Introduction
The human heart is a pivotal organ in the circulatory system, and it beats more than 2 billion times during normal life. This functioning of the heart depends on the cardiac conduction system, which includes impulse generators (e.g., sino-atrial node) and the impulse propagating (His-Purkinje) system. [1] The sinoatrial node acts as the natural pacemaker of the heart. The cells present in the sinus node have innate automaticity, which starts the electrical activity in the heart. This innate electrical potential moves from the sinoatrial node to the atrioventricular node and finally into the His-Purkinje system.[2] This movement of electric potential in an orderly manner controls the rhythmic contraction of the heart's chambers. The failure of this intrinsic electrical conduction in the heart can result in different arrhythmic problems. Several diseases and conditions affect the conduction system by involving impulse generation, impulse propagation, or both. Acquired conditions such as myocardial infarction, age-related degeneration, procedural complications, and drug toxicity are the major causes of the native conduction system malfunction.[3]
The current standard of care for symptomatic bradyarrhythmias due to conduction system diseases is the implantation of a cardiac implantable electronic device.[4] These pacing devices provide an external electrical stimulus that leads to depolarization of myocytes and helps maintain the electrical excitability of the heart tissue. This process leads to excitation-contraction coupling resulting in the contraction of myocardial tissue.[5]
Despite their success, electronic pacemakers have limitations, including complications related to implantation, limited battery life, the potential for infection, lack of physiologic autonomic responsiveness, and size restriction in younger patients.[6] The periodic evaluation of an implanted pacemaker is necessary to optimize programming and to identify correctable problems. This review will discuss the common pacing system problems of a cardiac implantable electronic device (pacemaker).
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Pacemakers consist of two main components: a pulse generator and the leads. Pulse generator houses the battery and other electronics which control the modes of the pacemaker. Pacemaker leads conduct the depolarizing potential to the myocardium. The sensing of the innate activity of the heart is also a function of the leads.[7]
Pacemaker Timing Cycle
A pacemaker has two primary functions, pacing (an electrical stimulus for myocardial depolarization) and sensing (detecting intrinsic electrical activity and wave of depolarization). Different timing cycles are programmed in a pacemaker for its functioning.[8]
- Lower Rate Limit: Base rate or slowest rate that the pacemaker will allow the patient's heart to go. It is programmable.
- Upper Rate Limit: The maximum rate at which a pacemaker can pace in the absence of intrinsic activity.
- Maximum Tracking Rate (MTR): Maximum atrial rate at which a dual-chamber pacemaker tracks the sensed atrial activity and paces the ventricle.
- Sensed AV Delay: It is a time duration after sensed atrial activity at which the pacemaker will pace the ventricle if it does not sense the intrinsic activity.
- Paced AV Delay: It is a time duration after atrial pacing at which the pacemaker will pace the ventricle if it does not sense the intrinsic activity.
- Post Ventricular Atrial Refractory Period (PVARP): It is a time duration after the ventricular event when a pacemaker does not react to a sensed atrial activity. It sees the activity but does not reset the timing cycle.
- Post Ventricular Atrial Blanking (PVAB): This is the time duration after the ventricular event when a pacemaker does not sense any atrial event. Pacemaker becomes blind for atrial events.
- Total Atrial Refractory Period (TARP): The sum of AV delay and post-ventricular atrial refractory period.
Issues of Concern
Pacemakers are electronic devices programmed to pace (deliver the depolarizing current) the specified cardiac chamber and sense the intrinsic cardiac activity in the respected chamber.[9] So, the patients with pacemakers generally face problems related to either sensing or pacing, and these problems can be grouped into the following categories.[10]
- Problems related to pacing.
- Output failure
- Failure to capture
- Problem related to sensing
- Under sensing
- Over sensing
- Pseudo-malfunction
- Safety pacing
- Pacemaker-mediated tachycardia
- Upper rate behavior
- Runaway pacemaker
Problems Related to Pacing
Output Failure
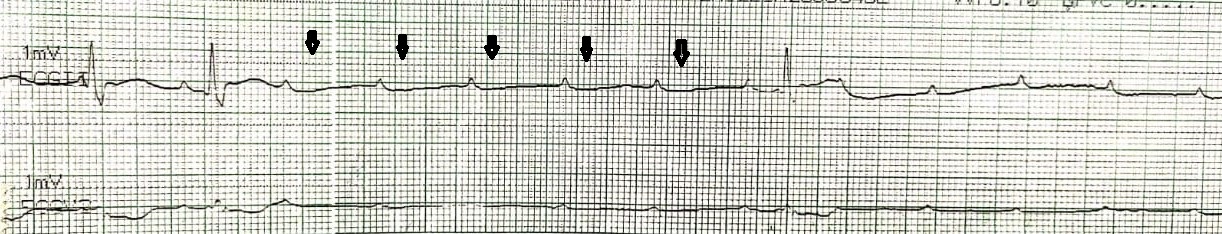
It is defined as the inability of the pacemaker to generate an impulse resulting in a heart rate lower than the programmed lower rate limit. It is characterized by the absence of a pacing spike on an electrocardiogram, and device interrogation confirms the diagnosis. (Figure.1) Causes of output failure include lead fracture, generator failure, and inhibition of pacing due to over-sensing and crosstalk.[11]
Failure to Capture
Failure to capture is defined as the inability of pacing impulse to produce an evoked potential. It is characterized by a pacing spike on the surface electrocardiogram at programmed heart rate, which is not followed by an evoked potential (P or a QRS). (Figure.2) Causes of failure to capture include lead dislodgment and elevated thresholds due to fibrosis or exit block at the site of lead implantation.[12] Acidosis and hyperkalemia may also lead to capture failure.[13]
These pacing problems could lead to life-threatening bradyarrhythmias as well as asystole in pacemaker-dependent patients.
Problems Related to Sensing
Under-sensing
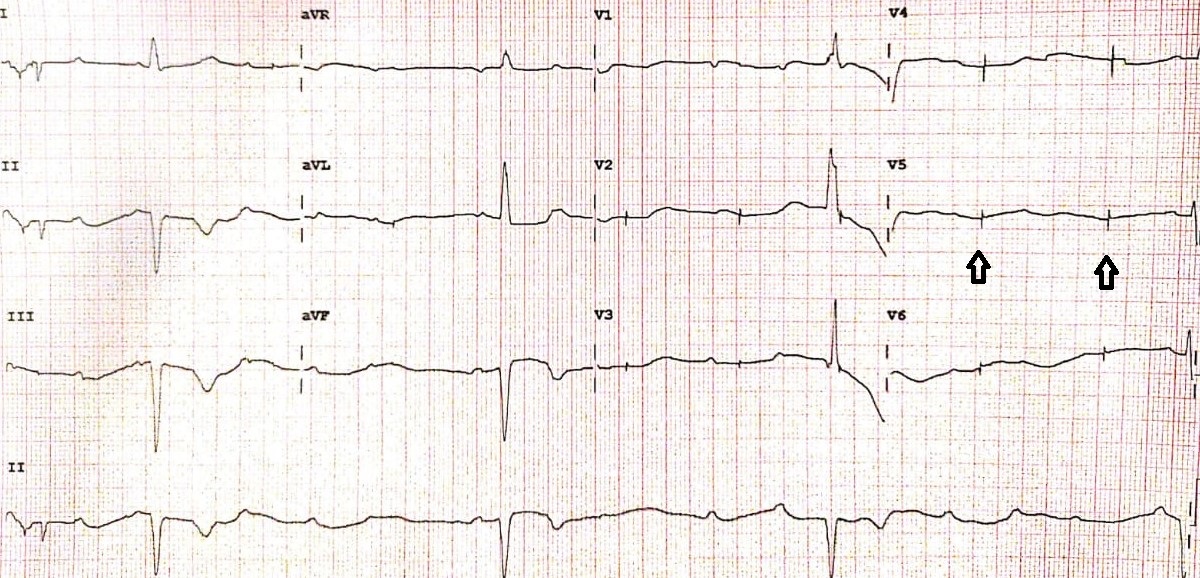
Under-sensing is defined by a failure of the pacemaker to see the spontaneous intrinsic activity, which results in asynchronous pacing. On a surface ECG, it is characterized by pacing spikes regardless of P waves or QRS complex. (Figure.3) The main causes of under-sensing include an improperly programmed sensing threshold (high sensing threshold), insufficient myocardial voltage signal, lead displacement, or pacemaker failure.[14]
Over-sensing
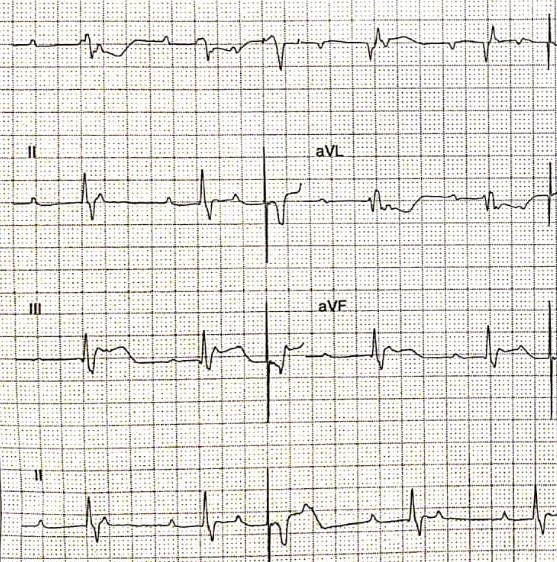
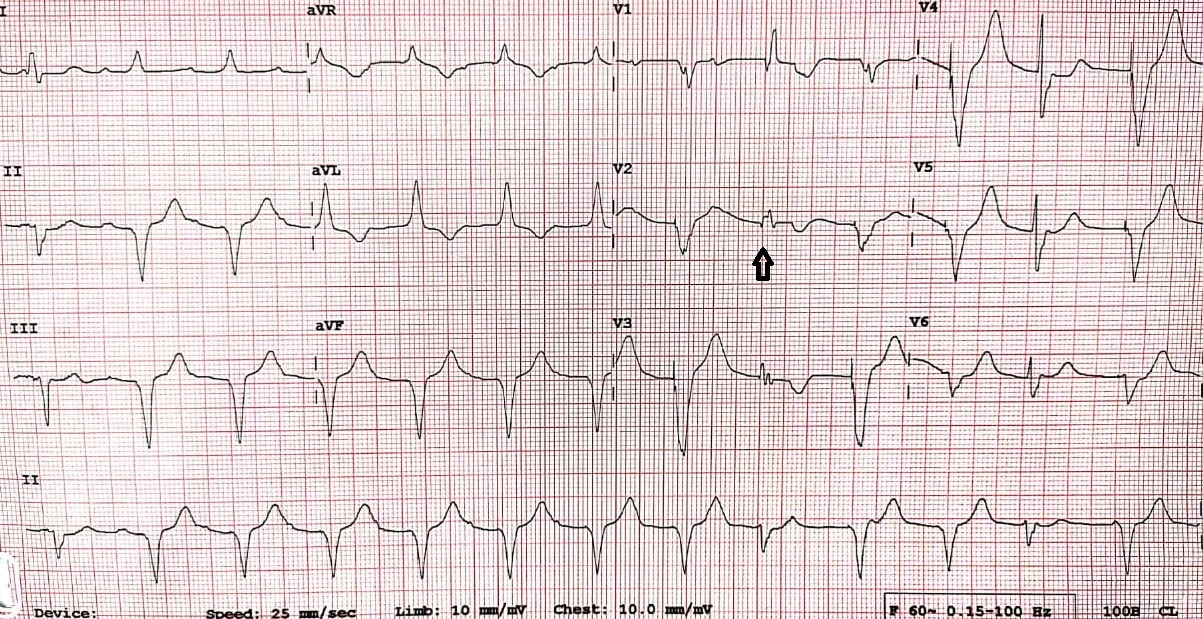
Over-sensing happens when the pacemaker detects an electrical signal which is not expected to be sensed. Over-sensing results in an inappropriate inhibition of the pacing stimulus leading to potentially life-threatening consequences. In addition to the native cardiac depolarization signals (P or R waves), any electrical activity with sufficient amplitude can be sensed by a pacemaker, inhibiting the pacing when required. Over-sensing can be caused either by a physiologic signal like T waves or by a non-physiologic signal like electromagnetic interference or a lead failure (an insulation break or a lead fracture.[15][16] Over-sensing is characterized by fewer pacing spikes than expected on a surface electrocardiogram. (Figure.4)
Sensing plays a major role in pacemakers, in patients with implantable cardioverter defibrillators (ICDs), sensing problems lead to inappropriate shocks.
Pseudomalformations
Fusion and Pseudofusion
Fusion is an electrical summation of an intrinsic beat and a depolarization from a pacing stimulus. The hallmark of the fusion phenomenon is that its morphology lies between a fully paced beat and an intrinsic beat. (Figure.5)
Pseudofusion occurs when the pacemaker spikes coincide with an intrinsic; however, it does not contribute to the actual depolarization. It is characterized by a morphology similar to an intrinsic beat. Fusion and pseudo-fusion beats are considered normal pacemaker behavior.[17]
Pacemaker Crosstalk
Pacemaker crosstalk is a feature of a dual-chamber pacemaker, characterized by detecting a paced signal in one chamber by the lead in another chamber and by the misrepresentation of the paced signal as a cardiac depolarization signal. This, in turn, results in inappropriate inhibition of pacing in the 2nd chamber.[18]
Ventricular Safety Pacing
During ventricular safety pacing, the pacemaker delivers a ventricular pacing stimulus after detecting a ventricular sensed event shortly after an atrial paced event. It is typical characterized by the appearance of two very closely spaced atrial and ventricular paced events on ECG. Safety pacing (SP) algorithms differ among pacemaker manufacturers.[19]
Pacemaker Mediated Tachycardia
Pacemaker-mediated tachycardia is a feature dual-chamber pacemaker with tracking mode (DDD, VDD). It is also called an endless-loop tachycardia characterized by atrial sensing followed by ventricular pacing at an upper tracking rate.[20] Pacemaker-mediated tachycardia requires the presence of retrograde (ventriculoatrial) conduction and a triggering event like premature ventricular contraction or loss of AV synchrony. A retrograde P wave produced by a premature ventricular complex is sensed by a pacemaker when it falls beyond the PVARP. This sensed atrial activity triggers AV delay, and the ventricle is paced at the end of programmed AV delay. This paced event again conducts retrograde and sensed as an atrial activity and triggers an AV delay again. (Figure.6) This endless loop tachycardia continues similar to a re-ent
rant tachycardia, except that the pacemaker forms part of the re-entrant circuit.[21] Pacemaker-mediated tachycardia could therefore be avoided by programming a sufficiently long post ventricular atrial refractory period (PVARP). Placing a magnet on the device during the PMT will change the pacemaker's mode to asynchronous dual-chamber pacing mode (in DOO, intrinsic P waves and R waves are ignored), which results in the termination of tachycardia by suspending the pacemaker's sensing function. This tachycardia is rare in the contemporary era due to advanced PMT algorithms programmed in the newer pacemakers.[22][23]
Upper Rate Behavior
Upper-rate behavior is also a feature of dual-chamber pacemakers with atrial tracking mode. In dual-chambered pacemakers, it is necessary to limit the atrial rate at which the device paces the ventricle. This limit is called the maximum tracking rate (MTR), and it is a programmable value. Upper rate behavior occurs when the atrial rate increases and approaches the maximum tracking rate.[24] When the atrial rate exceeds MTR, it results in pacemaker Wenckebach. If the atrial rate keeps increasing and exceeds the TARP, it will result in a pacemaker 2:1 AV block.
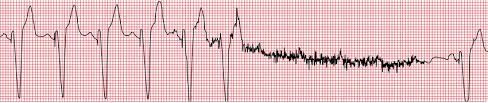
Runaway Pacemaker
Runaway pacemaker is a rare, life-threatening phenomenon caused by generator dysfunction, usually related to pacemaker battery depletion.[25] Other than the runaway phenomenon, the low battery voltage can provoke low rate stimuli, capture and sensing failures, and mode changes. Runaway pacemaker typically shows an ECG with captured beats alternating with non-captured high rate spikes. (Figure.7)
Clinical Significance
Advances in pacing device technology have led to its widespread use in treating patients with bradyarrhythmia and tachyarrhythmias.[26] It is imperative to have a comprehensive knowledge of normal pacemaker function to understand the pacemaker malfunction. Pacemaker malfunction can lead to potentially life-threatening situations, including syncope and even cardiac arrest.[27] Regular follow-up and programming of pacing devices are required for the basic understanding of their function, troubleshooting, and management of pacemaker malfunction. Comprehensive knowledge of pacemaker function and its management at the time of surgery or imaging enhances patient care.[28]
Other Issues
The topic of magnetic resonance imaging (MRI) in patients with cardiac implantable electronic devices (CIED) is still debatable. More than three-fourths of the patients require MRI at some point in time after implanting a pacing device.[29] The list of anticipated risks with MRI includes aberrant changes in the pacing output, changes in the programmed mode, and generation of current in the lead wires leading to heat-induced thermal damage at contact points and causing unintended cardiac stimulation.[30] A research study in patients with non-MRI-conditional devices concluded that there was no failure of the device or lead in these patients when undergoing non-thoracic MRI of approximately 1.5 teslas.[31]
MRI-conditional pacemakers are better able to handle the interference due to magnetic resonance imaging. The term MRI-conditional refers to devices with no known hazards or risks under specific magnetic resonance conditions. Notably, there are no “MRI-safe” devices, which are devices that have no known hazards or risks under all conditions. MRI-conditional devices have minimal ferromagnetic material, altered filtering, as well as specially designed lead conductors, which minimize current induction and heating of the tissue. MRI conditional leads are also required for a device to be labeled as MRI conditional.[32]
CT scan of the patient does not usually cause problems in the pacemakers. Therefore, the presence of an implanted pacemaker should not hinder such investigative imaging modality. In rare cases, it might lead to transient changes in the output of the pacemaker.[33]
Some CIEDs make use of piezoelectric crystal components in the circuitry or lead connections. Extracorporeal shock wave lithotripsy can damage such components due to its effect on those components leading to device malfunction.[34]
Therapeutic radiation can produce undesirable outcomes in patients with pacemakers. These outcomes include reprogramming resulting in aberrant behavior, resetting the device, or permanent malfunction due to damage to the semiconductor insulation. There will be precipitous output failure of the pacemaker in case of permanent damage to its components.[35] The volume of “scatter radiation” deemed safe for an implanted pacemaker is often provided by the manufacturer. However, in case of lack of that information, contacting the manufacturer for that information is the best next step.[36]
Enhancing Healthcare Team Outcomes
When planning therapeutic radiation for a patient with an implanted pacemaker, the status of the device requires monitoring by a healthcare provider who specializes in monitoring the pacemakers. Moreover, the radiation oncologist should assess the radiation dose to be received by the device in that particular case.[37]
If the malfunction of the device is due to lead dislodgement, the management depends on the timing of the event related to the timing of the implantation of the device, the severity of the dysfunction, the clinical situation of the patient, and the location of the displaced lead (atrial or ventricular). Reopening and reinstating the lead is a good management option in early displacements as the chances of fixation of the lead by the fibrous endocardial reaction are very low. Thus, allowing the manipulation of the lead.[38]
On the other hand, if the displacement of the lead is late, lead manipulation might not be an option. In such cases, introducing the lead in the heart chamber where the displacement has occurred is a good management plan if lead extraction is not possible. This new lead cancels the effect of previously displaced lead in that chamber.[39]
The best treatment of pacemaker dysfunction involves an interprofessional team of primary care clinicians, emergency medicine clinicians, cardiologists, cardiac surgeons, and cardiac nurses. Each of these disciplines needs to understand the function of pacemakers, be able to identify potential issues with pacemaker function, and engage in open information sharing with other team members to preclude adverse events and improve patient outcomes in those patients with pacemakers. [Level 5]
Different problems can arise during anesthesia, surgery, or ICU management of patients with cardiac implantable electrical devices (CIED). These problems include ventricular tachyarrhythmias, asystole, hypotension, and bradycardia.[40] Proper preoperative management is crucial to avoid such undesirable outcomes. The first step is to identify patients with CIED. The identification of such patients is followed by the clinical assessment, analysis of the functioning of the device. Hospitals should have pacemaker clinics or trained electrophysiology specialists who can properly assess the pacemakers before the surgery. In most cases, the modes of the pacemaker will require changing for undergoing surgical procedures.[11]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kennedy A, Finlay DD, Guldenring D, Bond R, Moran K, McLaughlin J. The Cardiac Conduction System: Generation and Conduction of the Cardiac Impulse. Critical care nursing clinics of North America. 2016 Sep:28(3):269-79. doi: 10.1016/j.cnc.2016.04.001. Epub 2016 Jun 22 [PubMed PMID: 27484656]
Anderson RH, Yanni J, Boyett MR, Chandler NJ, Dobrzynski H. The anatomy of the cardiac conduction system. Clinical anatomy (New York, N.Y.). 2009 Jan:22(1):99-113. doi: 10.1002/ca.20700. Epub [PubMed PMID: 18773472]
James TN. Normal variations and pathologic changes in structure of the cardiac conduction system and their functional significance. Journal of the American College of Cardiology. 1985 Jun:5(6 Suppl):71B-78B [PubMed PMID: 3998335]
Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2019 Aug 20:74(7):e51-e156. doi: 10.1016/j.jacc.2018.10.044. Epub 2018 Nov 6 [PubMed PMID: 30412709]
Level 1 (high-level) evidenceCingolani E, Goldhaber JI, Marbán E. Next-generation pacemakers: from small devices to biological pacemakers. Nature reviews. Cardiology. 2018 Mar:15(3):139-150. doi: 10.1038/nrcardio.2017.165. Epub 2017 Nov 16 [PubMed PMID: 29143810]
Cantillon DJ, Dukkipati SR, Ip JH, Exner DV, Niazi IK, Banker RS, Rashtian M, Plunkitt K, Tomassoni GF, Nabutovsky Y, Davis KJ, Reddy VY. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart rhythm. 2018 Jul:15(7):1023-1030. doi: 10.1016/j.hrthm.2018.04.022. Epub [PubMed PMID: 29957188]
Level 2 (mid-level) evidenceNelson GD. A brief history of cardiac pacing. Texas Heart Institute journal. 1993:20(1):12-8 [PubMed PMID: 8508058]
Hayes DL. Timing cycles of permanent pacemakers. Cardiology clinics. 1992 Nov:10(4):593-608 [PubMed PMID: 1423375]
Boink GJ, Christoffels VM, Robinson RB, Tan HL. The past, present, and future of pacemaker therapies. Trends in cardiovascular medicine. 2015 Nov:25(8):661-73. doi: 10.1016/j.tcm.2015.02.005. Epub 2015 Feb 20 [PubMed PMID: 26001958]
Hayes DL, Vlietstra RE. Pacemaker malfunction. Annals of internal medicine. 1993 Oct 15:119(8):828-35 [PubMed PMID: 8379604]
Atlee JL, Bernstein AD. Cardiac rhythm management devices (part II): perioperative management. Anesthesiology. 2001 Dec:95(6):1492-506 [PubMed PMID: 11748411]
Sabbagh E, Abdelfattah T, Karim MM, Farah A, Grubb B, Karim S. Causes of Failure to Capture in Pacemakers and Implantable Cardioverter-defibrillators. The Journal of innovations in cardiac rhythm management. 2020 Feb:11(2):4013-4017. doi: 10.19102/icrm.2020.110207. Epub 2020 Feb 15 [PubMed PMID: 32368374]
Wang YP, Chen BX, Su KJ, Sun LJ, Zhang Y, Guo LJ, Gao W. [Hyperkalemia-induced failure of pacemaker capture and sensing: a case report]. Beijing da xue xue bao. Yi xue ban = Journal of Peking University. Health sciences. 2014 Dec 18:46(6):980-2 [PubMed PMID: 25512296]
Level 3 (low-level) evidenceNguyên UC, Crijns HJGM. Undersensing, asynchronous pacing, and ventricular fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2019 Jul 1:21(7):1078. doi: 10.1093/europace/euz009. Epub [PubMed PMID: 30726912]
Furman S. Pacemaker sensing. Pacing and clinical electrophysiology : PACE. 1986 Mar:9(2):157 [PubMed PMID: 2419862]
Topf A, Motloch LJ, Kraus J, Danmayr F, Mirna M, Schernthaner C, Hoppe UC, Strohmer B. Exercise-related T-wave oversensing: an underestimated cause of reduced exercise capacity in a pacemaker-dependent patient-a case report and review of the literature. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2020 Oct:59(1):67-70. doi: 10.1007/s10840-019-00698-6. Epub 2020 Jan 23 [PubMed PMID: 31974858]
Level 3 (low-level) evidenceBoriani G, Biffi M, Schwarz T, Dong Y, Koenig A, Temporin S, Meyer S, Sperzel J. Evaluation of fusion beat detection with a new ventricular automatic capture algorithm in ICDs. Pacing and clinical electrophysiology : PACE. 2005 Jan:28 Suppl 1():S263-6 [PubMed PMID: 15683511]
Sweesy MW, Batey RL, Forney RC. Crosstalk during bipolar pacing. Pacing and clinical electrophysiology : PACE. 1988 Nov:11(11 Pt 1):1512-6 [PubMed PMID: 2462232]
Level 3 (low-level) evidenceSingh M, McCoy C, Daniels J. Ventricular Safety Pacing Triggered by Right Ventricular Lead Dislodgement. Circulation. 2019 Nov 19:140(21):1766-1768. doi: 10.1161/CIRCULATIONAHA.119.043267. Epub 2019 Nov 18 [PubMed PMID: 31738594]
Jastrzębski M. Pacemaker-mediated tachycardia: What is the mechanism? Pacing and clinical electrophysiology : PACE. 2018 Nov:41(11):1549-1551. doi: 10.1111/pace.13489. Epub 2018 Sep 19 [PubMed PMID: 30191581]
Alasti M, Machado C, Rangasamy K, Bittinger L, Healy S, Kotschet E, Adam D, Alison J. Pacemaker-mediated arrhythmias. Journal of arrhythmia. 2018 Oct:34(5):485-492. doi: 10.1002/joa3.12098. Epub 2018 Aug 3 [PubMed PMID: 30327693]
Ip JE, Lerman BB. Validation of device algorithm to differentiate pacemaker-mediated tachycardia from tachycardia due to atrial tracking. Heart rhythm. 2016 Aug:13(8):1612-7. doi: 10.1016/j.hrthm.2016.04.011. Epub 2016 Apr 19 [PubMed PMID: 27108937]
Level 1 (high-level) evidenceStrik M, Frontera A, Eschalier R, Defaye P, Mondoly P, Ritter P, Haïssaguerre M, Ploux S, Bordachar P. Accuracy of the pacemaker-mediated tachycardia algorithm in Boston Scientific devices. Journal of electrocardiology. 2016 Jul-Aug:49(4):522-9. doi: 10.1016/j.jelectrocard.2016.04.004. Epub 2016 Apr 22 [PubMed PMID: 27199031]
Furman S. Dual chamber pacemakers: upper rate behavior. Pacing and clinical electrophysiology : PACE. 1985 Mar:8(2):197-214 [PubMed PMID: 2580281]
Ortega DF, Sammartino MV, Pellegrino GM, Barja LD, Albina G, Segura EV, Balado R, Laiño R, Giniger AG. Runaway pacemaker: a forgotten phenomenon? Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2005 Nov:7(6):592-7 [PubMed PMID: 16216762]
Level 3 (low-level) evidenceWilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A, Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002 Dec 25:288(24):3115-23 [PubMed PMID: 12495391]
Level 1 (high-level) evidenceSteinbach K, Laczkovics A, Mohl W. [Sudden cardiac death in patients with pacemakers]. Acta medica Austriaca. 1978:5(1):1-5 [PubMed PMID: 685634]
Crossley GH, Poole JE, Rozner MA, Asirvatham SJ, Cheng A, Chung MK, Ferguson TB Jr, Gallagher JD, Gold MR, Hoyt RH, Irefin S, Kusumoto FM, Moorman LP, Thompson A. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart rhythm. 2011 Jul:8(7):1114-54. doi: 10.1016/j.hrthm.2010.12.023. Epub [PubMed PMID: 21722856]
Level 3 (low-level) evidenceKalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing and clinical electrophysiology : PACE. 2005 Apr:28(4):326-8 [PubMed PMID: 15826268]
Indik JH, Gimbel JR, Abe H, Alkmim-Teixeira R, Birgersdotter-Green U, Clarke GD, Dickfeld TL, Froelich JW, Grant J, Hayes DL, Heidbuchel H, Idriss SF, Kanal E, Lampert R, Machado CE, Mandrola JM, Nazarian S, Patton KK, Rozner MA, Russo RJ, Shen WK, Shinbane JS, Teo WS, Uribe W, Verma A, Wilkoff BL, Woodard PK. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart rhythm. 2017 Jul:14(7):e97-e153. doi: 10.1016/j.hrthm.2017.04.025. Epub 2017 May 11 [PubMed PMID: 28502708]
Level 3 (low-level) evidenceRusso RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, Boyle NG, Frabizzio JV, Birgersdotter-Green U, Higgins SL, Lampert R, Machado CE, Martin ET, Rivard AL, Rubenstein JC, Schaerf RH, Schwartz JD, Shah DJ, Tomassoni GF, Tominaga GT, Tonkin AE, Uretsky S, Wolff SD. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. The New England journal of medicine. 2017 Feb 23:376(8):755-764. doi: 10.1056/NEJMoa1603265. Epub [PubMed PMID: 28225684]
Jung W, Zvereva V, Hajredini B, Jäckle S. Safe magnetic resonance image scanning of the pacemaker patient: current technologies and future directions. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012 May:14(5):631-7. doi: 10.1093/europace/eur391. Epub 2012 Jan 10 [PubMed PMID: 22237585]
Level 3 (low-level) evidenceHenrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing and clinical electrophysiology : PACE. 2006 May:29(5):509-11 [PubMed PMID: 16689847]
Level 3 (low-level) evidencePlatonov MA, Gillis AM, Kavanagh KM. Pacemakers, implantable cardioverter/defibrillators, and extracorporeal shockwave lithotripsy: evidence-based guidelines for the modern era. Journal of endourology. 2008 Feb:22(2):243-7. doi: 10.1089/end.2007.0021. Epub [PubMed PMID: 18294028]
Level 1 (high-level) evidenceThomas D, Becker R, Katus HA, Schoels W, Karle CA. Radiation therapy-induced electrical reset of an implantable cardioverter defibrillator device located outside the irradiation field. Journal of electrocardiology. 2004 Jan:37(1):73-4 [PubMed PMID: 15132373]
Level 3 (low-level) evidenceKapa S, Fong L, Blackwell CR, Herman MG, Schomberg PJ, Hayes DL. Effects of scatter radiation on ICD and CRT function. Pacing and clinical electrophysiology : PACE. 2008 Jun:31(6):727-32. doi: 10.1111/j.1540-8159.2008.01077.x. Epub [PubMed PMID: 18507546]
Marbach JR, Sontag MR, Van Dyk J, Wolbarst AB. Management of radiation oncology patients with implanted cardiac pacemakers: report of AAPM Task Group No. 34. American Association of Physicists in Medicine. Medical physics. 1994 Jan:21(1):85-90 [PubMed PMID: 8164594]
Nawa S, Shimizu N, Kino K, Hayashi K. Spontaneous secure reimplantation of a dislodged pacemaker electrode onto the right ventricular outflow tract, reestablishing a sufficient pacing condition. Clinical cardiology. 1993 Mar:16(3):267-9 [PubMed PMID: 8444003]
Level 3 (low-level) evidenceFavale S, Nacci F. Percutaneous transcatheter repositioning of displaced permanent pacemaker lead. Pacing and clinical electrophysiology : PACE. 1999 Dec:22(12):1817-9 [PubMed PMID: 10642138]
Level 3 (low-level) evidenceEagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, Fleisher LA, Froehlich JB, Gusberg RJ, Leppo JA, Ryan T, Schlant RC, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC Jr, American College of Cardiology, American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Journal of the American College of Cardiology. 2002 Feb 6:39(3):542-53 [PubMed PMID: 11823097]
Level 1 (high-level) evidence