Pancreaticoduodenectomy (Whipple Procedure)
Pancreaticoduodenectomy (Whipple Procedure)
Introduction
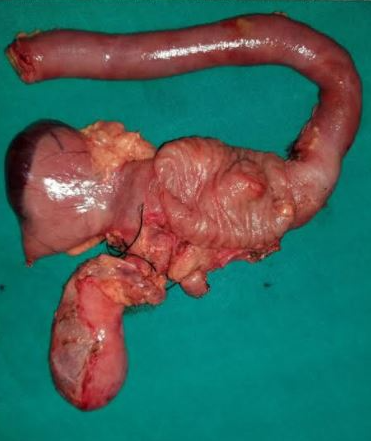
Pancreaticoduodenectomy, commonly known as the Whipple procedure, is a complex and technically challenging surgery primarily used to treat malignancies in the pancreatic head, periampullary region, and distal bile duct. The procedure involves the resection of the pancreatic head and uncinate process, duodenum, proximal jejunum, distal bile duct, gallbladder, and usually part of the stomach, followed by restoring bilioenteric continuity (see Image. Pancreaticoduodenectomy [Whipple Procedure]). While predominantly performed for malignant conditions, it is also indicated for benign conditions like chronic pancreatitis, large symptomatic cysts, or premalignant lesions such as intrapancreatic mucinous neoplasms.
The procedure was first performed by Walter Kausch in Germany and later refined by Allen Whipple in the United States and has become a cornerstone in managing pancreatic and periampullary cancers.[1] Advances in surgical techniques, including minimally invasive approaches like laparoscopy with or without robotic assistance, have improved outcomes, yet the Whipple procedure remains associated with significant morbidity and mortality.[2][3] Successful outcomes hinge on meticulous patient selection, comprehensive preoperative preparation, skilled surgical and anesthetic techniques, and coordinated postoperative care.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The pancreas, a retroperitoneal organ situated posterior to the stomach, is divided into the head, uncinate process, neck, body, and tail, with the head located within the C-loop of the duodenum (see Image. Pancreas and Duodenum, Anterior View).[4] The gland's arterial supply comes from the superior mesenteric artery (SMA) and the celiac trunk (see Image. Celiac Trunk). The gastroduodenal artery, a branch of the common hepatic division of the celiac trunk, runs inferiorly behind the pancreatic head, giving rise to the anterior and posterior superior pancreaticoduodenal arteries. Meanwhile, the SMA contributes to the anterior and posterior inferior pancreaticoduodenal arteries, creating an extensive anastomotic network within the pancreas. Venous drainage occurs through the pancreaticoduodenal veins into the superior mesenteric vein (SMV) or portal vein (PV), while the neck, body, and tail drain into the splenic vein.
The head of the pancreas is positioned anterior to the inferior vena cava (IVC) and left renal vein, with the PV lying posterior to the neck. The splenic artery winds along the upper margin of the pancreas, whereas the splenic vein travels behind the body and tail.[5] Lymphatic drainage follows the blood supply, with nodes along the vessels and within peripancreatic tissues and retropancreatic tissue (see Image. Lymphatics and Drainage of the Pancreas). Exocrine secretions from the pancreas enter the duodenum via the pancreatic ducts, with the main duct extending from the tail and merging with the common bile duct at the ampulla of Vater. The accessory pancreatic duct drains the lower part of the pancreas into the duodenum via the minor papilla.
Several critical anatomical factors must be considered during pancreatic surgery. The pancreas shares its blood supply with the C-loop of the duodenum, necessitating the removal of both during surgery. The uncinate process extends from the lower head of the pancreas, crossing posteriorly to the SMV and laterally to the SMA. Complete resection requires the skeletonization of the SMV and the right edge of the SMA. Additionally, the transverse mesocolon must be dissected from the pancreas's anterior surface to identify the inferior border and SMV. In cases where separation of the pancreas from the SMV/PV confluence is impossible, vein resection may be required. Comprehensive lymphadenectomy is also crucial in pancreaticoduodenectomy for cancer.[6][7]
Recognizing variations in vascular anatomy is essential before surgery. For example, a replaced right hepatic artery arising from the SMA occurs in approximately 12% of the population.[6] This variant can be particularly challenging, as it often runs behind the pancreatic head and bile duct. Identifying other vascular variants, such as accessory right and left hepatic arteries, is critical to minimize surgical complications.[6][7]
Indications
Indications for performing a pancreaticoduodenectomy include:
- Tumors involving the pancreatic head or uncinate process, including but not limited to:
- Pancreatic ductal adenocarcinoma [8]
- Pancreatic neuroendocrine tumors [9]
- Duodenal gastrointestinal stromal tumor [10]
- Intraductal papillary mucinous neoplasms [11][12]
- Periampullary cancer [13]
- Adenocarcinoma of the ampulla of Vater [14]
- Duodenal adenocarcinoma and other duodenal tumors [14]
- Chronic pancreatitis [15]
- Severe pancreatic trauma [16]
The resectability of pancreatic head neoplasms is based on radiographic criteria, which divides lesions into resectable, borderline resectable, and unresectable tumors, defined as:
- Resectable Disease
- No distant metastasis
- No radiographic evidence of PV or SMV distortion
- Clear dissection planes around the celiac trunk, hepatic artery, and SMA
- Borderline Resectable Disease
- SMV/PV involvement with distortion, narrowing, or occlusion, but the presence of suitable proximal and distal vessels for reconstruction
- Gastroduodenal artery encasement up to the hepatic artery with short segment encasement or abutment of the hepatic artery without extending to the celiac trunk
- Tumor abutment of the SMA less than 180° of the vessel wall circumference [17][18]
- Unresectable Disease
- Distant metastases
- Tumor encasement of SMA more than 180°
- Celiac trunk abutment
- IVC involvement
- Aortic involvement
- Irreparable SMV or PV occlusion [17]
Contraindications
Anatomic contraindications to a pancreaticoduodenectomy include unresectable disease and metastatic disease. Additionally, due to the notable morbidity and mortality associated with the procedure, a meticulous evaluation of a patient's capacity to withstand a pancreaticoduodenectomy is imperative. Individuals with significant comorbidities or other life-limiting illnesses may not be optimal candidates for surgery.
Equipment
Standard equipment used for an open pancreaticoduodenectomy includes, but is not limited to:
- Instruments for diagnostic laparoscopy and biopsy to be performed before the laparotomy
- A self-retaining retractor
- Laparotomy instrument set
- Surgical clips
- Suture materials, including fine monofilament sutures for potential vascular injury
- Scalpel
- Electrocautery
- Intestinal staplers
- Surgical drains
- A vessel-sealing device
- Vascular surgical instruments if vascular reconstruction is required
- Intraoperative ultrasound
Personnel
The personnel typically required to perform a pancreaticoduodenectomy include:
- Primary surgeon
- Surgical assistant or second surgeon
- Anesthesia personnel
- Surgical technician or operating room nurse
- Circulating or operating room nurse
Preparation
Preoperative preparation includes:
- Confirming resectability, vascular anatomy, and the relationship of the lesion to surrounding vasculature by a thorough review of preoperative imaging studies:
- Ideal imaging studies are triple-phase (arterial, venous, and portal venous phases) computed tomography (CT) scans with thin cuts or magnetic resonance imaging (MRI) with contrast.
- Optimizing preoperative nutrition with enteral or parenteral supplementation
- Correcting hyperbilirubinemia in patients that have biliary obstruction, which usually involves endoscopic biliary stenting
- A thorough discussion of the procedure's risks and benefits with the patient
- Preparing for postoperative pain control with anesthesia colleagues:
- The use of adjuncts such as epidural or intrathecal analgesia, nerve blocks, and other systemic nonopioid agents is essential.
- Prehabilitation in patients who are at high risk of postoperative complications
Perioperative preparation includes:
- Antibiotic prophylaxis within 30 minutes of skin incision
- Deep vein thrombosis prophylaxis
- Invasive monitoring if required (central venous access, arterial line)
- Available grafts or other potential conduits for vascular resections
- Ensuring normothermia and euglycemia
Technique or Treatment
This section outlines the conduct of a classic open pancreaticoduodenectomy. The resection and reconstruction have been ordered for easier understanding, appreciating that this is by no means the only approach. Anatomic factors and surgeon preferences may influence different approaches. Furthermore, the reconstruction techniques exhibit significant variability.
Staging Laparoscopy
Before proceeding to laparotomy for malignant disease, a staging laparoscopy is conducted to exclude metastatic disease and ensure resectability.[19] Staging laparoscopy has shown high success rates in detecting metastatic disease and can identify occult peritoneal surface disease radiologically. Typically, 2 or 3 5-mm ports are used to thoroughly inspect all parietal and visceral peritoneal surfaces. Careful attention is paid to areas that are difficult to visualize, such as the mesentery's root, the falciform ligament's insertion, and the diaphragmatic and pelvic peritoneal surfaces. Once the absence of metastatic disease is confirmed, the pancreaticoduodenectomy is commenced.
Pancreatic Resection
Pancreatic resection includes these procedural steps:
- Incision and exposure: The procedure begins with a vertical midline or bilateral subcostal incision, followed by the placement of a self-retaining retractor. A Kocher maneuver is performed to mobilize the duodenum and head of the pancreas from the retroperitoneum.[20] Filmy attachments between the pancreatic head and the IVC are separated until the origin of the left renal vein is identified. If necessary, the gonadal vein may be identified and ligated. The relationship of the tumor to the SMA and celiac trunk is confirmed by palpation. Partial mobilization of the hepatic flexure aids in separating the mesocolic attachments from the anterior pancreatic surface, with extensive right colon mobilization typically required only for planned vascular reconstruction. The lesser sac is accessed by opening the gastrocolic omentum lateral to the gastroepiploic arcade, exposing the anterior surface of the pancreas. Retrogastric pancreatic attachments are released to mobilize the stomach, and the transverse mesocolon is dissected off the anterior pancreatic surface, avoiding entry into the mesocolic tissue.
- Hepatoduodenal ligament dissection and cholecystectomy: Dissection proceeds to the hepatoduodenal ligament, which extends from the hepatic hilum to the lateral duodenum and contains portal structures. The common hepatic artery lymph node serves as a landmark for the dissection. The common hepatic artery is exposed and dissected toward the hepatic hilum, revealing the gastroduodenal artery, which is then encircled. After test clamping and confirming ongoing blood flow to the hepatic artery, the gastroduodenal artery is ligated and retracted to expose the PV. The PV is dissected from the artery to the right and the common bile duct to the left. The bile duct is then encircled, ensuring all fibrofatty lymphatic tissue is included. If the gallbladder is still present, cholecystectomy is performed next. The cystic duct and artery are ligated, and the gallbladder is removed as a separate specimen. The common bile duct is transected, with the transection site determined by the tumor location. The cut margin is sent for frozen section examination, and the bile duct is temporarily sutured to prevent bile leakage. Dissection of the PV continues toward the pancreatic head, including nodal tissue in the hepatoduodenal ligament distal to the common bile duct division.
- Retropancreatic tunnel creation: The middle colic vein is identified and traced back to its junction with the SMV, or the right gastroepiploic vein is traced backward. In some patients, a common insertion of the right gastroepiploic and middle colic veins forms the trunk of Henle.[21] The anterior surface of the SMV is gently separated from the pancreatic neck, creating a retropancreatic tunnel. This step is avoided if the SMV or PV is involved with the tumor. The cranial portion of the tunnel is created by separating the PV from the pancreatic neck and connecting it to the initial retropancreatic dissection over the SMV.
- Jejunal and gastric division: The transverse colon is elevated to reveal the ligament of Treitz, where the jejunum is divided 10 to 15 cm distal to the ligament using a linear stapler. The jejunal mesentery is ligated toward the ligament of Treitz until the jejunal limb is free and can be passed into the supramesocolic compartment. After clearing the omentum from the stomach wall, the distal stomach is transected using a linear stapler.
- Pancreatic division: Stay sutures are placed at the superior and inferior borders of the pancreas on either side of the planned transection line. The pancreas is then divided using sharp dissection or electrocautery, with bleeding controlled and the pancreatic duct margin sent for frozen section examination. The pancreatic head and uncinate process are carefully separated from the SMV, with small venous branches clipped or ligated. The final step involves dissecting the uncinate process from the SMV to the lateral border of the SMA. Once completed, the specimen is submitted for permanent pathology. Hemostasis is ensured, and preparation for reconstruction begins.
Pancreatic Reconstruction
Restoring bilioenteric continuity requires 3 key anastomotic connections:
- Pancreaticojejunostomy: A small mesocolic window brings The distal jejunal loop into the supramesocolic compartment. A jejunotomy is created, and duct-to-mucosa anastomosis is performed between the pancreatic duct and jejunal mucosa. The technique varies based on institutional preference, duct size, pancreatic consistency, and the surgeon's experience. The aim is to achieve a tension-free anastomosis with good vascularity. A pancreatic stent may be placed across the anastomosis.
- Hepaticojejunostomy: The same jejunal loop creates an anastomosis between the bile duct and jejunum. After a jejunotomy, this connection is made using interrupted or continuous sutures, typically in a single layer.
- Gastrojejunostomy: For the gastrojejunostomy, a jejunal loop distal to the pancreatic and biliary anastomoses is used. This loop is brought up to the stapled end of the stomach in an antecolic fashion, and the anastomosis is performed as a single-layer, double-layer, or stapled connection.
Closure and Postoperative Care
Following reconstruction, closed suction drains are placed near the pancreatic and biliary anastomoses to monitor postoperative drainage.[22] The decision to place a feeding jejunostomy is based on the surgeon's preference. The abdominal closure is performed in layers. A nasogastric tube is used overnight to decompress the stomach and is typically removed the following morning unless there is excessive output. The use of a nasogastric tube is not mandatory.[23] On postoperative days 1 and 3, the abdominal drain contents are evaluated for amylase levels. Elevated amylase (more than 3 times the upper limit of normal serum values) may indicate a pancreatic leak, requiring management based on the leak's volume, drain content, and the patient's clinical status.[24] Drains are gradually removed once output is minimal, there is no evidence of a pancreatic fistula, and the patient can tolerate a regular diet.
Other Considerations
- Pylorus-preserving pancreaticoduodenectomy: The stomach is retained in pylorus-preserving procedures, and the proximal duodenum is divided. The remainder of the operation follows the same protocol as the traditional approach. During reconstruction, a duodenojejunostomy is performed instead of a gastrojejunostomy. A Cochrane review comparing the 2 procedures found that pylorus preservation generally results in shorter operative times and less blood loss. However, the review noted that many of the included studies were of low quality, which limits the strength of these conclusions.[25]
- Vascular resection: When tumors invade the SMV or PV, vein resection may be necessary. Often, the tumor can be carefully separated from the vein, or a portion of the vein wall can be resected without requiring reconstruction. If a short segment of the vein (typically less than 2 cm) is affected, it can usually be reconstructed by mobilizing the 2 ends and performing a primary anastomosis. For defects larger than 2 cm, graft interposition is required. Options for grafting include autologous veins (eg, left renal vein, internal jugular vein, saphenous vein), cadaveric veins, and prosthetic materials.
- Minimally invasive resection: Advances in minimally invasive techniques, such as laparoscopic and robotic-assisted laparoscopic pancreaticoduodenectomy, are becoming more common. Outcomes from these techniques are comparable to those of traditional open surgery, particularly in high-volume centers with experienced surgeons. These approaches offer the potential benefits of reduced postoperative pain, shorter recovery times, and minimal scarring.
Complications
The overall morbidity and mortality in patients undergoing pancreaticoduodenectomy remains high, although it has improved significantly over the decades. Current mortality rates vary from 2% to 10%, with morbidity rates reported as high as 60%. This section focuses on some of the complications unique to pancreaticoduodenectomy. Naturally, all the major complications associated with major abdominal surgery are applicable as well.
Delayed Gastric Emptying
Delayed gastric emptying is characterized by the inability to tolerate a solid diet or the continued need for a nasogastric tube several days postsurgery. Although specific criteria for this condition have been established, they are not covered in this chapter. Management typically involves extended nasogastric decompression, postpyloric feeding or parenteral nutrition, and prokinetic agents like metoclopramide. Delayed gastric emptying often results from a pancreatic leak and generally improves once the underlying issue is resolved.[26]
Pancreatic Fistula
A pancreatic fistula is defined by amylase levels greater than 3 times the upper limit of normal serum values in the pancreatic drain after surgery, usually after postoperative day 3. Fistulae are graded based on severity:
- Grade A: biochemical leak, no clinical relevance
- Grade B: persistent amylase-rich drainage that alters postoperative management, usually persisting beyond 3 weeks
- Grade C: fistula with organ failure
Most pancreatic leaks are grade A and resolve with appropriate drainage, resuscitation, and nutrition. Occasionally, they require additional drain placement or antibiotics for undrained collections. Grade B and C leaks may require prolonged nutrition and repeated drainage or endoscopic interventions.[27]
Visceral Artery Pseudoaneurysm
Visceral artery pseudoaneurysms commonly involve the gastroduodenal artery and often arise in the context of a pancreatic leak. In this scenario, pancreatic enzymes erode the ligated stump of the gastroduodenal artery, gradually leading to pseudoaneurysm formation. A gastroduodenal artery pseudoaneurysm represents a true surgical emergency, as rupture can result in life-threatening hemorrhage. Management typically involves embolization or stenting across the pseudoaneurysm, with surgery reserved as a last resort due to its high failure rate. The sudden presence of blood in a pancreatic drain should be treated as a potential pseudoaneurysm until ruled out.[28]
Exocrine Insufficiency
Exocrine insufficiency typically presents with symptoms such as diarrhea, bloating, and fatty stools, often exacerbated by high-fat meals. While the condition is usually diagnosed clinically, it can be confirmed through stool testing. Management involves the oral administration of pancreatic enzyme supplements to alleviate symptoms and improve nutrient absorption.[29]
Endocrine Insufficiency: Diabetes develops in approximately 20% of patients postoperatively, with the rate much higher in patients who have impaired glucose tolerance preoperatively.[30][31]
Bile Leaks: Most small bile leaks heal spontaneously, but more significant ones that appear in the immediate postoperative period may require reoperation.
Biliary Strictures at the Hepaticojejunostomy: These are late complications from a narrow anastomosis, ischemia, or tumor recurrence.[26]
Clinical Significance
Pancreaticoduodenectomy is the sole curative treatment option for most tumors affecting the pancreatic head and uncinate process, the periampullary region, and the distal bile duct. While it is a complex procedure, having a comprehensive understanding of anatomy, diagnostic imaging, interventions, surgical procedures, perioperative and postoperative management, and early recognition and treatment of complications is paramount for optimal patient outcomes. Adequate education for clinicians, nurses, and the entire healthcare team is imperative, emphasizing the necessity of a multidisciplinary approach.
Enhancing Healthcare Team Outcomes
Pancreaticoduodenectomy, commonly known as the Whipple procedure, is a highly complex surgical intervention typically performed to treat malignancies of the pancreas, duodenum, or bile duct. Successful management of these cases requires a well-coordinated, interprofessional team approach, with clear communication and collaboration among surgeons, gastroenterologists, oncologists, radiologists, anesthesiologists, and specialized nursing staff. To minimize complications, physicians and advanced practitioners must ensure accurate preoperative assessment, optimal patient selection, and meticulous intraoperative technique. Concurrently, anesthesiologists play a crucial role in managing fluid balance, hemodynamic stability, and pain control, while perioperative nurses assist in patient preparation, operative support, and postoperative monitoring.
Pharmacists are essential in managing complex medication regimens, including antibiotics, analgesics, and prophylactic agents to prevent infection or thromboembolism. Dietitians collaborate to optimize nutritional status, as malnutrition is a common concern in these patients. Physical and occupational therapists contribute by facilitating early mobilization, enhancing postoperative recovery, and reducing hospital stays. Effective interprofessional communication is crucial for timely intervention and complication management, such as addressing pancreatic fistulae or delayed gastric emptying. By using each team member’s expertise, care coordination can significantly improve patient outcomes, reduce morbidity, and enhance overall safety in pancreaticoduodenectomy management.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)

Pancreas and Duodenum, Anterior View. The pancreas is a retroperitoneal organ lying posterior to the stomach, divided arbitrarily into the head, neck, body, tail, and uncinate processes. The head lies within the C-loop of the duodenum.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

Celiac Trunk. The image shows the celiac trunk and its branches around the stomach, pancreas, spleen, and duodenum.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

Lymphatics and Drainage of the Pancreas. This includes the subpyloric glands, pancreas, duodenum, stomach, inferior gastric, pancreaticolienal, and supragastric glands.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011 Jun:13(6):377-84. doi: 10.1111/j.1477-2574.2011.00305.x. Epub 2011 Mar 31 [PubMed PMID: 21609369]
Jiang YL, Zhang RC, Zhou YC. Comparison of overall survival and perioperative outcomes of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. BMC cancer. 2019 Aug 7:19(1):781. doi: 10.1186/s12885-019-6001-x. Epub 2019 Aug 7 [PubMed PMID: 31391085]
Level 1 (high-level) evidenceKimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Annals of surgery. 2014 Apr:259(4):773-80. doi: 10.1097/SLA.0000000000000263. Epub [PubMed PMID: 24253151]
Level 3 (low-level) evidenceSkandalakis LJ, Rowe JS Jr, Gray SW, Skandalakis JE. Surgical embryology and anatomy of the pancreas. The Surgical clinics of North America. 1993 Aug:73(4):661-97 [PubMed PMID: 8378816]
Negoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. Surgical Anatomy of the Superior Mesenteric Vessels Related to Colon and Pancreatic Surgery: A Systematic Review and Meta-Analysis. Scientific reports. 2018 Mar 8:8(1):4184. doi: 10.1038/s41598-018-22641-x. Epub 2018 Mar 8 [PubMed PMID: 29520096]
Level 1 (high-level) evidenceCovey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002 Aug:224(2):542-7 [PubMed PMID: 12147854]
Rubio-Manzanares-Dorado M, Marín-Gómez LM, Aparicio-Sánchez D, Suárez-Artacho G, Bellido C, Álamo JM, Serrano-Díaz-Canedo J, Padillo-Ruiz FJ, Gómez-Bravo MÁ. Implication of the presence of a variant hepatic artery during the Whipple procedure. Revista espanola de enfermedades digestivas. 2015 Jul:107(7):417-22 [PubMed PMID: 26140634]
Liang B, Chen Y, Li M, Dong X, Yao S, Liu T. Total laparoscopic duodenum-preserving pancreatic head resection for solid pseudopaillary neoplasm of pancreas: A case report. Medicine. 2019 May:98(21):e15823. doi: 10.1097/MD.0000000000015823. Epub [PubMed PMID: 31124984]
Level 3 (low-level) evidenceKim H, Song KB, Hwang DW, Lee JH, Alshammary S, Kim SC. Laparoscopic versus open pancreaticoduodenectomy for pancreatic neuroendocrine tumors: a single-center experience. Surgical endoscopy. 2019 Dec:33(12):4177-4185. doi: 10.1007/s00464-019-06969-7. Epub 2019 Jul 12 [PubMed PMID: 31300907]
Zhou Y, Wang X, Si X, Wang S, Cai Z. Surgery for duodenal gastrointestinal stromal tumor: A systematic review and meta-analysis of pancreaticoduodenectomy versus local resection. Asian journal of surgery. 2020 Jan:43(1):1-8. doi: 10.1016/j.asjsur.2019.02.006. Epub 2019 Mar 8 [PubMed PMID: 30853211]
Level 1 (high-level) evidenceHüttner FJ, Capdeville L, Pianka F, Ulrich A, Hackert T, Büchler MW, Probst P, Diener MK. Systematic review of the quantity and quality of randomized clinical trials in pancreatic surgery. The British journal of surgery. 2019 Jan:106(1):23-31. doi: 10.1002/bjs.11030. Epub [PubMed PMID: 30582642]
Level 2 (mid-level) evidenceMenon G, Hoilat GJ, Babiker HM, Mukkamalla SKR. Mucinous Cystic Pancreatic Neoplasms. StatPearls. 2024 Jan:(): [PubMed PMID: 28846358]
Morales E, Zimmitti G, Codignola C, Manzoni A, Garatti M, Sega V, Rosso E. Follow "the superior mesenteric artery": laparoscopic approach for total mesopancreas excision during pancreaticoduodenectomy. Surgical endoscopy. 2019 Dec:33(12):4186-4191. doi: 10.1007/s00464-019-06994-6. Epub 2019 Jul 22 [PubMed PMID: 31332566]
Yoshimachi S, Ohtsuka H, Aoki T, Miura T, Ariake K, Masuda K, Ishida M, Mizuma M, Hayashi H, Nakagawa K, Morikawa T, Motoi F, Kanno A, Masamune A, Fujishima F, Sasano H, Kamei T, Naitoh T, Unno M. Mixed adenoneuroendocrine carcinoma of the ampulla of Vater: a case report and literature review. Clinical journal of gastroenterology. 2020 Feb:13(1):37-45. doi: 10.1007/s12328-019-01009-2. Epub 2019 Jul 24 [PubMed PMID: 31342462]
Level 3 (low-level) evidenceMikulić D, Bubalo T, Mrzljak A, Škrtić A, Jadrijević S, Kanižaj TF, Kocman B. Role of total pancreatectomy in the treatment of paraduodenal pancreatitis: A case report. World journal of gastrointestinal surgery. 2019 Jun 27:11(6):296-302. doi: 10.4240/wjgs.v11.i6.296. Epub [PubMed PMID: 31367277]
Level 3 (low-level) evidenceRios G, Conrad A, Cole D, Adams D, Leveen M, O'Brien P, Baron P. Trends in indications and outcomes in the Whipple procedure over a 40-year period. The American surgeon. 1999 Sep:65(9):889-93 [PubMed PMID: 10484097]
Level 2 (mid-level) evidenceTempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB 3rd, Binder E, Cardin DB, Cha C, Chiorean EG, Chung V, Czito B, Dillhoff M, Dotan E, Ferrone CR, Hardacre J, Hawkins WG, Herman J, Ko AH, Komanduri S, Koong A, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Scaife C, Thayer S, Weekes CD, Wolff RA, Wolpin BM, Burns J, Darlow S. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2017 Aug:15(8):1028-1061. doi: 10.6004/jnccn.2017.0131. Epub [PubMed PMID: 28784865]
Level 1 (high-level) evidenceMullapudi B, Hawkes PJ, Patel A, Are C, Misra S. Borderline resectable pancreatic cancer. Indian journal of surgical oncology. 2015 Mar:6(1):63-68. doi: 10.1007/s13193-014-0374-8. Epub 2015 Jan 4 [PubMed PMID: 25937766]
Ta R, O'Connor DB, Sulistijo A, Chung B, Conlon KC. The Role of Staging Laparoscopy in Resectable and Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Digestive surgery. 2019:36(3):251-260. doi: 10.1159/000488372. Epub 2018 Apr 12 [PubMed PMID: 29649825]
Level 1 (high-level) evidenceVasiliadis KD. "Mesopancreas-first" radical resection of pancreatic head cancer following the Cattell-Braasch-Valdoni maneuver: Appreciating the legacy of pioneers in visceral surgery. Annals of hepato-biliary-pancreatic surgery. 2021 Aug 31:25(3):376-385. doi: 10.14701/ahbps.2021.25.3.376. Epub [PubMed PMID: 34402439]
Gao Y, Lu Y. Variations of Gastrocolic Trunk of Henle and Its Significance in Gastrocolic Surgery. Gastroenterology research and practice. 2018:2018():3573680. doi: 10.1155/2018/3573680. Epub 2018 Jun 6 [PubMed PMID: 29977286]
Andersson R, Søreide K, Ansari D. The Dilemma of Drains after Pancreatoduodenectomy: Still an Issue? Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2020 Dec:109(4):359-361. doi: 10.1177/1457496919866014. Epub 2019 Aug 1 [PubMed PMID: 31370750]
Bergeat D, Merdrignac A, Robin F, Gaignard E, Rayar M, Meunier B, Beloeil H, Boudjema K, Laviolle B, Sulpice L. Nasogastric Decompression vs No Decompression After Pancreaticoduodenectomy: The Randomized Clinical IPOD Trial. JAMA surgery. 2020 Sep 1:155(9):e202291. doi: 10.1001/jamasurg.2020.2291. Epub 2020 Sep 16 [PubMed PMID: 32667635]
Level 1 (high-level) evidenceKoek S, Wiegele S, Ballal M. Drain fluid amylase and lipase as a predictive factor of postoperative pancreatic fistula. ANZ journal of surgery. 2022 Mar:92(3):414-418. doi: 10.1111/ans.17296. Epub 2021 Oct 22 [PubMed PMID: 34676961]
Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, Büchler MW. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. The Cochrane database of systematic reviews. 2011 May 11:(5):CD006053. doi: 10.1002/14651858.CD006053.pub4. Epub 2011 May 11 [PubMed PMID: 21563148]
Level 1 (high-level) evidenceSimon R. Complications After Pancreaticoduodenectomy. The Surgical clinics of North America. 2021 Oct:101(5):865-874. doi: 10.1016/j.suc.2021.06.011. Epub 2021 Jul 29 [PubMed PMID: 34537148]
Nahm CB, Connor SJ, Samra JS, Mittal A. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clinical and experimental gastroenterology. 2018:11():105-118. doi: 10.2147/CEG.S120217. Epub 2018 Mar 15 [PubMed PMID: 29588609]
Feng F, Cao X, Liu X, Qin J, Xing Z, Duan J, Liu C, Liu J. Two forms of one complication: Late erosive and nonerosive postpancreatectomy hemorrhage following laparoscopic pancreaticoduodenectomy. Medicine. 2019 Jul:98(30):e16394. doi: 10.1097/MD.0000000000016394. Epub [PubMed PMID: 31348239]
Level 2 (mid-level) evidenceGoess R, Ceyhan GO, Friess H. Pancreatic exocrine insufficiency after pancreatic surgery. Panminerva medica. 2016 Jun:58(2):151-9 [PubMed PMID: 27058237]
Maxwell DW, Jajja MR, Tariq M, Mahmooth Z, Galindo RJ, Sweeney JF, Sarmiento JM. Development of Diabetes after Pancreaticoduodenectomy: Results of a 10-Year Series Using Prospective Endocrine Evaluation. Journal of the American College of Surgeons. 2019 Apr:228(4):400-412.e2. doi: 10.1016/j.jamcollsurg.2018.12.042. Epub 2019 Jan 26 [PubMed PMID: 30690075]
Oh HM, Yoon YS, Han HS, Kim JH, Cho JY, Hwang DW. Risk factors for pancreatogenic diabetes after pancreaticoduodenectomy. Korean journal of hepato-biliary-pancreatic surgery. 2012 Nov:16(4):167-71. doi: 10.14701/kjhbps.2012.16.4.167. Epub 2012 Nov 30 [PubMed PMID: 26388929]