Introduction
Injury of the pars interarticularis is among the most common causes of low back pain, especially in adolescent athletes. Sometimes these lesions develop in an asymptomatic manner, and they are detected in adulthood when the injury becomes chronic and symptomatic.[1]
The spectrum of pathologies in the pars interarticularis ranges from bone stress and pars fracture (spondylolysis) to isthmic spondylolisthesis, representing an anterior vertebral slippage. Bone stress is considered the earliest sign of disease. Repetitive bone stress causes bone remodeling and may result in spondylolysis, a non-displaced fracture of the pars interarticularis.[2] Also, radiographically visualized spondylolysis is associated with spondylolisthesis in about 25% of cases.[3][4]
Spondylolisthesis, a related condition to spondylolysis, is defined by the forward displacement of the upper vertebra relative to the caudal vertebra. In 1976 Wiltse et al.[5] classified spondylolisthesis into five types:
- Type I or dysplastic: is attributed to congenital dysplasia of the superior articular process of the sacrum.
- Type II or isthmic: is due to a lesion in the pars interarticularis; these subclassify as:
- (a) Lytic, when a fatigue pars fracture is present
- (b) Pars elongation due to multiple healed stress fractures
- (c) Acute pars fracture
- Type III or degenerative: originates from facet instability without a pars fracture.
- Type IV or traumatic: the displacement is due to an acute posterior arch fracture other than pars.
- Type V or pathological: is due to posterior vertebral arch bone disease.[5]
- Type VI or iatrogenic: it is a potential sequel to spinal surgery.
For this activity, the focus will be on type II or isthmic spondylolisthesis.
Spondylolisthesis was classified by Meyerding et al. into five subtypes according to the magnitude of slippage on plain lateral lumbar radiographs measured by the movement of the upper vertebra relative to the inferior vertebra.[6]
- Grade I, less than 25% of displacement,
- Grade II, between 25 and 50%,
- Grade III, between 50 and 75%,
- Grade IV, between 75 and 100% and
- Grade V or spondyloptosis, when there is no contact between the vertebrae endplates. The commonly used Grade V, representing more than 100% slip or spondyloptosis, is not part of the original grading system.
Most pars lesions or spondylolysis occur at L5 (85 to 95%), with L4 being the second most commonly affected vertebra (5 to 15%). The other lumbar levels are less often affected.[3][7][8][9][10] The defect is unilateral in 22% of the cases.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pars defect is an acquired condition that develops at a young age.[3][7] However, there is a strong association between pars defects and congenital anomalies such as spina bifida occulta.[11][12][13]
Congenital pars injury has never been described.[14] Even though the acquired theory is the most accepted, there is evidence that a genetic component is likely to contribute. Wynne-Davies et al. found that first-degree relatives of those affected by spondylolysis had a higher incidence (19%) of spondylolysis compared to the general population.[15]
Isthmic spondylolysis represents a fatigue fracture in the pars interarticularis isthmus; this is the weakest part of the posterior vertebral arch. Hyperextension and rotatory forces may result in repetitive stress in this region. An isolated traumatic event is less common as a cause of spondylolysis; however, it could potentially complete a developing pars fracture.[16][17][18]
Athletes encompass the majority of patients presenting with spondylolysis. Sport-specific maneuvers with repetitive twisting rotation and extension increase the load on the spine and may result in stress injury. There are reports that the strength of the neural arch increases through adulthood until the age of 50. Children and adolescents are prone to a higher risk of fatigue pars fractures.[19]
Epidemiology
The incidence of spondylolysis is about 3 to 6%.[20][3][7] It is higher in elite adolescent athletes (8 to 15%).[13][21] The male-to-female ratio is 2 or 3 to 1.[20][3] Divers, rowers, gymnasts, weight lifters, wrestlers, and throwing track and field athletes have higher rates within this group.[9][10] Roche and Rowe reported an overall incidence of 4.2%, with rates of 6.4% for White males and 2.3% for White females, while the prevalence for African-American males was 2.8% and 1.1% for African-American females.[7]
Fredrickson et al. found an increased incidence in the population sorted by age.[3] The overall incidence at age six was 4.4%, 5.2% by age 12, and 6% by adulthood. No newborn within 500 studied patients showed radiographic evidence of spondylolysis. Family members of affected patients have a higher rate of spondylolysis than the rest of the population.
Approximately 80% of patients with spondylolysis at the L5 level have the isthmic type of spondylolisthesis, and 20% of these show slippage that exceeds 25%.[22]
History and Physical
The general population has a relatively high incidence of radiographically identified spondylolysis. However, clinicians find the majority of these lesions in asymptomatic patients.[16][3]
Symptomatic pars lesions appear to be a clinical problem in adolescents, especially athletes.[16] The mechanism of trauma, the patient's history, and physical examination findings may lead to suspicion of pars interarticularis injuries. The mechanism of injury involves hyperextension and twisting rotation activities such as weight lifting or aerobic gymnastics. However, any sporting activity may increase the risk of pars injury.
The most frequent complaint is low back pain, either localized or diffuse. Trunk hyperextension or rotation exercises usually exacerbate the pain and rest partially relieves it. It is usually located at the lower lumbar back. However, patients may complain about buttocks or radiating pain in one or both legs.[23][24][25][18] The outset of pain can be gradual for a long period with mild symptoms or start sharply after acute trauma.[17]
During the physical examination, patients tend to express a hyperlordotic posture. Tight hamstrings and knee contractures are common.[26][19] Sometimes, patients may demonstrate a flattened lumbar lordosis, a palpable step off of the spinous process, or limitation of lumbar flexion and extension.
The Stork test or one-legged hyperextension maneuver is thought to be the only pathognomonic finding during examination. It involves asking the patient to stand on one leg and extend the low back. When properly done, the leg is straight, and the trunk tilted, resulting from the lumbosacral extension. Concurrent ipsilateral knee flexion may produce trunk tilt without low back extension, producing lower test sensitivity. Pain indicates possible spondylolysis on the ipsilateral side.[17][18][26]
If patients present an isolated spondylolysis, the neurological examination should be normal. The most frequent neurological symptoms are radicular pain, weakness, tingling, and numbness in the lower limbs. They are related to spondylolisthesis rather than spondylolysis. Sometimes patients complain about symptom exacerbation when performing activities that involve low back extension. As a result, patients will express a kyphotic lumbar posture to relieve nerve roots by lumbar flexion and foraminal widening. If radicular pain is present, the L5 nerve root is the most commonly affected, and the straight leg raise test may be positive. Bowel and bladder symptoms or cauda equina syndrome are rare.
Evaluation
Multiple imaging studies may assist in the diagnosis of a pars lesion.[16] Once pars injury is suspected from the history and physical examination, radiographic studies are the next step. Anterior-posterior and lateral lumbar X-rays must be requested. Although lateral lumbosacral radiographs may show the defect in pars in 80% of the cases, the classical oblique X-ray views have been mentioned as important studies to diagnose this condition. The defect in isthmic spondylolysis appears as a lucency in the region of the pars interarticularis. The common description of the radiographic appearance of the lesion on lateral oblique radiographs is commonly that of a collar or a broken neck on the “Scotty dog.” See Image. Oblique Plain Radiograph of the Lumbar Spine. Radiographic studies have limited sensitivity to diagnose acute pars injury compared with other imaging modalities. When an X-ray is not diagnostic, further studies merit consideration.
Scintigraphy is an excellent screening tool for low back pain in children or adolescents. It has shown high sensitivity for detecting acute injuries and bone stress reactions in the pars. However, some lesions may not display an increased contrast uptake.
A computed tomography scan (CT) may be helpful in some cases due to its higher specificity. The tomographic finding of an acute injury include the margin reabsorption in the pars; pars sclerosis may indicate chronic stress, and marginal sclerosis with widening may indicate a chronic condition. Identifying patients with a normal CT scan and abnormalities on bone scintigraphy or Single-photon emission computed tomography (SPECT) is important as these patients are presumed to be in a very early stage of the disease and have a higher chance of healing with timely conservative treatment.
SPECT is considered the best diagnostic adjunct when plain radiographs are negative. Several of the abnormalities identified on SPECT proved to represent spinal pathology other than spondylolysis, including infection and osteoid osteoma. Although these modalities may present with increased sensitivity in detecting pars lesions compared with plain film, they are not necessarily highly specific for this disorder. Repetitive stress causes local bone remodeling and abnormal uptake of scintigraphic tracer. SPECT has 10 to 20 times more contrast than planar bone scintigraphy; it is more sensitive than radiography and planar bone scans and improves anatomic localization of skeletal lesions without exposing the patient to additional radiation.[2]
Magnetic resonance imaging (MRI) offers advantages in terms of visualizing other types of pathology present in the lumbar spine and may potentially detect pars edema secondary to stress in their clinical course.[27] The lack of ionizing radiation with MRI may also make it a desirable modality for studying pars lesions, especially in the female adolescent population.[28] However, it is worth noting that MRI, like CT, does not assess whether a bony lesion is metabolically active and is less sensitive and specific than scintigraphy or SPECT.
The optimal diagnostic algorithm remains undefined in the current literature. When an acute pars injury is suspected, the recommendation is for an X-ray to be the first diagnostic study, followed by scintigraphy or SPE T. If SPECT is positive, a CT scan should be run after SPECT to confirm the pars lesion suspicion and diagnose the stage of the lesion. If SPECT is negative, pars injury has almost certainly been ruled out as the cause of the low back pain, and MRI can be the next study to search for other possible entities.[16]
Treatment / Management
There is no consensus about the conservative or surgical management of pars injury.
To gain an increased understanding of healing rates and to stratify patients, Morita et al. and Sairyo et al. attempted to assess the relationship between bony healing and the radiographic stage of the pars lesion.[29][30] Their classification assigned the pars lesions into early, progressive, and terminal stages based on either plain radiographs or CT. They found much higher healing rates in early-stage lesions with very little or no healing in terminal-stage defects.[29][30] They defined the early stage as a thin line of bony absorption at the level of the pars, a widening of that line, and small bone fragments within, as suggestive of a progressive stage, while sclerotic changes determined the terminal stage. The healing rate with conservative treatment in the early stage was 73%; it descends to 38.5% in the progressive stage and 0% in the terminal-stage patients.[29] (B2)
Both of these studies, plus the study by Blanda et al., revealed higher healing rates for unilateral pars defects than for bilateral lesions.[8] Sakai et al. reported similar results in their study on 60 pediatric patients; they found a 100% healing rate in the very early stage, 93.8% in the early stage, and 80% in the progressive stage, and did not report results in the terminal patients as there was no conservative treatment attempted in these patients.[31](B2)
Young patients with spondylolysis or low-grade spondylolisthesis generally receive conservative management as their initial treatment.[32] It includes bracing, activity restriction, physical therapy, and pain control.[16][8] (B2)
The treatment regimen of the brace varies widely; it usually consists of 23 hours a day of usage for six months, with a subsequent six-month weaning period and physical therapy. TLSO bracing indications include when an acute pars stress reaction spondylolysis is present, for isthmic spondylolysis, and low-grade spondylolisthesis that has failed to improve with physical therapy.
Physical therapy should be done for six months and include hamstring stretching, pelvic tilts, abdominal strengthening, and close follow-up of low-grade dysplastic lysis as there is a higher chance of progression.
Surgical treatment for spondylolysis is generally only for patients who fail to respond to conservative treatment or those in the terminal stage. Surgery is necessary in about 9 to 15% of cases of spondylolysis and/or low-grade spondylolisthesis.[8][33] Other indications for surgical intervention include progressive slip, intractable pain, development of neurological deficits, and segmental instability associated with pain.[23][18] (B2)
The most common surgical options are direct repair of the pars defect or fusion of the lumbar segment. Direct repair is the preferred surgical treatment as it avoids a decreased range of motion and adjacent segment disease reported in fusion surgery, with rates from 5.2 to 18.5%.[34][35] There are many surgical techniques; Buck´s technique with a single lag screw, Scott´s technique with cerclage wire, Morscher´s technique that utilizes a hook screw fixation, Louis´s fixation technique with a butterfly plate, Tokuhashi´s technique based on pedicle screw hook fixation, and Ulibarri´s fixation technique with pedicle screw rod system.[36][37][38][39][40] (B2)
Of the surgical treatment options listed here, the most favorable option is the pedicle screw hook technique and the pedicle screw rod technique. This success is due to the high success rate, low rate of hardware failure, the lack of required postoperative bracing, and sufficient maintenance of reduction during flexion, extension, torsion, and side bending.[1]
L5-S1 in-situ posterolateral fusion with bone grafting is the recommended approach in patients with L5 spondylolysis or low-grade spondylolisthesis (Meyerding Grade I and II) that have failed nonoperative treatment or demonstrated progression, neurologic deficits, and dysplastic changes with a high propensity for progression. The fusion is possible with or without instrumentation. Postoperative immobilization in a TLSO is a suggested approach, and decompression is indicated if clinical symptoms of stenosis or radiculopathy are present.
L4-S1 posterolateral fusion, with or without reduction, is considered for high-grade isthmic spondylolisthesis (Meyerding Grade III, IV, V). The reduction is extremely controversial, with no accepted guidelines. It may take place with instrumentation or positioning. It is possible to restore sagittal alignment and reduce lumbosacral kyphosis, even though there is a significant risk of complications (8 to 30%), including L5 nerve root injury as the most common complication, sexual dysfunction, or catastrophic neurologic injury. Fusion is usually an instrumented intervention.
Lately, low-intensity pulsed ultrasound (LIPUS), in addition to conservative treatment, appears to be very promising for achieving a higher rate of bony union. LIPUS requires more supporting studies but may become a standard of care in the future.[1] Arima et al. evaluated the use of LIPUS versus conventional conservative treatment in patients with the progressive stage of spondylolysis.[41] The experimental group consisted of 9 adolescent patients that received a combination of LIPUS for 20 minutes every day in addition to conventional conservative treatment. The control group consisted of 10 adolescent patients who received only conventional conservative treatment. The experimental group treated with LIPUS achieved a union rate of 66.7% with a mean treatment time of 3.8 months. The control group treated with conventional conservative treatment achieved a union rate of 10% with a treatment time of 3.8 months.
Differential Diagnosis
The differential diagnosis for back pain is extensive and includes degenerative disease, infection, inflammation, tumors, and trauma.
Pediatric dogma suggests that children presenting with back pain may have serious pathology, including malignancy and infection. However, most instances of back pain are non-specific and self-limiting. The onset of symptoms will help differentiate between acute trauma, chronic overuse injury, and postural and developmental abnormalities.
Abdominal and pelvic pathologies may refer pain to the back; laboratory tests are necessary for evaluating patients presenting with back pain and high suspicion of infection or systemic disease. White cell count, erythrocyte sedimentation rate (ESR), or C reactive protein, blood, and joint cultures are necessary if an infection is suspected.
The following is a list of the most common differential diagnoses:
- Pain Originating from the Posterior Elements of the Spine
Spondylolysis and Spondylolisthesis
Posterior element overuse syndrome: It includes injuries to the ligaments, joint capsules, muscle-tendon units, and facet joints. It is also known as hyperlordotic back pain, spondylotic back pain, or lumbar facet syndrome. Patients will present with extension-related low back pain, weak core muscles, decreased lumbar range of motion, and tight hamstrings with no evidence of spondylolysis. Conservative management with cryotherapy and non-steroidal anti-inflammatory drugs is indicated, including physiotherapy focusing on core strength and hamstring flexibility.
- Sacroiliac joint (SI) Pain
This syndrome may be present in children with insidious onset of low back and buttock pain, worsened by extension, prolonged standing, or sitting. It can result from inflammation or stress fracture of the sacrum or SI joint. Rest, ice, and non-steroidal anti-inflammatory drugs are indicated. A rheumatoid origin of the inflammation must be ruled out.
- Pain Originating from the Anterior Elements of the Spine
Scheuermann disease: This entity, with an incidence rate of 1 to 8%, is the most common cause of an adolescent kyphotic deformity. Both females and males are affected equally, and its etiology is unknown. The main difference between Scheuermann disease and postural kyphosis is that the first one is a fixed kyphosis during hyperextension and flexion of the spine.
Lumbar disk herniation is relatively uncommon in children and adolescents, with a rate of 3.5%. Children complain about low back or leg pain worsened by bending forward or Valsalva (coughing, sneezing).
- Scoliosis
Idiopathic scoliosis demonstrates a Cobb angle above 10 degrees on a standing coronal radiograph. It affects 1 to 3% of the school-age population. However, it is usually painless in this age group.
- Infectious Disorders
Discitis and vertebral osteomyelitis are uncommon entities. Discitis results from a bacterial infection (the most common germen isolated is Staphylococcus aureus) and usually affects the lumbar region, mainly in children under five years old. Children may present with unspecific symptoms, irritability, refusal to bear weight, and back or abdominal pain. Fever is not constant. Vertebral osteomyelitis is yet more uncommon in children, representing only 1 to 2% of all cases of pediatric osteomyelitis.
- Inflammatory Disorders
Spondyloarthropathy includes juvenile idiopathic arthritis, psoriatic arthritis, reactive arthritis, and inflammatory bowel disease. They are more common in boys, and the genetic marker HLA-B27 should be ruled out.
- Pain Amplification Syndromes
These syndromes do not have an identified cause. They provoke severe chronic musculoskeletal pain and sometimes are associated with minor trauma, underlying chronic illnesses, and psychological distress.
- Tumors
Benign and malignant tumors require exclusion in every child presenting with low back pain, even though they are very rare. The physician should look for severe pain and night pain, fever, weight loss, or neurologic symptoms. The most common benign lesions are osteoid osteoma, bone cysts, osteoblastoma, and Langerhans cell histiocytosis. Malignant bone tumors include osteosarcoma, leukemia, Ewing sarcoma, and metastases from rhabdomyosarcoma or neuroblastoma.
Prognosis
Progressive spondylolisthesis is among the primary concerns in patients with pars defects. Overall, the risk of progression is small, running at about 3 to 4%.[12][42] A progressive slip was predominantly noted during the adolescent growth spurt and was associated with the presence of spina bifida occulta in this study.[43] Progression after the age of 20 years is much less common; this is likely due to the ossification of the growth plate. Sietsalo et al. demonstrated an increased tendency to progress with an initial slip greater than 20%.[44]
In low-grade spondylolisthesis, the pelvic incidence is a significant predictor of the risk progression.[45][46] while in high-grade spondylolisthesis, the pelvic incidence is always high, so sacral slope and pelvic tilt became more relevant.[47]
The majority of adolescents return to activities after six months of conservative treatment. Skeletally immature children and adolescents with bilateral spondylolysis or spondylolisthesis require standing lateral radiographs to assess the progression of slippage every 6 to 12 months until reaching skeletal maturity. Adolescents with over 50% slip (Grade 3 or 4) warrant orthopedic referral and possible surgical treatment.
Complications
Complications of this condition, as well as from the postoperative course, appear below.
A chronic pars defect or non-fusion is a possible evolution after the non-operative treatment as the potential for healing decreases. This condition can be asymptomatic or produce chronic low back pain.
Another possible evolution from a spondylolysis is the slippage of the lower vertebral body (spondylolisthesis). This problem can be associated with low back pain and neurologic symptoms.
Complications derived from surgical treatment are:
- Neurologic deficit: L5 deficit resulting in motor weakness has been mentioned with a prevalence of 30% in cases of high-grade spondylolisthesis after reduction; most cases are transitory and resolve completely.
- Isthmus non-union: Represents a failure in the consolidation process. It is more common in patients with progressive or terminal stages.[29][30]
- Progression of slippage
- Hardware failure
Deterrence and Patient Education
As a patient, it is crucial to have a general practitioner consultation in the presence of low back pain, particularly when it results from repetitive sports activities and is not relieved by rest. Pars interarticularis injury is a common cause of low back pain in young people. Prompt recognition and treatment may help improve short- and long-term outcomes and avoid complications from wrong or delayed diagnosis.
Enhancing Healthcare Team Outcomes
The pars interarticularis injury is a common cause of low back pain, especially among young people. It is frequently misdiagnosed and can potentially affect clinical outcomes and patient satisfaction. Thus, an interprofessional team approach is essential to make a prompt and accurate diagnosis. While the orthopedic surgeon usually manages the primary injury, other clinicians and nurses should coordinate the patient's education on lifestyle changes and appropriate exercises. Patients need to stretch before physical activity, maintain healthy body weight, discontinue smoking, and not lead a sedentary life.
Patients with low back pain, especially after repetitive sports activities, require a general practitioner consultation. If the healthcare professional considers this diagnosis a cause of low back pain, referral to a spine specialist is vital. Patients may also have this condition discovered by a chiropractor, who should work collaboratively with orthopedic specialists on a way forward.
Entering a rehabilitation program may be necessary for all patients to restore muscle strength and joint flexibility. Physical therapists need to keep the healthcare team informed regarding progress. The pharmacist should work with the team by educating the patient on alternatives to opiates for pain control, verifying all dosing, and performing medication reconciliation. Nursing can monitor progress at follow-up visits, monitor treatment progress, and note any adverse medication effects. Only with such an interprofessional team approach can the morbidity of this disorder be reduced. [Level 5]
All interprofessional team members must have open communication channels to consult with other team members, especially if they note any changes in the patient's condition that may require intervention. Further, all providers must maintain meticulous records of all interactions and observations of the patient's progress so that everyone on the interprofessional team is operating from the same updated data.
Media
(Click Image to Enlarge)
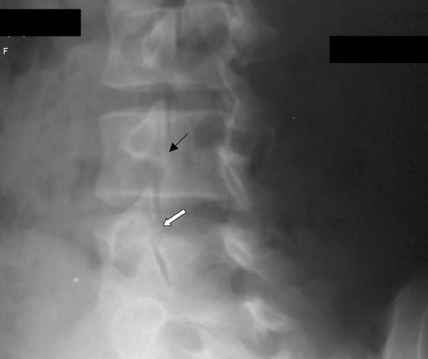
Oblique Plain Radiograph of the Lumbar Spine. The black arrow demonstrates normal pars interarticularis. The white arrow demonstrates lucency in the pars interarticularis, suggestive of fracture. The scottie dog features: transverse process being the nose, the pedicle forming the eye, the inferior articular facet being the front leg, the superior articular facet representing the ear, the pars interarticularis equivalent to the neck of the dog. The "collar" represents the pars fracture.
Contributed by J Taylor Mansfield, DO
References
Gagnet P, Kern K, Andrews K, Elgafy H, Ebraheim N. Spondylolysis and spondylolisthesis: A review of the literature. Journal of orthopaedics. 2018 Jun:15(2):404-407. doi: 10.1016/j.jor.2018.03.008. Epub 2018 Mar 17 [PubMed PMID: 29881164]
Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. Journal of orthopaedic surgery and research. 2010 Mar 3:5():13. doi: 10.1186/1749-799X-5-13. Epub 2010 Mar 3 [PubMed PMID: 20199678]
Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. The Journal of bone and joint surgery. American volume. 1984 Jun:66(5):699-707 [PubMed PMID: 6373773]
Libson E,Bloom RA,Dinari G, Symptomatic and asymptomatic spondylolysis and spondylolisthesis in young adults. International orthopaedics. 1982; [PubMed PMID: 6222997]
Level 2 (mid-level) evidenceWiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clinical orthopaedics and related research. 1976 Jun:(117):23-9 [PubMed PMID: 1277669]
MEYERDING HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. The Journal of the International College of Surgeons. 1956 Nov:26(5 Part 1):566-91 [PubMed PMID: 13367505]
ROCHE MB, ROWE GG. The incidence of separate neural arch and coincident bone variations; a survey of 4,200 skeletons. The Anatomical record. 1951 Feb:109(2):233-52 [PubMed PMID: 14811059]
Level 3 (low-level) evidenceBlanda J,Bethem D,Moats W,Lew M, Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. Journal of spinal disorders. 1993 Oct; [PubMed PMID: 8274809]
Level 2 (mid-level) evidenceRossi F. Spondylolysis, spondylolisthesis and sports. The Journal of sports medicine and physical fitness. 1978 Dec:18(4):317-40 [PubMed PMID: 745379]
Soler T, Calderón C. The prevalence of spondylolysis in the Spanish elite athlete. The American journal of sports medicine. 2000 Jan-Feb:28(1):57-62 [PubMed PMID: 10653544]
Turner RH, Bianco AJ Jr. Spondylolysis and spondylolisthesis in children and teen-agers. The Journal of bone and joint surgery. American volume. 1971 Oct:53(7):1298-306 [PubMed PMID: 4939956]
Danielson BI,Frennered AK,Irstam LK, Radiologic progression of isthmic lumbar spondylolisthesis in young patients. Spine. 1991 Apr; [PubMed PMID: 2047916]
Level 2 (mid-level) evidenceJackson DW, Wiltse LL, Cirincoine RJ. Spondylolysis in the female gymnast. Clinical orthopaedics and related research. 1976 Jun:(117):68-73 [PubMed PMID: 132328]
Hensinger RN. Spondylolysis and spondylolisthesis in children and adolescents. The Journal of bone and joint surgery. American volume. 1989 Aug:71(7):1098-107 [PubMed PMID: 2668295]
Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. The Journal of bone and joint surgery. British volume. 1979 Aug:61-B(3):301-5 [PubMed PMID: 383720]
Level 3 (low-level) evidenceStandaert CJ, Herring SA. Spondylolysis: a critical review. British journal of sports medicine. 2000 Dec:34(6):415-22 [PubMed PMID: 11131228]
Wiltse LL, Widell EH Jr, Jackson DW. Fatigue fracture: the basic lesion is inthmic spondylolisthesis. The Journal of bone and joint surgery. American volume. 1975 Jan:57(1):17-22 [PubMed PMID: 1123367]
Ciullo JV, Jackson DW. Pars interarticularis stress reaction, spondylolysis, and spondylolisthesis in gymnasts. Clinics in sports medicine. 1985 Jan:4(1):95-110 [PubMed PMID: 3967315]
Kayser R, Mahlfeld K, Heyde CE, Grasshoff H, Mellerowicz H. Tight hamstring syndrome and extra- or intraspinal diseases in childhood: a multicenter study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006 Apr:15(4):403-8 [PubMed PMID: 15912349]
Level 2 (mid-level) evidenceAmato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984 Dec:153(3):627-9 [PubMed PMID: 6494460]
Herman MJ, Pizzutillo PD, Cavalier R. Spondylolysis and spondylolisthesis in the child and adolescent athlete. The Orthopedic clinics of North America. 2003 Jul:34(3):461-7, vii [PubMed PMID: 12974495]
Saraste H. Spondylolysis and spondylolisthesis. Acta orthopaedica Scandinavica. Supplementum. 1993:251():84-6 [PubMed PMID: 8451998]
Hanson EC, Shook JE, Wiesseman GJ, Wood VE. Congenital pedicle defects of the axis vertebra. Report of a case. Spine. 1990 Mar:15(3):236-8 [PubMed PMID: 2353264]
Level 3 (low-level) evidenceMicheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Archives of pediatrics & adolescent medicine. 1995 Jan:149(1):15-8 [PubMed PMID: 7827653]
Level 2 (mid-level) evidenceComstock CP, Carragee EJ, O'Sullivan GS. Spondylolisthesis in the Young Athlete. The Physician and sportsmedicine. 1994 Dec:22(12):39-46. doi: 10.1080/00913847.1994.11947717. Epub [PubMed PMID: 29272968]
Micheli LJ. Back injuries in gymnastics. Clinics in sports medicine. 1985 Jan:4(1):85-93 [PubMed PMID: 3155669]
Yamane T, Yoshida T, Mimatsu K. Early diagnosis of lumbar spondylolysis by MRI. The Journal of bone and joint surgery. British volume. 1993 Sep:75(5):764-8 [PubMed PMID: 8376435]
Harvey CJ, Richenberg JL, Saifuddin A, Wolman RL. The radiological investigation of lumbar spondylolysis. Clinical radiology. 1998 Oct:53(10):723-8 [PubMed PMID: 9817088]
Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. The Journal of bone and joint surgery. British volume. 1995 Jul:77(4):620-5 [PubMed PMID: 7615609]
Level 2 (mid-level) evidenceSairyo K, Katoh S, Ikata T, Fujii K, Kajiura K, Goel VK. Development of spondylolytic olisthesis in adolescents. The spine journal : official journal of the North American Spine Society. 2001 May-Jun:1(3):171-5 [PubMed PMID: 14588344]
Level 2 (mid-level) evidenceSakai T, Tezuka F, Yamashita K, Takata Y, Higashino K, Nagamachi A, Sairyo K. Conservative Treatment for Bony Healing in Pediatric Lumbar Spondylolysis. Spine. 2017 Jun 15:42(12):E716-E720. doi: 10.1097/BRS.0000000000001931. Epub [PubMed PMID: 27755499]
Lim MR, Yoon SC, Green DW. Symptomatic spondylolysis: diagnosis and treatment. Current opinion in pediatrics. 2004 Feb:16(1):37-46 [PubMed PMID: 14758112]
Level 3 (low-level) evidenceSteiner ME,Micheli LJ, Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985 Dec; [PubMed PMID: 3914087]
Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004 Sep 1:29(17):1938-44 [PubMed PMID: 15534420]
Level 2 (mid-level) evidenceCheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007 Sep 15:32(20):2253-7 [PubMed PMID: 17873819]
Level 2 (mid-level) evidenceBuck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. The Journal of bone and joint surgery. British volume. 1970 Aug:52(3):432-7 [PubMed PMID: 4916960]
Morscher E, Gerber B, Fasel J. Surgical treatment of spondylolisthesis by bone grafting and direct stabilization of spondylolysis by means of a hook screw. Archives of orthopaedic and traumatic surgery. Archiv fur orthopadische und Unfall-Chirurgie. 1984:103(3):175-8 [PubMed PMID: 6497607]
Louis R. [Pars interarticularis reconstruction of spondylolysis using plates and screws with grafting without arthrodesis. Apropos of 78 cases]. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 1988:74(6):549-57 [PubMed PMID: 3070652]
Level 3 (low-level) evidenceTokuhashi Y, Matsuzaki H. Repair of defects in spondylolysis by segmental pedicular screw hook fixation. A preliminary report. Spine. 1996 Sep 1:21(17):2041-5 [PubMed PMID: 8883209]
Ulibarri JA, Anderson PA, Escarcega T, Mann D, Noonan KJ. Biomechanical and clinical evaluation of a novel technique for surgical repair of spondylolysis in adolescents. Spine. 2006 Aug 15:31(18):2067-72 [PubMed PMID: 16915090]
Arima H, Suzuki Y, Togawa D, Mihara Y, Murata H, Matsuyama Y. Low-intensity pulsed ultrasound is effective for progressive-stage lumbar spondylolysis with MRI high-signal change. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2017 Dec:26(12):3122-3128. doi: 10.1007/s00586-017-5081-z. Epub 2017 Apr 8 [PubMed PMID: 28391380]
Frennered AK, Danielson BI, Nachemson AL. Natural history of symptomatic isthmic low-grade spondylolisthesis in children and adolescents: a seven-year follow-up study. Journal of pediatric orthopedics. 1991 Mar-Apr:11(2):209-13 [PubMed PMID: 2010523]
Blackburne JS, Velikas EP. Spondylolisthesis in children and adolescents. The Journal of bone and joint surgery. British volume. 1977 Nov:59-B(4):490-4 [PubMed PMID: 925059]
Seitsalo S, Osterman K, Hyvãrinen H, Tallroth K, Schlenzka D, Poussa M. Progression of spondylolisthesis in children and adolescents. A long-term follow-up of 272 patients. Spine. 1991 Apr:16(4):417-21 [PubMed PMID: 2047915]
Hanson DS, Bridwell KH, Rhee JM, Lenke LG. Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine. 2002 Sep 15:27(18):2026-9 [PubMed PMID: 12634563]
Level 1 (high-level) evidenceLabelle H, Roussouly P, Berthonnaud E, Transfeldt E, O'Brien M, Chopin D, Hresko T, Dimnet J. Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine. 2004 Sep 15:29(18):2049-54 [PubMed PMID: 15371707]
Level 2 (mid-level) evidenceHresko MT, Labelle H, Roussouly P, Berthonnaud E. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine. 2007 Sep 15:32(20):2208-13 [PubMed PMID: 17873812]
Level 2 (mid-level) evidence