Introduction
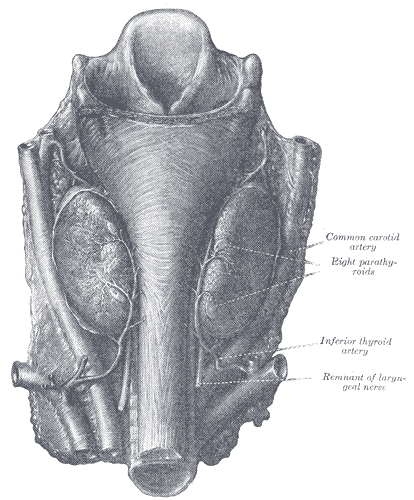
The parathyroid glands are a group of endocrine glands located on the posterior thyroid. These glands are responsible for the secretion of parathyroid hormone (PTH), and play a role in calcium homeostasis. Typically, there are four glands divided into pairs - the superior parathyroid glands and the inferior parathyroid glands. The superior parathyroids generally are located superior to the inferior thyroid artery and posterolaterally to the recurrent laryngeal nerve, bilaterally. The inferior parathyroids are most commonly near the inferior pole of the thyroid and anteromedially to the recurrent laryngeal nerve, bilaterally. Branches of the inferior thyroid artery deliver arterial blood supply. Venous drainage is via the thyroid veins (superior, middle, inferior).[1] The number and location of the parathyroid glands can be variable, with patients presenting with anywhere from three to eight parathyroid glands. Ectopic glands generally appear along the path of embryologic descent, within the carotid sheath, inside the thyroid gland, or within the superior mediastinum.[2]
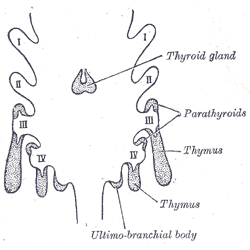
Embryologically, the parathyroid glands derive from the endoderm of the third and fourth pharyngeal pouches. The third pharyngeal pouch gives rise to the inferior parathyroid glands, while the superior parathyroids arise from the fourth pharyngeal pouch. Due to their relatively long course of descent, the final location of the inferior glands is more variable.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
Much of the human head/neck development derives from the framework of the pharyngeal arches, which appear during the fourth to fifth weeks of development. There are a total of six pharyngeal arches, and the spaces between the arches are the pharyngeal clefts. The pharyngeal pouches develop due to lateral migration of the endoderm and present as outpouchings of the primitive pharynx. The first pharyngeal pouch contributes to the formation of the middle ear and the mastoids, while the second pharyngeal pouch forms the epithelial lining of the palatine tonsil. The third and fourth pouches are responsible for the development of the parathyroids, the thymus, and a portion of the thyroid gland. The pharyngeal pouches are derived from the endoderm, while the pharyngeal arches are composed of mesoderm, and the clefts are ectoderm.[3]
Third Pharyngeal Pouch - The third pharyngeal pouch divides into dorsal and ventral wings. The dorsal wing gives rise to the inferior parathyroid gland, while the ventral wing leads to the formation of the thymus. The thymus loses its connection with the pharyngeal wall and descends inferomedially to fuse with the contralateral portion in the anterior mediastinum. In the seventh week of gestation, the inferior parathyroid glands separate from the posterior pharyngeal wall and follow the path of the thymus, to reach their final destination on the posterior thyroid.
Fourth Pharyngeal Pouch - The fourth pharyngeal pouch divides into dorsal and ventral wings. The dorsal wing differentiates into the superior parathyroid gland, while the ventral wing differentiates into the ultimobranchial body. The ultimobranchial bodies fuse with the posterolateral thyroid and give rise to the parafollicular C-cells of the thyroid.[4] In the seventh week of development, the parathyroid glands separate from the pharyngeal wall and attach to the posterior surface of the thyroid. Due to the comparatively longer course, the final location of the inferior parathyroid is more variable, and ectopic parathyroid tissue can be found anywhere along their pathway of descent.
Cellular
The parathyroid glands are composed of two cell types - chief cells and oxyphil cells.[5]
Chief Cells - Chief cells are the functional cells of the parathyroids, responsible for the synthesis and secretion of PTH. During low-calcium states, a calcium-sensitive receptor (CaSR) on the surface of the parathyroid gland activates a G-protein messenger pathway, leading to PTH synthesis/secretion. During hypercalcemic states, this pathway becomes inhibited, leading to decreased PTH synthesis/secretion.
Oxyphil Cells - Oxyphil cells are poorly understood, and their function is unclear. Research has suggested that they produce/secrete PTH in cases of secondary hyperparathyroidism.[6] They are larger than chief cells microscopically and become more abundant with increasing age.
Biochemical
Primary hyperparathyroidism (PHPT), according to recent studies, could be caused by an alteration during the development of the semaphorin3d glycoprotein (Sema3d). The latter regulates the excessive formation of cells of the parathyroid gland. If Sema3d is ineffective, it could be an important cause of PHPT development; glycoprotein works by inhibiting the epidermal growth factor receptor (EGFR)/Erb-B2 (receptor tyrosine kinase 2 or also known as HER2/neu).
Isolated parathyroid aplasia is caused by a mutation in the Glial cells missing (Drosophila) homolog b (GCM2) gene or by a mutation in the SRY-Box Transcription Factor 3 (SOX3) protein.
DiGeorge syndrome comes from a 22q11 chromosome aberration (chromosome 22q11.2123), from a loss of the Tbox transcription factor gene 1 (TBX1), an alteration of chromosome 10p13 containing the nebulette gene (NEBL) gene.
Hypoparathyroidism comes from an alteration of chromosome 8q12.2 with a lack of the chromodomain helicase DNA binding protein type 7 (CDH7) gene and the aberration of the gene encoding the semaphorin 3E protein (SEMA3E). Other variations of hypothyroidism concern an anomaly of chromosome 10p14-15, which will cause a haploinsufficiency of the GATA binding protein-3 gene (GATA3); an anomaly of chromosome 1q42–43 which contains the gene for the tubulin-specific protein chaperone E (TBCE); an aberration of the family with sequence similarity 111, member A (FAM111A) gene for DNA replication. Mutation of chromosome 11p15.3-p15.1 alters the function of the parathyroid hormone, as well as in the chromosomal area p.S23P and p.S23X. Other causes result from a mutation of mitochondrial development.
Molecular Level
Pharyngeal pouch formation begins with lateral migration of the endoderm, and this process becomes stimulated by several fibroblast-growth-factors (FGFs). The predominant factors vary based on the location within the pouches - FGF8 expresses in the anterior region of each pouch, bone-morphogenic-protein-7 (BMP7) expresses in the posterior region, and paired-box-protein-1 (PAX1) expresses in the dorsal area of each pouch. In the second and third pouches, SHH (sonic-hedgehog-signaling-molecule) expresses in the posterior endoderm. Endodermal tissue migration is a key step in the development of the pharyngeal arches, neural crest cell migration, and the differentiation of skeletal structures in the head/neck.[3]
Function
Parathyroid Gland Physiology [7]
The parathyroid glands play a vital role in calcium homeostasis.[8] They contain calcium-sensitive-receptors (CaSRs) that sense the level of serum calcium. In states of hypocalcemia, the glands increase the secretion of PTH to increase serum calcium. Increased serum phosphate, decreased levels of activated Vitamin D, and cases of minor hypomagnesemia also stimulate PTH secretion. Elevated serum calcium, low levels of serum phosphate, and severe hypomagnesemia lead to decreased PTH secretion.
Parathyroid Hormone (PTH) Physiology [9]
The initial synthesis of parathyroid hormone takes place within the parathyroid gland as pre-pro-PTH (a 115-amino-acid polypeptide). It is then cleaved into pro-PTH (90-amino-acids). A second cleavage leads to an 84-amino-acid mature PTH, with a half-life of approximately 3 minutes, allowing for rapid control of serum calcium. PTH exerts its effects primarily through action in the kidneys, bones, and the GI tract.
- PTH Effect in the Kidney - Multiple effects
- Increased calcium absorption in the distal convoluted tubule
- Increased phosphate excretion in the proximal convoluted tubule
- Increased activation of Vitamin D to its active form, calcitriol
- PTH Effect in the Bones - Stimulation of RANKL leading to osteoclast differentiation
- Increased bone resorption - increasing serum calcium and phosphate
- PTH Effect in the Intestines - Indirect effect secondary to increased calcitriol
- Increased calcium absorption in the small intestine
Testing
Ultrasound - Can be used to identify the parathyroid glands on the posterior thyroid. The parathyroids appear as small, oval masses on the posterior thyroid. They are comparatively homogenous and hypoechoic when compared to the thyroid, allowing for the identification of intra-thyroid parathyroid tissue. It is useful to screen for parathyroid adenomas. Normal parathyroid glands are approximately 5 mm in size and weigh 30 to 50 mg, while parathyroid adenomas are 1 to 2 cm in size, and weigh 500 to 1000 mg. Ultrasound has poor sensitivity for the identification of parathyroid hyperplasia.
Technetium-99 (sestamibi) Scan - Technetium-99 (99mTc) is a radiotracer used in nuclear medicine studies.[10] It is also useful for myocardial perfusion scans, detection of breast cancer, detection of multi-drug resistance proteins in certain cancers, and identification of parathyroid tissue. It is particularly useful in the localization of ectopic parathyroid tissue. Technetium-99 is taken up by tissues with high metabolic activity and blood flow. In cases of primary hyperparathyroidism, normal parathyroid tissue undergoes suppression by negative feedback and will have low metabolic activity, while parathyroid adenomas/hyperplasia will not be suppressed and will have high metabolic activity. Therefore, abnormal parathyroid tissue will be demonstrated radiographically. Of note, The thyroid is often suppressed before sestamibi scanning to decrease its metabolic activity and 99mTc uptake.[11]
4D CT Scan - This is a specialized imaging technique with higher sensitivity/specificity for parathyroid adenomas than sestamibi scanning.[12] It has utility in identifying smaller adenomas (as small as 1mm x 6mm) It is particularly useful in false-negative sestamibi scans (identifying the abnormal gland in 80% of cases) and in patients needing reoperation, (identifying 91% of abnormal glands as compared to 45% for sestamibi scanning).[13] 4D CT is the combination of standard CT scanning while evaluating contrast changes over time (the fourth 'dimension'). Often three phases are used, with pre-contrast, arterial phase, and delayed phases. Parathyroid adenomas have low attenuation in the pre-contrast phase, intense/maximal enhancement in the arterial phase, and rapid washout during the delayed phase. One-or-two phase protocols have been developed to reduce radiation exposure.
Pathophysiology
Hyperparathyroidism
- Primary Hyperparathyroidism [14] - This occurs when 1+ parathyroid glands secrete PTH autonomously, regardless of serum calcium levels. This condition is often discovered incidentally on routine labs, with mild elevations in serum calcium. Occasionally, patients can present with symptoms of hypercalcemia.[15] The most common cause is a parathyroid adenoma (approximately 85% of cases), followed by parathyroid hyperplasia (about 15%) and parathyroid carcinoma (less than 1%).[16] Typically, parathyroid adenomas affect a single gland (but can be multiple in rare cases), while parathyroid hyperplasia is multi-glandular. In primary hyperparathyroidism, both serum calcium and PTH are elevated.
- Secondary hyperparathyroidism - occurs most frequently in patients with chronic renal failure. Impaired renal function leads to an inability to reabsorb calcium or excrete phosphate properly. Potentially compounding this is a failure to activate vitamin D, further disrupting calcium homeostasis. Other, less common causes include severe vitamin D deficiency, malabsorption syndromes, and inadequate sun exposure. In secondary hyperparathyroidism, serum PTH is elevated, but serum calcium is low-normal to decreased.
- Tertiary Hyperparathyroidism - Most commonly occurs when hyperplastic parathyroid glands in secondary hyperparathyroidism patients begin to secrete PTH autonomously. This condition is often discovered when the chronic process causing secondary disease has resolved, e.g., in a renal transplant patient with a persistent increase in PTH. Lab results in tertiary hyperparathyroidism mimic primary hyperparathyroidism, with increases in both PTH and serum calcium.
Hypoparathyroidism [17] - This can occur secondary to a variety of causes, most commonly following head/neck surgery, but can also occur due to abnormal parathyroid development, severe hypomagnesemia, autoimmune destruction of the parathyroids and other causes. This condition most commonly presents with symptoms of hypocalcemia, including perioral paresthesias, tetany/muscle cramps, and Chvostek's sign (facial muscle spasm in response to mechanical stimulation).[18][19] In patients receiving bilateral thyroidectomy, low intraoperative PTH levels can be a good predictor for postoperative hypocalcemia.[20]
DiGeorge Syndrome [21] - a development defect caused by a microdeletion at 22q11, which can lead to a failure of the development of the third and fourth pharyngeal pouches, affecting the thymus and parathyroid glands. Poor thymic development leads to abnormal immune system development and deficiencies in T-cells. Improper parathyroid gland development leads to hypocalcemia. Patients with DiGeorge Syndrome can also present with congenital cardiac defects (particularly of the outflow tracts), abnormal facial development, and cleft palates.
Multiple Endocrine Neoplasia - a rare, genetically inherited syndrome, leading to proliferative lesions in various endocrine organs. There are several forms defined by the genes involved, but all have an autosomal dominant inheritance pattern.
- MEN 1 [22] - Aka Wermer syndrome, this syndrome results from a mutation in the MEN1 tumor suppressor gene on chromosome 11. Approximately 90% of cases are inherited, and 10% are due to random mutation. This mutation can lead to lesions of the '3 Ps' - the parathyroids, pancreas, and pituitary. The most common parathyroid lesion is an adenoma. Pancreatic tumors are most commonly gastrinomas, but insulinomas and glucagonomas can also present. Pituitary adenomas most commonly secrete prolactin.
- MEN 2A [23] - Aka Sipple syndrome, this syndrome results from a mutation in the RET oncogene, a tyrosine-kinase localized to chromosome 10q11. MEN 2A can also affect the parathyroids, but more commonly causes parathyroid hyperplasia than adenomas. This syndrome also presents with medullary thyroid carcinoma and pheochromocytoma;100% of patients with (+) RET mutation will develop medullary thyroid cancer in their lifetime, so prophylactic thyroidectomy is the recommendation.
- MEN 2B [23] - This also results from a mutation in the RET oncogene, located at chromosome 10q11. MEN 2B can lead to medullary thyroid carcinoma, pheochromocytomas, similarly to MEN2A. It can also present with marfanoid body habitus and mucosal neuromas of the skin, oral mucosa, and intestines. 100% of patients with (+) RET mutation will develop medullary thyroid cancer in their lifetime, so here again, the recommendation is for prophylactic thyroidectomy.
Clinical Significance
Ectopic Parathyroid Glands - These result from abnormal embryologic development and migration of the parathyroid glands. They occur in approximately 15% of patients and are the most common etiology of persistent/recurrent disease following surgical parathyroidectomy. [24] The inferior parathyroids are more likely to be ectopic, secondary to their prolonged course of descent during development. Ectopic superior parathyroids are most common in the tracheoesophageal groove (approximately 45%), retro-esophageal area (approximately 20%), or posterior mediastinum (approximately 15%). Ectopic inferior parathyroid glands most commonly present within the thymus (approximately 30%), the anterior mediastinum (approximately 20%), within the thyroid (approximately 20%), or within the thyrothymic ligament (approximately 15%). [25] Mediastinal ectopic glands >6cm below the superior clavicle will likely require a thoracic approach, but those less than 6 cm can undergo successful resection through a cervical approach.[26] Other locations for ectopic tissue have been described, including a case of an adenoma anterior to the pericardium or within the sternohyoid muscles in a patient with MEN1.[27][28] Some patients present with more than four parathyroid glands, often as a result of fragmentation during development. In a case study of patients with parathyroid hyperplasia who underwent surgical resection, 46% of patients possessed ectopic glands, supernumerary glands, or both.[29]
Parathyroid Surgery
- Overview - Surgery is performed to remove abnormal parathyroid tissue while leaving sufficient normal tissue behind to maintain normal calcium homeostasis. Parathyroid adenomas treatment is by resection of the abnormal gland(s). Parathyroid hyperplasia is manageable with total resection with parathyroid autotransplantation or a subtotal (3.5 glands) resection in parathyroid hyperplasia.[30] Cervical thymectomy can be performed in parathyroid hyperplasia, as well, to remove additional parathyroid tissue. In cases of parathyroid carcinoma, the tumor is resected, along with the ipsilateral lobe of the thyroid, adjacent soft tissue, and regional lymph nodes.
- Guidelines for Performing Surgery - The National Institute of Health (NIH) recommends parathyroidectomy in all patients with symptomatic hyperparathyroidism and select patients with asymptomatic disease. These criteria include patients under 50-years-old, those with serum Ca greater than 1 mg/dL above the upper-limit-of-normal, urinary calcium excretion greater than 400 mg/24 hours, a 30% reduction in creatinine clearance and those with a DEXA-scan below 2.5. Studies have shown that compliance with the NIH guidelines is low, but age, serum calcium, and hypercalciuria were the most common indications for surgical intervention. Patients with only one criterion received surgery, approximately 33% of the time, while patients meeting 3+ criteria underwent surgery 82% of the time.[31]
- Open Neck Exploration - The old gold standard for parathyroid surgery, this procedure involved bilateral exploration and visualization of all four parathyroid glands. As radiographic localization has improved, and most cases of hyperparathyroidism result from a single adenoma (about 85%), open exploration is becoming less common.
- Minimally Invasive Parathyroidectomy [32] - An increasingly popular procedure, due to a lower complication rate, improved cosmetic outcomes, decreased time-under-anesthesia, and shorter hospital stays. Minimally-invasive procedures require a localization study (99mTC vs. 4D CT) to identify abnormal tissue. Minimally invasive approaches are contraindicated in patients suspected to have parathyroid hyperplasia (multiglandular disease), parathyroid carcinoma or concomitant thyroid disease (requiring radical resection), and previous neck surgery/irradiation (increased difficulty of the procedure).
- Intraoperative PTH Monitoring (ioPTH) - Rapid PTH is used to confirm the complete removal of hyperfunctioning parathyroid tissue. Serum PTH is drawn preoperatively, and at 0, 5, and 10 minutes post-tissue resection. Per the Miami criteria, a decrease of PTH over 50% from preoperative levels at 10 minutes is considered adequate resection. ioPTH increases the detection of multiglandular disease, minimizing the need for recurrent operations. [33] However, localization studies and ioPTH are no replacement for strong comprehension of the anatomy/embryology of the parathyroid glands.[34]
- Complications - Complications of parathyroid surgery are infrequent, and even more so in minimally-invasive surgery (3.1% for open, 1.2% for minimally invasive).
- Transient Hypoparathyroidism (50%) - Seen commonly, this occurs because the resected/abnormal parathyroid tissue has suppressed the remaining/normal parathyroid tissue. Eventually, the normal glands achieve calcium homeostasis, and hypoparathyroidism/hypocalcemia resolves.
- Recurrent Laryngeal Nerve Injury (10 to 15%) - Unilateral injury can lead to voice hoarseness, while bilateral injury can lead to airway occlusion. Damage can be transient in cases of nerve compression or stretching, or permanent if the nerve is transected or suffers sufficient thermal injury. In a 2017 study, 10.6% of thyroid/parathyroid surgeries led to transient recurrent laryngeal nerve injuries, while 1.1% of surgical patients suffered permanent injury.[35]
- Persistent Hypercalcemia (1 to 5%) - Generally, this is due to a failure to identify all adenomas or resect all of the hyperplastic tissue. This complication has become less common with increasing usage of localization studies and ioPTH.
- Permanent Hypoparathyroidism (less than 1%) - Most commonly seen in parathyroid hyperplasia, where all the parathyroid tissue has been removed.
Media
(Click Image to Enlarge)
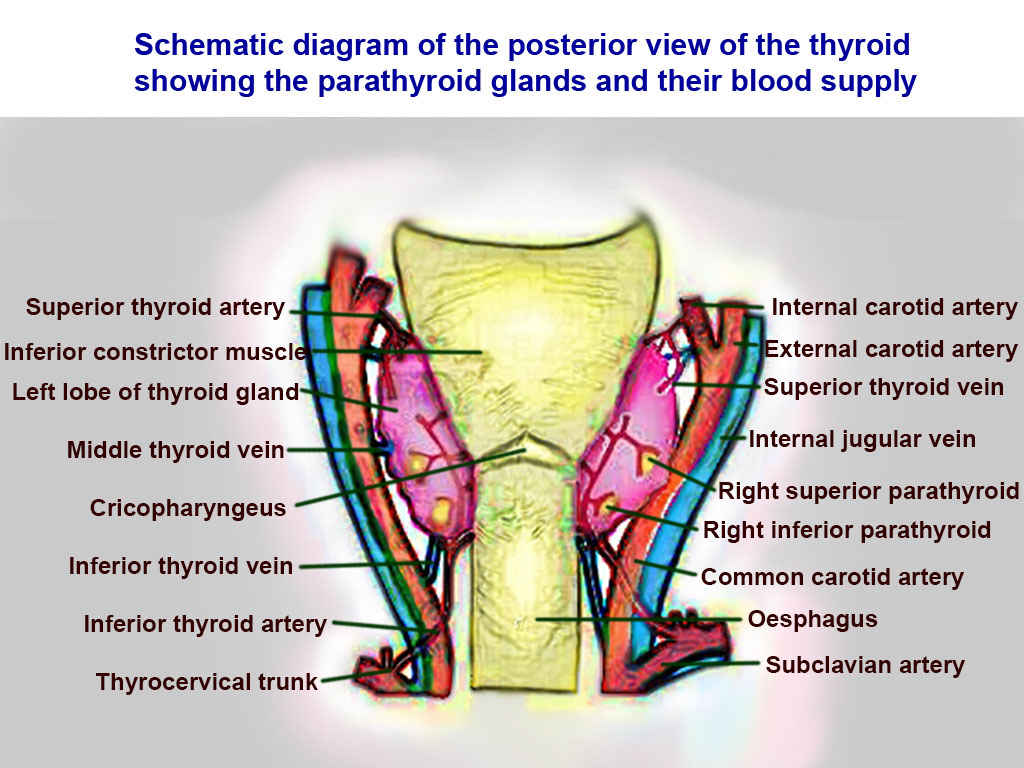
Thyroid Arteries, Veins, and Muscles. Posterior view of thyroid includes superior thyroid, inferior constrictor, left lobe of thyroid, middle thyroid vein, cricopharyngeaus, inferior thyroid vein, inferior thyroid vein, inferior thyroid artery, thyrocervical trunk, internal carotid, external carotid, superior thyroid, internal jugular, right superior parathyroid, right inferior parathyroid, common carotid, oesphagus, and subclavian.
Contributed by T Silappathikaram
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Ilahi A, Muco E, Ilahi TB. Anatomy, Head and Neck, Parathyroid. StatPearls. 2024 Jan:(): [PubMed PMID: 30725888]
Georgakopoulos B, Al Khalili Y. Anatomy, Head and Neck, Parathyroid, Ectopic Glands. StatPearls. 2023 Jan:(): [PubMed PMID: 31082016]
Casale J, Giwa AO. Embryology, Branchial Arches. StatPearls. 2023 Jan:(): [PubMed PMID: 30860722]
Rosen RD, Sapra A. Embryology, Thyroid. StatPearls. 2023 Jan:(): [PubMed PMID: 31869075]
Brown MB, Limaiem F. Histology, Parathyroid Gland. StatPearls. 2023 Jan:(): [PubMed PMID: 31536203]
Tanaka Y, Funahashi H, Imai T, Seo H, Tominaga Y, Takagi H. Oxyphil cell function in secondary parathyroid hyperplasia. Nephron. 1996:73(4):580-6 [PubMed PMID: 8856255]
Level 3 (low-level) evidenceLofrese JJ, Basit H, Lappin SL. Physiology, Parathyroid. StatPearls. 2023 Jan:(): [PubMed PMID: 29494116]
Yu E, Sharma S. Physiology, Calcium. StatPearls. 2024 Jan:(): [PubMed PMID: 29489276]
Khan M, Jose A, Sharma S. Physiology, Parathyroid Hormone. StatPearls. 2024 Jan:(): [PubMed PMID: 29763115]
Rizk TH, Nagalli S. Technetium (99mTc) Sestamibi. StatPearls. 2023 Jan:(): [PubMed PMID: 31985941]
Blanco I, Carril JM, Banzo I, Quirce R, Gutierrez C, Uriarte I, Montero A, Vallina NK. Double-phase Tc-99m sestamibi scintigraphy in the preoperative location of lesions causing hyperparathyroidism. Clinical nuclear medicine. 1998 May:23(5):291-7 [PubMed PMID: 9596153]
Bann DV, Zacharia T, Goldenberg D, Goyal N. Parathyroid localization using 4D-computed tomography. Ear, nose, & throat journal. 2015 Apr-May:94(4-5):E55-7 [PubMed PMID: 25923289]
Brown SJ, Lee JC, Christie J, Maher R, Sidhu SB, Sywak MS, Delbridge LW. Four-dimensional computed tomography for parathyroid localization: a new imaging modality. ANZ journal of surgery. 2015 Jun:85(6):483-7. doi: 10.1111/ans.12571. Epub 2014 Mar 27 [PubMed PMID: 24674300]
Level 2 (mid-level) evidencePokhrel B, Leslie SW, Levine SN. Primary Hyperparathyroidism. StatPearls. 2024 Jan:(): [PubMed PMID: 28722924]
Sadiq NM, Naganathan S, Badireddy M. Hypercalcemia. StatPearls. 2024 Jan:(): [PubMed PMID: 28613465]
Wolfe SA, Sharma S. Parathyroid Adenoma. StatPearls. 2023 Jan:(): [PubMed PMID: 29939647]
Hans SK, Levine SN. Hypoparathyroidism. StatPearls. 2024 Jan:(): [PubMed PMID: 28722928]
Goyal A, Anastasopoulou C, Ngu M, Singh S. Hypocalcemia. StatPearls. 2024 Jan:(): [PubMed PMID: 28613662]
Omerovic S, M Das J. Chvostek Sign. StatPearls. 2023 Jan:(): [PubMed PMID: 31194466]
Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002 May:131(5):515-20 [PubMed PMID: 12019404]
Lackey AE, Muzio MR. DiGeorge Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 31747205]
Singh G, Mulji NJ, Jialal I. Multiple Endocrine Neoplasia Type 1. StatPearls. 2024 Jan:(): [PubMed PMID: 30725665]
Yasir M, Mulji NJ, Kasi A. Multiple Endocrine Neoplasias Type 2. StatPearls. 2024 Jan:(): [PubMed PMID: 30085596]
Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2012 Nov:120(10):604-10. doi: 10.1055/s-0032-1327628. Epub 2012 Nov 22 [PubMed PMID: 23174995]
Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. American journal of surgery. 2006 Mar:191(3):418-23 [PubMed PMID: 16490559]
Callender GG, Grubbs EG, Vu T, Hofstetter WL, Fleming JB, Woodburn KL, Lee JE, Evans DB, Perrier ND. The fallen one: the inferior parathyroid gland that descends into the mediastinum. Journal of the American College of Surgeons. 2009 May:208(5):887-93; discussion 893-5. doi: 10.1016/j.jamcollsurg.2009.01.032. Epub [PubMed PMID: 19476855]
Level 2 (mid-level) evidencePatrinos A, Zarokosta M, Piperos T, Tsiaoussis J, Noussios G, Mariolis-Sapsakos T. An anatomic aberration and a surgical challenge: Mediastinal parathyroid adenoma anterior the pericardium. A case report. International journal of surgery case reports. 2019:58():153-156. doi: 10.1016/j.ijscr.2019.04.005. Epub 2019 Apr 6 [PubMed PMID: 31048210]
Level 3 (low-level) evidenceMiura D. Ectopic parathyroid tumor in the sternohyoid muscles: supernumerary gland in a patient with MEN type 1. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005 Aug:20(8):1478-9 [PubMed PMID: 16007345]
Level 3 (low-level) evidenceLiechty RD, Weil R 3rd. Parathyroid anatomy in hyperplasia. Archives of surgery (Chicago, Ill. : 1960). 1992 Jul:127(7):813-5; discussion 815-6 [PubMed PMID: 1524481]
Baumann DS, Wells SA Jr. Parathyroid autotransplantation. Surgery. 1993 Feb:113(2):130-3 [PubMed PMID: 8430361]
Kuo EJ, Al-Alusi MA, Du L, Shieh A, Livhits MJ, Leung AM, Yeh MW. Surgery for Primary Hyperparathyroidism: Adherence to Consensus Guidelines in an Academic Health System. Annals of surgery. 2019 Jan:269(1):158-162. doi: 10.1097/SLA.0000000000002474. Epub [PubMed PMID: 28806302]
Level 3 (low-level) evidenceWolfe SA, Fingeret A. Parathyroid Minimally Invasive Surgery. StatPearls. 2024 Jan:(): [PubMed PMID: 29939611]
Dobrinja C, Santandrea G, Giacca M, Stenner E, Ruscio M, de Manzini N. Effectiveness of Intraoperative Parathyroid Monitoring (ioPTH) in predicting a multiglandular or malignant parathyroid disease. International journal of surgery (London, England). 2017 May:41 Suppl 1():S26-S33. doi: 10.1016/j.ijsu.2017.02.063. Epub [PubMed PMID: 28506410]
Yeung M. Parathyroidectomy Without the Utilisation of iPTH: The Gold Standard is Still a Good Operation-How Understanding the Anatomy and a Simple US Can Help. World journal of surgery. 2020 Feb:44(2):622-624. doi: 10.1007/s00268-019-05217-2. Epub [PubMed PMID: 31602517]
Level 3 (low-level) evidenceJoliat GR, Guarnero V, Demartines N, Schweizer V, Matter M. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Incidence and postoperative evolution assessment. Medicine. 2017 Apr:96(17):e6674. doi: 10.1097/MD.0000000000006674. Epub [PubMed PMID: 28445266]