Introduction
Ascites, characterized by abnormal fluid accumulation in the peritoneal cavity, often emerges as a grim harbinger of underlying liver cirrhosis, a condition with a daunting prognosis.[1] Paracentesis, a pivotal medical procedure, is the key to understanding and managing this complex condition. Diagnostic paracentesis provides a window into the origins of ascites, enabling healthcare professionals to pinpoint its underlying cause and rule out peritoneal fluid infection. In contrast, therapeutic paracentesis is a powerful tool for alleviating the distressing symptoms associated with ascites by safely removing substantial volumes of ascitic fluid.
This review addresses the indications, contraindications, and potential complications of paracentesis, providing invaluable insights for healthcare practitioners. Additionally, it underscores the crucial role of an interprofessional team in managing patients with ascites, as early diagnosis and intervention can significantly impact patient outcomes. In a world where liver cirrhosis remains a leading cause of ascites, understanding the nuances of paracentesis becomes an essential skill for healthcare professionals.
Paracentesis is a procedure performed in patients with ascites, during which a needle is inserted into the peritoneal cavity to obtain ascitic fluid.[2] The removal and testing of the ascitic fluid to diagnose the etiology of ascites or to rule out an infection of peritoneal fluid is called diagnostic paracentesis. Therapeutic paracentesis refers to removing large quantities of ascitic fluid to treat the patient's symptoms caused by ascites.
The most common cause of ascites is cirrhosis of the liver.[3] Patients who develop ascites due to liver cirrhosis have an estimated one-year mortality rate of 20% compared to a one-year mortality rate of 7% in patients with cirrhosis and without the development of ascites.[4] Therefore, the ascitic fluid should be sampled in all patients with new-onset ascites.
Ascites can be of 2 types: exudative and transudative. Regarding the differentiation between transudate and exudate, the preferred way to distinguish ascites is the serum-ascitic albumin gradient (SAAG).[5] The SAAG is directly related to portal pressure. The SAAG level greater than or equal to 1.1 g/dL indicates portal hypertension and transudative ascites. If this level is less than 1.1 g/dL, it means exudative ascites. The causes of transudative ascites include the following:[6]
- Hepatic cirrhosis
- Heart failure
- Alcoholic hepatitis
- Fulminant hepatic failure
- Nephrotic syndrome
- Portal vein thrombosis
The causes of exudative ascites include the following:
- Peritoneal carcinomatosis
- Pancreatitis
- Peritonitis
- Ischemic colitis
- Intestinal obstruction
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Paracentesis can be performed in a lateral decubitus or supine position, with or without imaging guidance. When performing a paracentesis without imaging guidance, it is recommended to use the left lower quadrant of the abdominal wall as the entry point for the needle. This location is considered the safest and most favorable due to the thinner abdominal wall and deeper pocket of fluid, according to a study conducted by Sakai H et al.[7]
The following are some essential anatomical considerations that should be taken into account while performing paracentesis:
- Surgical scars
- Spleen
- Inferior epigastric arteries
- Cecum - if performing paracentesis in the right lower quadrant
The ascitic fluid level is percussed to perform a paracentesis, and a needle is inserted in the midline or lower quadrant (lateral to rectus abdominis muscle, 2 to 4 cm superomedial to the anterior superior iliac spine).[8][2] This approach avoids puncture of the inferior epigastric arteries. When inserting the needle, the healthcare provider must avoid visible surface veins or surgical scars. The needle should be inserted at a 45-degree angle or using the z-tracking technique to minimize the chance of an ascites fluid leak.
Indications
Diagnostic paracentesis can be performed in the following situations:
- Rule out spontaneous bacterial peritonitis in patients with known ascites presenting with concerning symptoms such as abdominal pain, fever, gastrointestinal bleeding, worsening encephalopathy, new or worsening renal or liver failure, hypotension, or other signs of infection or sepsis
- Identify the etiology of new-onset ascites. Fluid evaluation helps determine etiology, distinguish between transudate and exudate, detect cancerous cells, or address other possibilities.
Therapeutic paracentesis is helpful when alleviating abdominal discomfort or respiratory distress in hemodynamically stable patients with tense ascites or ascites refractory to diuretics.[8][2]
Large-volume paracentesis (LVP) is required in patients with refractory ascites. Bureau et al described a low-flow pump system that can move the fluid from the peritoneal cavity into the urinary bladder, which is removed through micturition.[9] This demonstrated improved quality of life and reduced need for repeated LVP in patients with ascites.
Patients with abdominal compartment syndrome generally require a laparotomy for emergency surgical decompression. However, LVP can help reduce intra-abdominal pressure in a patient with tense ascites that may lead to ACS.[10] Advanced hepatic cirrhosis may cause moderate-to-severe ascites, leading to impaired respiration. Wittmer et al reported the benefits of paracentesis in 30 patients with liver cirrhosis and ascites.[11] They reported improvement in these patients' abdominal breathing patterns and ventilatory variables. Paracentesis also led to improvement in thoracoabdominal mobility and a reduction in dyspnea and fatigue, causing increased peripheral oxygen saturation.
Contraindications
There are few absolute contraindications for paracentesis.[12] Parcentesis is absolutely contraindicated in the following:
- Disseminated intravascular coagulation
- An acute abdomen requiring surgery
The relative contraindications of paracentesis include the following:
- Pregnancy
- Organomegaly
- Ileus
- Intestinal obstruction
- Distended bladder
- Clotting derangements (ie, severe thrombocytopenia where platelets are less than 20 × 103/μL and an international normalized ratio (INR) more than 2.0)
Coagulopathy and thrombocytopenia (common in cirrhotic patients) are not absolute contraindications, as the incidence of bleeding complications from the procedure is very low.[13] Those with severe thrombocytopenia should receive platelets before the procedure, and those with an increased INR should receive fresh-frozen plasma (FFP). One method often employed in the case of an elevated INR is to administer one unit of FFP before the procedure and the second unit during the procedure. In patients with no prior history and without clinical evidence of active bleeding, tests such as prothrombin time, activated partial thromboplastin time, and platelet count may not be required before the procedure.[13]
During the procedure, providers should avoid passing the needle/catheter through sites of skin infection, surgical scars, visibly engorged abdominal wall vessels, or abdominal wall hematomas.
Equipment
Prepackaged paracentesis kits with plastic sheath cannulas attached to a syringe and a stopcock are available. Alternatively, traditional large-bore intravenous (IV) catheters or 18-gauge to 20-gauge standard or spinal needles can be used. These can be attached to a syringe for aspiration and IV tubing for fluid drainage. If these prepackaged kits are not available, one will need the following:
- Sterile gloves
- Antiseptic swab sticks
- Scalpel, No. 11 blade
- Sterile drapes/towels
- Chlorhexidine or betadine
- Lidocaine 1%, 5-mL ampule
- Two injection needles, 22-gauge
- Catheter, 8 French, over 18-gauge × 7.5-inch (19-cm) needle with 3-way stopcock and self-sealing valve
- Injection needle, 25-gauge
- Introducer needle, 20-gauge
- 20 ml or 60 ml syringe to collect a sample of fluid
- Tubing set with roller clamp
- Drainage bag or vacuum container
- Gauze, 4 × 4 in. (10 × 10 cm)
- Three specimen vials or collection bottles (hematology, chemistry, and microbiology sample tubes plus blood culture bottles)[12]
Preparation
Consent
Explain the procedure, risks, benefits, complications, and alternate options to the patient or their representative, and obtain signed informed consent.
Portal of Entry
The preferred site for paracentesis is the right or left lower quadrant of the abdomen lateral to the rectus sheath and superior to the anterior superior iliac spine (ASIS). The 2 recommended areas of entry for paracentesis are the following:
- 2 cm below the umbilicus through the linea alba in the midline
- 5 cm superior and medial to ASIS on either side
Positioning
The lateral decubitus position is preferred because air-filled loops of the bowel float in a distended abdominal cavity. However, patients with severe ascites may need to be positioned supine. Placing the patient in the lateral decubitus position can aid in identifying fluid pockets in patients with lower fluid volumes. Ask the patient to empty their bladder before starting the procedure.
Imaging
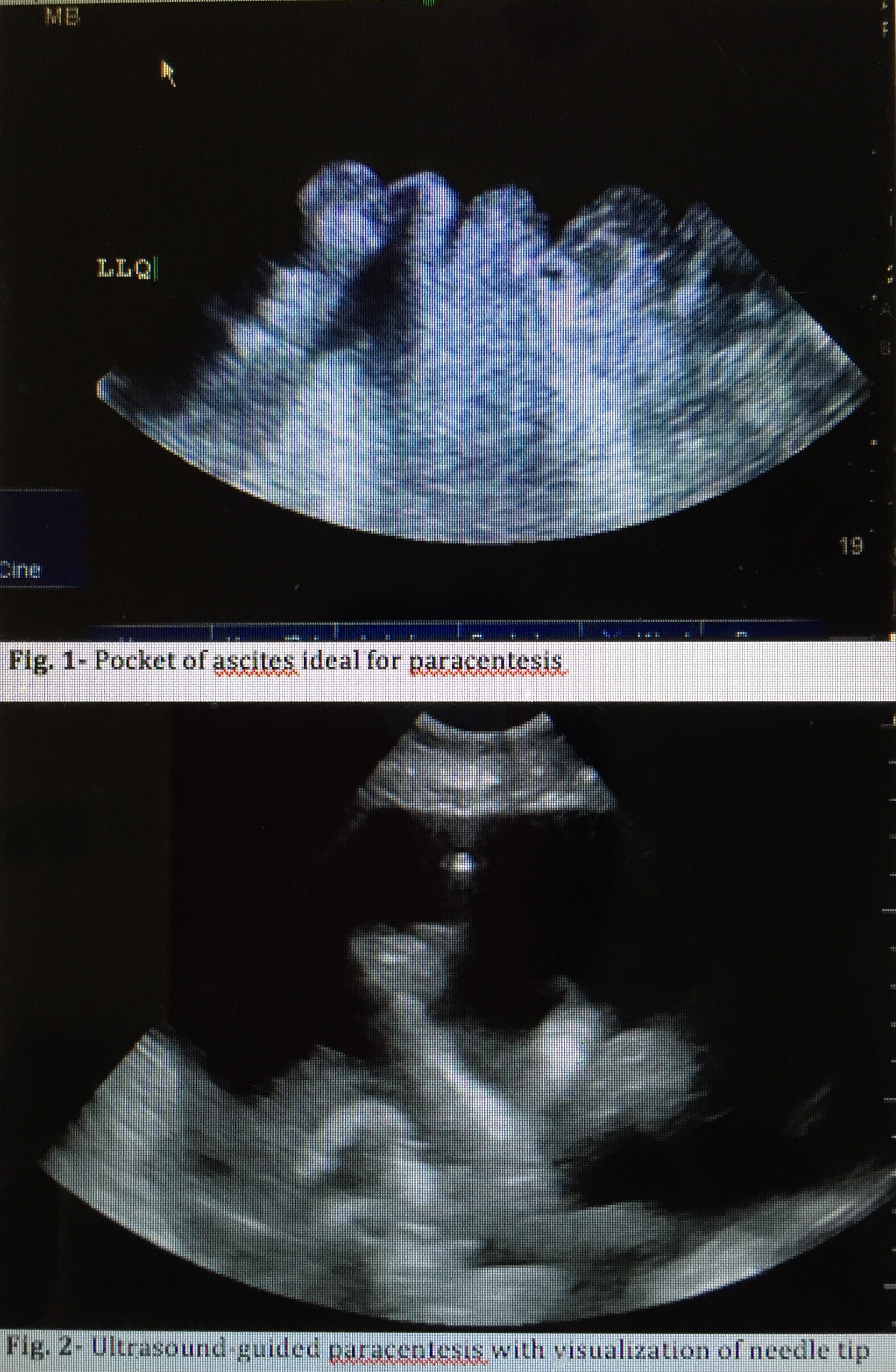
Bedside ultrasound guidance is recommended to identify an appropriate location for the procedure.[14] Ultrasound can confirm the presence of fluid (see Image. Ultrasound for Paracentesis Uses. Fig 1) and identify an area with sufficient fluid for aspiration, thereby decreasing the incidence of unsuccessful aspiration and complications. Ultrasound increases the success rate of paracentesis and helps prevent an unnecessary invasive procedure in some patients.[15] The procedure can be performed either after marking the insertion site or in real-time by advancing the needle under direct ultrasound guidance (see Image. Ultrasound for Paracentesis Uses. Fig 2). Performing an ultrasound also helps the provider avoid small bowel adhesions or a distended urinary bladder below the entry point. Avoiding areas of prominent veins (caput medusae), scar tissue, and infected skin is critical to minimize complications.
Technique or Treatment
Historically, paracentesis under ultrasonographic guidance has been used for hospital palliative care. However, some patients may have difficulty reaching the hospital. Home-based palliative paracentesis (HBPP) is a safe, effective, and convenient option for such patients. This was reported by Ota et al. in a case series of patients with ascites.[16] Home-based abdominal paracentesis in refractory congestive heart failure (CHF) cases is a better alternative to periodic percutaneous paracentesis. A study by Kunin et al demonstrated that it improved symptoms in patients on peritoneal dialysis without needing peritoneal exchanges for fluid.[17]
Before beginning the procedure, ensure the patient's urinary bladder is empty. Prepare and drape the patient in a sterile fashion. Cleanse the skin with an antiseptic solution. Administer local anesthesia to the skin and soft tissue (down to the peritoneum) at the planned needle or catheter insertion site. Use a 25-gauge needle and the 5 ml syringe to raise a small skin wheal with lidocaine around the skin entry site. Insert the needle or IV catheter attached to a syringe or the prepackaged catheter directly perpendicular to the skin or use the z-track method, which is thought to decrease the chance of fluid leakage after the procedure. This method entails puncturing and pulling the skin caudally before advancing the needle through the soft tissue and peritoneum. If using a catheter kit, making a small nick in the skin using an 11-blade scalpel is recommended to advance the catheter through the skin and soft tissue smoothly.
Apply negative pressure to the syringe during needle or catheter insertion until a loss of resistance is felt and a steady flow of ascitic fluid is obtained. This is paramount to detect unwanted entry into a vessel or other structure rapidly. Advance the catheter over the needle into the peritoneal cavity. After collecting sufficient fluid in the syringe for fluid analysis, either remove the needle if performing a diagnostic tap or connect the collecting tubing to it or the catheter's stopcock to drain larger volumes of fluid into a vacuum container, plastic canister, or drainage bag. After draining the desired amount of fluid, remove the catheter and hold pressure to stop bleeding from the insertion site.[8][12]
Kelil et al. reported that wall suction and plastic canisters for drainage and collection of fluid during therapeutic paracentesis were safe alternatives to evacuated glass bottles. They also reduced per-procedure costs.[18]
Peritoneal Fluid Analysis
Send ascitic fluid for laboratory testing, including cell count with differential, Gram stain, and fluid culture. Placing some fluid into bacterial culture bottles can help to increase culture sensitivity.[2] Spontaneous bacterial peritonitis is diagnosed when the absolute neutrophil count (PMN) is 250 cells/mm3 or more.[2][12] This is calculated by multiplying the number of white cells by the percentage of neutrophils reported in the differential. Empiric antibiotics, typically a third-generation cephalosporin or a fluoroquinolone, should be started in patients with ascites and a high suspicion for spontaneous bacterial peritonitis regardless of the absolute neutrophil count or in patients with an absolute neutrophil count above the cut-off range.
Additional tests that can aid in inpatient management include albumin, total protein, lactate dehydrogenase (LDH), glucose, cytology, and tumor markers. Albumin, in particular, can be used to calculate SAAG, which can assist in determining the etiology of ascites by classifying it as either exudative or transudative. SAAG helps identify the presence of portal hypertension. It is calculated by subtracting the ascitic fluid albumin from the serum albumin obtained on the same day. SAAG greater than 1.1 indicates a high probability of portal hypertension. SAAG less than 1.1 rules out portal hypertension.[2][19]
Complications
Paracentesis is a safe procedure; however possible complications include the following:[20]
- Persistent leakage of ascitic fluid at the needle insertion site. This can often be addressed with a single skin suture.
- Abdominal wall hematoma or bleeding
- Wound infection
- Perforation of surrounding vessels or viscera (extremely rare)
- Hypotension after large volume fluid removal (more than 5 L to 6 L). Albumin is often administered after removing more than 5 L of fluid to prevent this complication.[12][21]
- Spontaneous hemoperitoneum
- Catheter laceration and loss in the abdominal cavity
- Hepatorenal syndrome[22]
- Subcutaneous effusion due to ascitic fluid leakage[23]
Clinical Significance
The development of fluid in the peritoneal space, known as ascites, can occur due to many different disease states. Performing a paracentesis will help determine the etiology of a patient's ascites. Draining the peritoneal fluid may help identify infection, causes of liver disease, or portal hypertension and relieve symptoms by removing a large volume of fluid. Using bedside ultrasound to identify an ideal pocket of fluid will increase the likelihood of a successful procedure.
According to a retrospective study of 97 patients, early paracentesis can decrease mortality rates in cases of SBP.[24] The study showed that emergency physicians often ordered early paracentesis.
Enhancing Healthcare Team Outcomes
Paracentesis is a relatively simple procedure performed at the bedside with or without ultrasound guidance to remove ascitic fluid from a patient's abdomen. The procedure is often performed by the internist, emergency department physician, radiologist, general surgeon, or intensivist. The procedure is either performed for diagnostic or therapeutic reasons. When performed as a diagnostic tool, it can help to identify certain conditions the patient may have. When performed for therapeutic reasons, it can quickly relieve symptoms. Unfortunately, ascites often recur and require multiple paracenteses, significantly impacting a person's quality of life.
Patients need close monitoring by nursing during and after the procedure, as it can be associated with hypotension, tachycardia, hemorrhage, fluid leakage, infection, and bowel perforation.[25][26] They can then communicate any untoward signs or symptoms to the physician so appropriate action can be taken. A pharmacist can ensure that all medications used during the procedure are accurately dosed. Close communication between the interprofessional team is vital to ensure good outcomes.
Media
(Click Image to Enlarge)
References
Ong JP. Paracentesis. The American journal of gastroenterology. 2006 Sep:101(9):1954-5 [PubMed PMID: 16968501]
Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology (Baltimore, Md.). 2009 Jun:49(6):2087-107. doi: 10.1002/hep.22853. Epub [PubMed PMID: 19475696]
Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021 Jan:70(1):9-29. doi: 10.1136/gutjnl-2020-321790. Epub 2020 Oct 16 [PubMed PMID: 33067334]
Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Alimentary pharmacology & therapeutics. 2010 Dec:32(11-12):1343-50. doi: 10.1111/j.1365-2036.2010.04473.x. Epub 2010 Oct 4 [PubMed PMID: 21050236]
Level 2 (mid-level) evidenceLichoska-Josifovikj F, Grivceva-Stardelova K, Todorovska B, Andreevski V, Nikolov F, Adem D. THE VALUE OF SERUM-ASCITES ALBUMIN GRADIENT AS A PREDICTOR OF SPONTANEOUS BACTERIAL PERITONITIS IN PATIENTS WITH LIVER CIRRHOSIS AND ASCITES. Georgian medical news. 2022 Sep:(330):23-25 [PubMed PMID: 36427835]
Angeleri A, Rocher A, Caracciolo B, Pandolfo M, Palaoro L, Perazzi B. New Biochemical Parameters in the Differential Diagnosis of Ascitic Fluids. Gastroenterology research. 2016 Feb:9(1):17-21 [PubMed PMID: 27785319]
Sakai H, Sheer TA, Mendler MH, Runyon BA. Choosing the location for non-image guided abdominal paracentesis. Liver international : official journal of the International Association for the Study of the Liver. 2005 Oct:25(5):984-6 [PubMed PMID: 16162157]
Thomsen TW, Shaffer RW, White B, Setnik GS. Videos in clinical medicine. Paracentesis. The New England journal of medicine. 2006 Nov 9:355(19):e21 [PubMed PMID: 17093242]
Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rosi S, MacDonald S, Malago M, Stepanova M, Younossi ZM, Trepte C, Watson R, Borisenko O, Sun S, Inhaber N, Jalan R. Alfapump® system vs. large volume paracentesis for refractory ascites: A multicenter randomized controlled study. Journal of hepatology. 2017 Nov:67(5):940-949. doi: 10.1016/j.jhep.2017.06.010. Epub 2017 Jun 21 [PubMed PMID: 28645737]
Level 1 (high-level) evidenceAllen R, Sarani B. Evaluation and management of intraabdominal hypertension. Current opinion in critical care. 2020 Apr:26(2):192-196. doi: 10.1097/MCC.0000000000000701. Epub [PubMed PMID: 32004192]
Level 3 (low-level) evidenceWittmer VL, Lima RT, Maia MC, Duarte H, Paro FM. RESPIRATORY AND SYMPTOMATIC IMPACT OF ASCITES RELIEF BY PARACENTESIS IN PATIENTS WITH HEPATIC CIRRHOSIS. Arquivos de gastroenterologia. 2020 Jan-Mar:57(1):64-68. doi: 10.1590/S0004-2803.202000000-11. Epub [PubMed PMID: 32294737]
McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Digestive diseases and sciences. 2007 Dec:52(12):3307-15 [PubMed PMID: 17393312]
McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991 Feb:31(2):164-71 [PubMed PMID: 1996485]
Level 2 (mid-level) evidenceMillington SJ, Koenig S. Better With Ultrasound: Paracentesis. Chest. 2018 Jul:154(1):177-184. doi: 10.1016/j.chest.2018.03.034. Epub 2018 Apr 7 [PubMed PMID: 29630894]
Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. The American journal of emergency medicine. 2005 May:23(3):363-7 [PubMed PMID: 15915415]
Level 1 (high-level) evidenceOta KS, Schultz N, Segaline NA. Palliative Paracentesis in the Home Setting: A Case Series. The American journal of hospice & palliative care. 2021 Aug:38(8):1042-1045. doi: 10.1177/1049909120963075. Epub 2020 Sep 30 [PubMed PMID: 32996326]
Level 2 (mid-level) evidenceKunin M, Mini S, Abu-Amer N, Beckerman P. Regular at-home abdominal paracentesis via Tenckhoff catheter in patients with refractory congestive heart failure. International journal of clinical practice. 2021 Dec:75(12):e14924. doi: 10.1111/ijcp.14924. Epub 2021 Oct 5 [PubMed PMID: 34581465]
Kelil T, Shyn PB, Wu LE, Levesque VM, Kacher D, Khorasani R, Silverman SG. Wall suction-assisted image-guided therapeutic paracentesis: a safe and less expensive alternative to evacuated bottles. Abdominal radiology (New York). 2016 Jul:41(7):1333-7. doi: 10.1007/s00261-016-0634-x. Epub [PubMed PMID: 27315094]
Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World journal of hepatology. 2016 Feb 28:8(6):307-21. doi: 10.4254/wjh.v8.i6.307. Epub [PubMed PMID: 26962397]
Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Archives of internal medicine. 1986 Nov:146(11):2259-61 [PubMed PMID: 2946271]
De Gottardi A, Thévenot T, Spahr L, Morard I, Bresson-Hadni S, Torres F, Giostra E, Hadengue A. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009 Aug:7(8):906-9. doi: 10.1016/j.cgh.2009.05.004. Epub 2009 May 15 [PubMed PMID: 19447197]
Duggal P, Farah KF, Anghel G, Marcus RJ, Lupetin AR, Babich MM, Sandroni SE, McGill RL. Safety of paracentesis in inpatients. Clinical nephrology. 2006 Sep:66(3):171-6 [PubMed PMID: 16995339]
Otsuka Y, Nagaoka H, Nakano Y, Sakae H, Hasegawa K, Otsuka F. Subcutaneous edema as rare complication of abdominal paracentesis. Clinical case reports. 2021 Nov:9(11):e05116. doi: 10.1002/ccr3.5116. Epub 2021 Nov 19 [PubMed PMID: 34824856]
Level 3 (low-level) evidenceAbdu B, Akolkar S, Picking C, Boura J, Piper M. Factors Associated with Delayed Paracentesis in Patients with Spontaneous Bacterial Peritonitis. Digestive diseases and sciences. 2021 Nov:66(11):4035-4045. doi: 10.1007/s10620-020-06750-0. Epub 2020 Dec 3 [PubMed PMID: 33274417]
Ning S, Yang Y, Wang C, Luo F. Pseudomyxoma peritonei induced by low-grade appendiceal mucinous neoplasm accompanied by rectal cancer: a case report and literature review. BMC surgery. 2019 Apr 25:19(1):42. doi: 10.1186/s12893-019-0508-6. Epub 2019 Apr 25 [PubMed PMID: 31023277]
Level 3 (low-level) evidenceJun Jie NG, Teo KA, Shabbir A, Yeo TT. Widespread Intra-abdominal Carcinomatosis from a Rhabdoid Meningioma after Placement of a Ventriculoperitoneal Shunt: A Case Report and Review of the Literature. Asian journal of neurosurgery. 2018 Jan-Mar:13(1):176-183. doi: 10.4103/1793-5482.181128. Epub [PubMed PMID: 29492156]
Level 3 (low-level) evidence