level) evidenceAscoli P, Cavagnini F. Hypopituitarism. Pituitary. 2006:9(4):335-42 [PubMed PMID: 17077946]
Cuesta M, Thompson CJ. The syndrome of inappropriate antidiuresis (SIAD). Best practice & research. Clinical endocrinology & metabolism. 2016 Mar:30(2):175-87. doi: 10.1016/j.beem.2016.02.009. Epub 2016 Feb 27 [PubMed PMID: 27156757]
Tee K, Dang J. The suspect - SIADH. Australian family physician. 2017 Sep:46(9):677-680 [PubMed PMID: 28892600]