Introduction
Bone infection is called osteomyelitis. It is an acute or chronic inflammatory process involving the bone and its structures secondary to infection with pyogenic organisms, including bacteria, fungi, and mycobacteria. Interestingly, archeological finds showed animal fossils with evidence of bone infection, making this a relatively old disease.[1] Various terms were used to describe infected bone over the years until Nelaton came up with the term osteomyelitis in 1844.[1] Before the introduction of penicillin in the 1940s, management of osteomyelitis was mainly surgically consisting of extensive debridement, saucerization, and wound packing following which the affected area is left to heal by secondary intention[1] resulting in high mortality from sepsis. Since the availability of antibiotics, mortality rates from osteomyelitis, including staphylococcal osteomyelitis, has improved significantly.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Healthy intact bone is resistant to infection. The bone becomes susceptible to disease with the introduction of a large inoculum of bacteria, from trauma, ischemia, or the presence of foreign bodies because bone sites to which microorganisms can bind are exposed.[2] Certain bacteria such as Staphylococcus aureus adhere to the bone by expressing receptors, called adhesins, for some components of the bone matrix, including laminin, collagen, fibronectin, and bone sialoglycoprotein. S. aureus expresses a collagen-binding adhesin, which permits its attachment to bone cartilage while the fibronectin-binding adhesin's role in attachment of bacteria to surgically implanted devices in bone was recently discovered.[2] Also interesting to note is that S. aureus can survive intracellularly after being internalized by cultured osteoblasts. Some bacteria create a protective biofilm coating around themselves and underlying surfaces. This characteristic of some bacteria to adhere to the bone and surgically implanted devices following which they express phenotypic resistance to antibiotic therapy and their ability to survive intracellularly may explain the persistence of bone infections and high failure rates of shorter courses of antimicrobial treatment.[2]
Epidemiology
The overall incidence of osteomyelitis in the United States is mostly unknown, but reports show it to be as high as 1 in 675 US hospital admissions each year or about 50,000 cases annually.[3] Other studies show an overall incidence of osteomyelitis of 21.8 cases per 100,000 person-years.[4] The incidence was higher in men for unknown reasons but increases with age, mainly due to an increase in the prevalence of comorbid factors such as diabetes mellitus and peripheral vascular disease.[4] Also, an increase in the availability of sensitive imaging tests, such as magnetic resonance imaging (MRI) and bone scintigraphy has improved diagnostic accuracy and the ability to characterize the infection.[5]
Pathophysiology
Bone can get infected via the hematogenous route of infection through bacteremic seeding of bone from a distant source of infection, contiguous spread from surrounding tissue and joints, or direct inoculation of bone from trauma or surgery.[1][2] Hematogenous osteomyelitis occurs more frequently in children compared to adults, and long bones are usually affected.[5] In adults, hematogenous osteomyelitis affects the vertebrae most commonly. Contiguous osteomyelitis in young adults usually occurs in the setting of trauma and related surgery, while in older adults, infection is typically related to decubitus ulcers and infected joint arthroplasties.[1] Osteomyelitis associated with vascular insufficiency frequently occurs in the presence of underlying diabetes mellitus.
In patients with diabetes, osteomyelitis usually results from compromised blood supply to the lower extremities, which contributes to impaired local immunity and skin healing, promoting the spread of infection. Sensory neuropathy in the setting of diabetes mellitus predisposes to the formation of skin ulceration at pressure and trauma points, complicating matters even worse.[1][2][5]
Contiguous osteomyelitis frequently develops in debilitated patients who are wheelchair or bedbound and are predisposed to pressure-related skin ulcerations, especially in the sacrum, buttock, hips, and heel. These ulcers are typically colonized by polymicrobial flora from the skin and gastrointestinal tract such that soft tissue infection can quickly spread to the underlying bone.[1] Other sources of contiguous osteomyelitis are trauma leading to infected, exposed skin, and soft tissue. Osteomyelitis with direct inoculation of bacteria may occur in the setting of open fractures, bone reconstructive surgery, or with placing orthopedic hardware.[1][2]
Histopathology
The well-perfused metaphyses, which have scarce functioning phagocytes, are the most common site of infection in hematogenous osteomyelitis affecting the long bones. The blood supply to the long bones penetrates the bone at the mid-shaft then splits into two traveling to both metaphyseal endplates. Slowing of blood flow in vascular loops at the metaphysis encourages the deposition of microbes and the establishment of infection. To contain the disease, phagocytes release enzymes that lyse bone, creating an inflammatory response. This inflammatory response forms pus (a protein-rich exudate containing dead phagocytes, tissue debris, and microorganisms) and causes increased intramedullary pressure. The inflammatory exudate can rupture through the cortex to the periosteum if left unchecked. Disruption of the periosteum impairs the periosteal blood supply leading to bone ischemia then necrosis.[1][2] Separated pieces of necrotic bone are called sequestra, which occasionally contain pus. New bone formation over the injured periosteum is called involucrum, and it may partially surround a sequestrum. Discharge from a sequestrum can lead to sinus tract formation.
The main histopathological finding in acute osteomyelitis are microorganisms, congested or thrombosed blood vessels, and infiltrates of neutrophils. On the other hand, the hallmark histopathological finding in chronic osteomyelitis is necrotic bone. Other features of chronic osteomyelitis include a predominance of mononuclear cells, replacement of osteoclast resorbed bone by granulation, and fibrous tissue leading to bone loss and the formation of sinus tracts, which is pathognomic.
Native vertebral osteomyelitis (NVO) is usually a hematogenous infection affecting two adjacent vertebral endplates.[6] This presentation is explained by the blood supply of the vertebrae as segmental arteries typically bifurcate to supply two adjacent endplates of contiguous vertebrae.[1] Venous drainage from Batson's plexus may explain spondylodiscitis metastasizing from a urinary tract focus, especially in senior men.
The categorization of osteomyelitis as acute or chronic is based on the histopathological findings rather than the duration of illness.[5] Acute osteomyelitis refers to infection occurring before the development of sequestra and usually takes place within two weeks of disease onset. However, the development of sequestra may be slow in some settings, such as vertebral osteomyelitis, and rapid in others as when associated with prosthetic devices or open fractures. Following the development of necrotic bone and the formation of sequestra, the infection is considered chronic.
There are two major classification schemes for osteomyelitis. The first is by Lew and Waldvogel, while the other is by Cierny and Mader. Lew and Waldvogel classified osteomyelitis based on the duration of illness as acute or chronic and by the mechanism of infection (either hematogenous or contiguous infection). Contiguous infection is further classified based on the presence or absence of associated vascular insufficiency.
The Cierny and Mader scheme provides guidance in patient management. In this scheme, the classification of osteomyelitis is by anatomic stage and the host health status.
The categories and corresponding anatomic types are:
- Stage 1: Disease confined to the medullary of the bone
- Stage 2: Superficial disease
- Stage 3: Localized spread
- Stage 4: Diffuse disease
The local and systemic factors which define host health status are:
- A: Normal host
- Bs: Host with systemic compromising factors
- Bl: Host with local compromising factors
- Bsl: Host with both local and systemic compromising factors
- C: Host for whom treatment of the osteomyelitis is worse than the disease itself.
Causes of systemic host compromise are malnutrition, renal and hepatic failure, diabetes mellitus, chronic hypoxia, neoplasm, and immunodeficiency disease. Local compromising factors of the host health status are chronic lymphedema, venous stasis, major and small vessel disease, arteritis, peripheral neuropathy, and tobacco use.
History and Physical
The clinical presentation of osteomyelitis depends on the etiology. Sometimes diagnosis in adults can be tricky, and it requires a high index of suspicion. A good history and physical is always the right place to start and are essential parts of the initial evaluation. Some patients are at high risk for osteomyelitis, and these include those with bacteremia, endocarditis, intravenous drug use, trauma, and open fractures. Also, patients with chronic poorly healing wounds in the setting of diabetes mellitus, peripheral vascular disease, peripheral neuropathy, or orthopedic hardware are at increased risk.[1] Acute osteomyelitis may present gradually with onset over a few days but usually manifests within two weeks. Patients may have local symptoms such as erythema, swelling, and warmth at the site of infection. There may be a dull pain with or without motion and sometimes constitutional symptoms such as fever or chills. In subacute presentations, some patients may have generalized malaise, mild pain over several weeks with minimal fever, or other constitutional symptoms. Acute osteomyelitis may also present as septic arthritis, especially if the metaphyses of the bone is within the infected joint capsule. Septic arthritis of the elbow, shoulder and hip joints may complicate osteomyelitis of the proximal radius, humerus, and femur, respectively. New or worsening neck or back pain in a patient with fever, elevated inflammatory markers (CRP, erythrocyte sedimentation rate [ESR]),[6] bacteremia, or endocarditis should raise the suspicion for native vertebral osteomyelitis (NVO).[7]
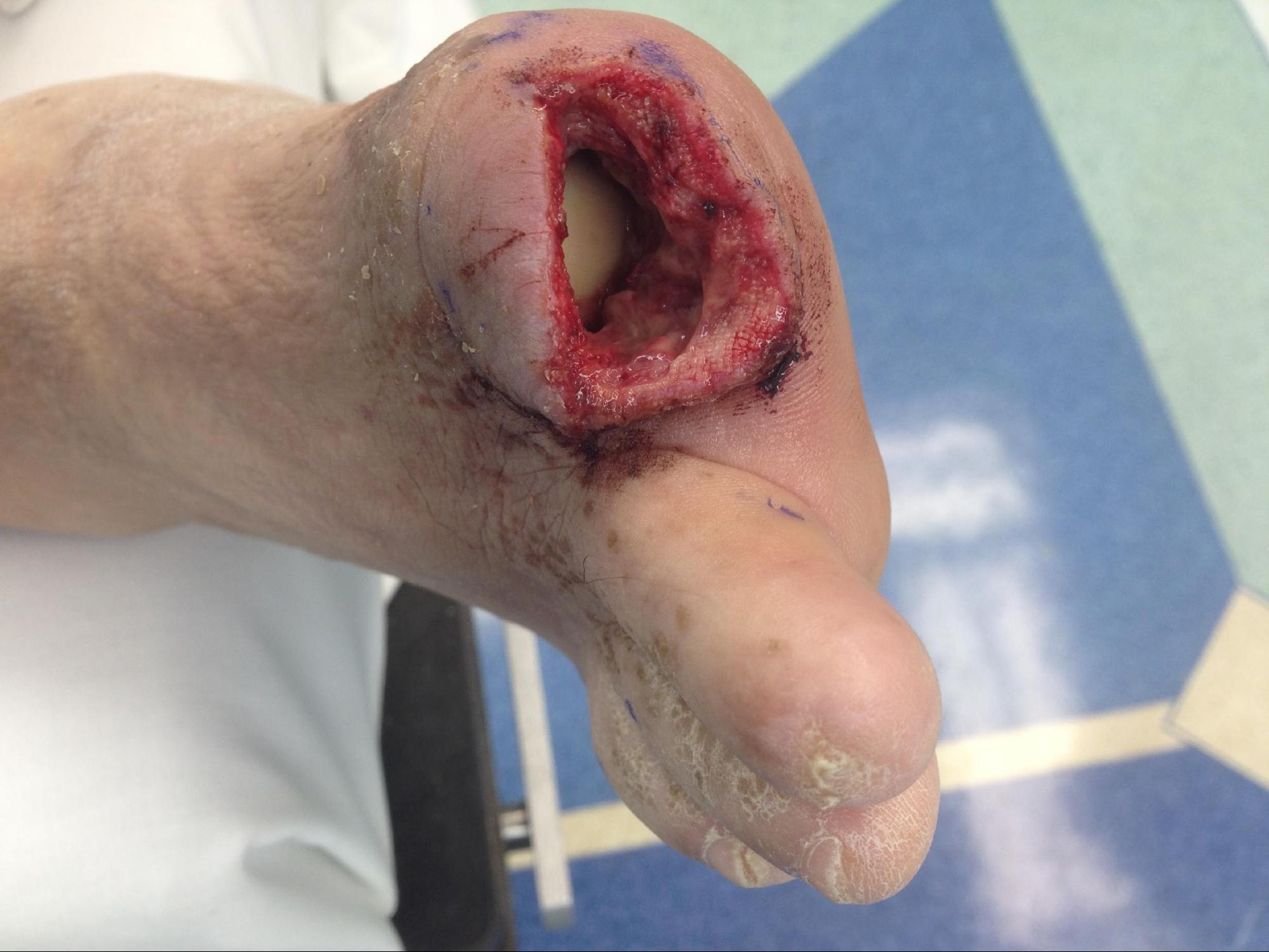
In chronic osteomyelitis, symptoms may occur over a longer duration of time, usually more than two weeks. As with acute osteomyelitis, patients may also present with swelling, pain, and erythema at the site of infection, but constitutional symptoms like fever are less common. Patients who have deep or extensive ulcers that do not heal after several weeks of appropriate therapy, especially in people with diabetes or debilitated patients, should raise the suspicion for osteomyelitis. Physical examination should focus primarily on finding a possible nidus of infection, assessing sensory function, and peripheral vasculature. Tenderness to palpation over vertebral bone may be a significant finding in vertebral osteomyelitis. The ability to probe an ulcer to the bone with a blunt sterile instrument is highly suggestive of osteomyelitis.[2] The probe to bone test is a screening tool in conjunction with the patient's pretest probability for osteomyelitis to determine whether additional diagnostic tests such as radiographic imaging or bone biopsy are required for therapeutic decisions.[8]
Evaluation
Laboratory data can be useful in the assessment of osteomyelitis but are usually nonspecific for osteomyelitis. There may or may not be leukocytosis, the elevation of ESR, and C-reactive protein (CRP). The CRP level correlates with clinical response to therapy and may be used to monitor treatment. Blood cultures may be positive, especially in hematogenous osteomyelitis involving the vertebrae, clavicle, or pubis.
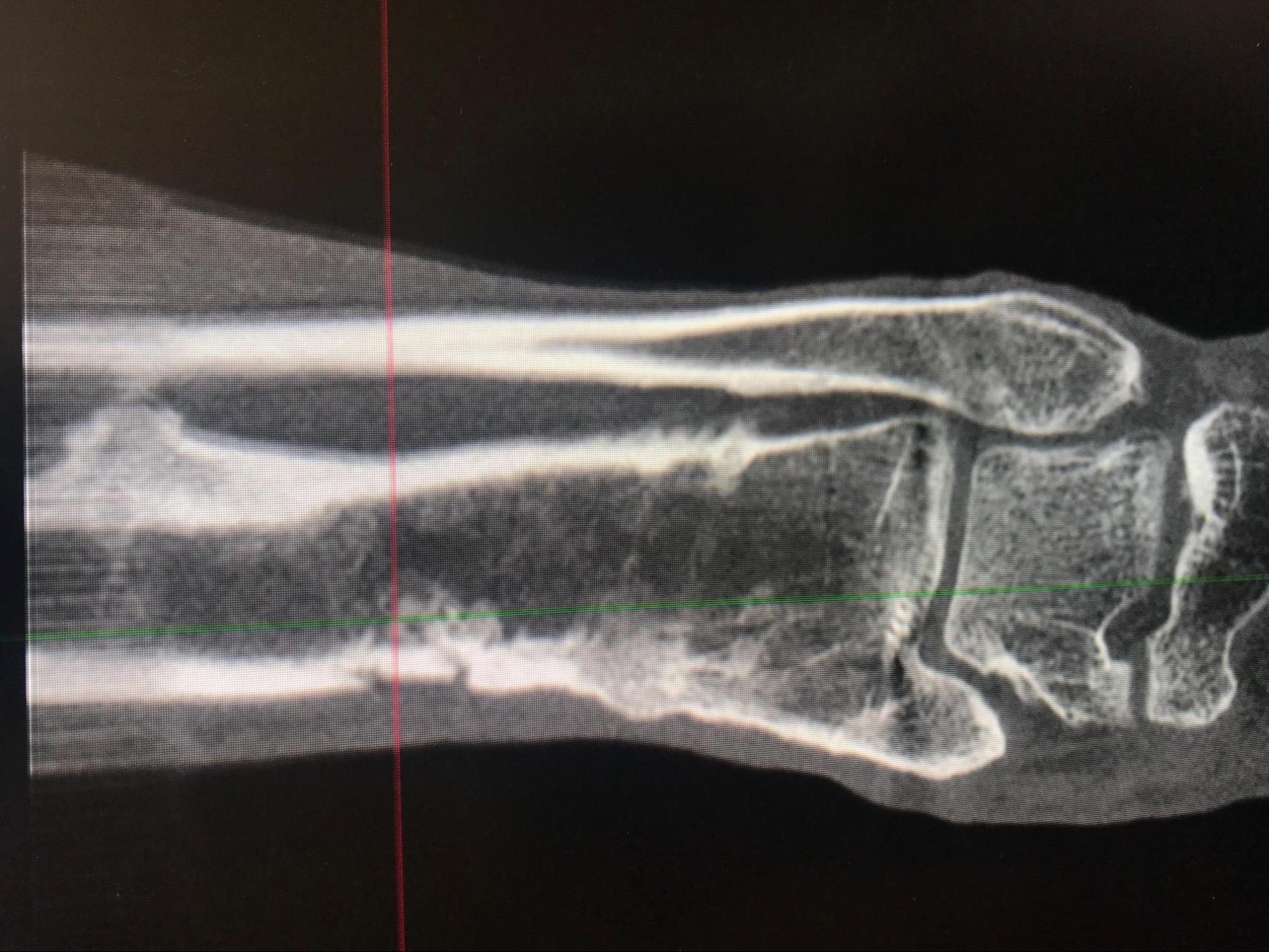
Radiographic imaging is an essential component of the evaluation of a patient with suspected osteomyelitis. Clinically, the most useful studies are plain radiographs, magnetic resonance imaging (MRI), and technetium-99 bone scintigraphy.[5] A plain radiograph is usually the initial imaging of choice but may have a delay of about 14 days before the appearance of findings suggestive of osteomyelitis.[1][5] They are, however, used to rule out other potential causes of symptoms such as metastasis or osteoporotic fractures. Typically seen are soft tissue swelling, osteopenia, osteolysis, bony destruction, and nonspecific periosteal reaction. Lytic lesions are detectable on plain radiographs after approximately 50% to 75% of the bone matrix has been lost, making this modality inadequate for the detection of early bone disease.[5] Rarely, Brodie's abscess, which is a well-circumscribed lytic lesion, may be seen in a child who has been in pain for months without fever.
Of all the imaging modalities currently in use, MRI has the highest combined sensitivity and specificity (78% to 90% and 60 % to 90% respectively) for detecting osteomyelitis. It can detect early bone infection within 3 to 5 days of disease onset [5][6], but its use is limited in the setting of surgical hardware. MRI has a high negative predictive value, so a negative result is sufficient for the exclusion of disease if symptoms have been present for at least one week. The use of intravenous contrast does not improve the detection of disease but helps provide the distinction between a phlegmon, necrotic tissue, and abscess. Nuclear imaging has a high sensitivity for detecting early evidence of bone disease but has very poor specificity. It is especially useful if metal hardware prevents the use of MRI. Three-phase technetium-99 bone scan and tagged white blood cell scans are the modalities commonly used.
Other imaging modalities less commonly used are positron emission tomography (PET), which is expensive and not routinely available, leukocyte scintigraphy, gallium scan, and computed tomography (CT scan). A CT scan is more sensitive than a plain radiograph for assessing cortical and trabecular integrity, periosteal reaction, intraosseous and soft tissue gas, the extent of a sinus tract, and is superior to MRI in detecting necrotic bone fragments. However, though the CT scan is readily available compared to the MRI, it is more expensive than plain radiograph and has a limited role in the diagnosis of osteomyelitis. It should be used mainly to determine the extent of bony destruction (especially in the spine) to guide biopsies or in patients with contraindications to MRI.
Bone biopsy (either open or percutaneously) is essential to establish the histopathological diagnosis in osteomyelitis, identify the causative pathogen, and provide susceptibility data that helps direct antibiotic therapy. Superficial wound cultures or material from needle puncture or sinus tracts should not be used in diagnosis as these specimens do not correlate well with bone biopsy results.[1] In patients with positive blood cultures and radiographic evidence of osteomyelitis, a bone biopsy may not be very useful[7]. An open bone biopsy is preferred over percutaneous biopsy, if possible. Cessation of antibiotics 48 to 72 hours before open bone biopsy may increase microbiological yield but is not routinely necessary as bone cultures are often positive regardless of prior antibiotic therapy because these infections occur in areas of infection-induced infarction or necrosis. Percutaneous biopsy should be done through intact skin to prevent sampling errors, and fluoroscopic or CT guidance is preferable. Percutaneous biopsy should be done ideally before initiation of antibiotic therapy, if possible, to increase the microbiological yield. Usually recommended is the collection of 2 samples, one for histopathology and the other for culture and gram stain.
Treatment / Management
Hematogenous osteomyelitis is primarily monomicrobial, while osteomyelitis due to contiguous spread or direct inoculation is usually polymicrobial or monomicrobial.[1] The most common pathogens in osteomyelitis depend on the patient's age. Staphylococcus aureus is the most common cause of acute and chronic hematogenous osteomyelitis in adults and children.[1][5] Increasingly isolated from patients with osteomyelitis is methicillin-resistant Staphylococcus aureus (MRSA). In some studies, MRSA accounted for over one-third of all staphylococcal isolates.[5] Other common pathogens are coagulase-negative staphylococcus, beta-hemolytic streptococcus, enterococci, aerobic gram-negative bacilli (including Pseudomonas species, Enterobacter species, Escherichia coli), and anaerobic gram-negative bacilli (such as Peptostreptococcus, Clostridium species, Bacteroides).
It is crucial to consider less common pathogens in the appropriate epidemiological setting and immunocompromised patients. These include Mycobacterium tuberculosis, which may spread to the spine from the lungs; nontuberculous mycobacteria (Mycobacterium avium intracellulare, Bacille Calmette-Guerin, which may complicate intravesical therapy for bladder cancer); Candida species; fungi such as Blastomyces, Coccidiodes, Cryptococcus, and Aspergillus. Infection with Actinomyces and Sporothrix usually follow traumatic inoculation. Salmonella and S. aureus are implicated in hematogenous osteomyelitis in sickle cell disease, while Brucella and Salmonella occur in spinal infections.[1][2] Bartonella henselae may be associated with HIV-related osteomyelitis and Pasteurella multocida or Eikenella corrodens seen in human or animal bites.
Effective treatment of osteomyelitis involves a collaborative effort among various medical and surgical specialties. The two main aspects of therapy are surgical containment of the infection and prolonged antibiotics. Surgical debridement of all diseased bone is often required as antibiotics penetrate poorly into infected fluid collections such as abscesses and injured or necrotic bone.[1] Thus, the removal of necrotic tissue and bone is usually indicated where feasible.[2][1] Preoperative use of imaging modalities such as MRI allows for delineation of the extent of the infection, but intraoperatively it is still difficult for the surgeon to determine if all necrotic bone and tissue have been successfully removed. Examination of the pathology report helps determine if repeat debridement is necessary. In osteomyelitis associated with prosthetic joints, removal of the hardware is indicated.[2] However, if the infected prosthesis is in a stable joint such as the hip and is infected with a very susceptible organism such as streptococci, therapy with an extended antibiotic course for several months without removing the device has been successful.[2] When the prosthesis has to be removed, a two-stage exchange arthroplasty is more commonly used as this carries less risk of recurrent infection compared to the 1-stage arthroplasty, especially if more virulent bacteria such as S. aureus is involved.[2] If surgical debridement is not feasible based on the location of the infection, e.g., some cases of pelvic osteomyelitis, then extended antibiotic therapy for months may be used. NVO rarely requires surgical debridement except if there are associated neurological complications necessitating relief of spinal cord compression, failure of medical treatment, or need to drain epidural or paravertebral abscesses.[2] Debridement, however, is needed in cases of osteomyelitis associated with spinal implants.[6]
Also important is the need for revascularization of the affected limb before surgical intervention if there is evidence of significant peripheral vascular disease, control diabetes mellitus and address other host factors that may impede wound healing, including tobacco use, malnutrition, chronic hypoxia, immunodeficiency states, chronic lymphedema and peripheral neuropathy[2].
Prolonged antibiotic therapy is the cornerstone of treatment for osteomyelitis. Results of culture and sensitivity should guide antibiotic treatment if possible, but in the absence of this data, it is reasonable to start empiric antibiotics. A commonly used broad-spectrum empiric antibiotic regimen against both gram-positive and negative organisms, including MRSA, is vancomycin (15 mg/kg intravenously [IV] every 12 hours) plus a third a generation cephalosporin (e.g., ceftriaxone 2 gm IV daily) or a beta-lactam/beta-lactamase inhibitor combination (e.g., piperacillin/tazobactam 3.375 IV every 8 hours).[1] Once sensitivity data becomes available, then the antibiotic therapy should be narrowed for targeted coverage of the susceptible organisms.
Pathogen-Specific Antibiotic Therapy for Osteomyelitis in Adults[1][5][2][6]
Staphylococcus aureus penicillin-sensitive
Treatment of choice is penicillin G 4 million units every 6 hours
Alternative regimens are a first-generation cephalosporin, e.g., cefazolin 2 g IV every 8 hours, clindamycin 900 mg IV every 8 hours, vancomycin 15 mg/kg IV every 12 hours, oxacillin or nafcillin 2 g IV every 4 hours
Staphylococcus aureus penicillin-resistant
Treatment of choice is nafcillin 2 gm IV every 4 hours
Alternative therapies are cefazolin, clindamycin, or vancomycin (doses as above)
Staphylococcus aureus methicillin-resistant
Treatment of choice is vancomycin IV
An alternative regimen is linezolid 600 mg IV every 12 hours.
Streptococci (group A, B, Beta hemolytic, Streptococcus pneumoniae)
Treatment of choice is penicillin G 4 million units every 6 hours
Alternative regimens include ceftriaxone 2 gm IV daily, clindamycin IV, vancomycin IV, cefazolin IV (doses as above)
Enterobacteriaceae quinolone sensitive
Treatment of choice is ciprofloxacin 400 mg IV twice per day (bid) or 750 mg orally (PO) bid, levofloxacin 500 to 750 mg PO or IV daily
Alternative regimens include ceftriaxone 2g IV daily, cefepime 2 gm IV every 12 hours, ceftazidime 2 gm IV every 8 hours
Enterobacteriaceae, quinolone-resistant (Escherichia coli)
Treatment of choice is piperacillin/tazobactam 3.375 g IV every 8 hours, Ticarcillin/clavulanate 3.1 gm IV every 4 hours
An alternative regimen is ceftriaxone 2 g IV daily
Pseudomonas aeruginosa
Treatment of choice is cefepime 2 gm IV every 12 hours, ceftazidime 2 gm IV every 8 hours
Alternative regimens include meropenem 1 gm IV every 8 hours, Imipenem 500 mg IV every 6 hours, ciprofloxacin 400 mg IV every 12 hours, or 750 mg PO daily
Enterococci
Treatment of choice is penicillin G 4 million units every 6 hours
Alternatively, vancomycin 15 mg/kg every 12 hours, daptomycin 6 mg/kg IV daily, linezolid 600 mg IV or PO every 12 hours
Anaerobes
Treatment of choice is clindamycin 900 mg IV every 8 hours, ticarcillin/clavulanate 3.1 gm IV every 4 hours
Alternatively, metronidazole 500 mg IV every 8 hours (for gram-negative anaerobes)[1][2][5]
The recommended duration of treatment for osteomyelitis in adults is 4 to 6 weeks of parenteral antibiotic therapy to achieve acceptable cure rates with a decreased risk of recurrence.[7][6][2] In cases where the infected bone is wholly debrided or amputated with clean disease-free margins documented, a shorter duration of antibiotic therapy is acceptable. A 2-week course of antibiotics postoperatively is sufficient to allow for the treatment of any residual tissue infection and wound healing of the surgical site.[1](A1)
Vacuum-assisted wound closure devices are used in the right clinical setting, especially where large or deep wounds are left after extensive debridement. These devices have been shown to promote healing by both direct and indirect effects on the wound.[9] Hyperbaric oxygen therapy is not routinely recommended in the treatment of osteomyelitis.
Differential Diagnosis
The differential diagnosis for osteomyelitis is broad, and it is essential to keep these in mind during the evaluation of a patient with a suspected bone infection. These differentials include:
- Charcot arthropathy especially in people with diabetes
- SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis)
- Arthritis including rheumatoid arthritis
- Metastatic bone disease
- Fracture, including pathological and stress fractures.
- Gout
- Avascular necrosis of the bone
- Bursitis
- Sickle cell vaso-occlusive pain crises
Prognosis
With aggressive early treatment, the prognosis of acute osteomyelitis is good. However, there is a possibility that the infection could recur years after successful treatment if there is new trauma to the same area or if host immunity is compromised.[10] In adults, the recurrence rate of chronic osteomyelitis is about 30% at 12 months, but in cases involving P. aeruginosa, the recurrence rate may be as high as 50%.[5] Cases involving prosthetic devices are more difficult to treat, causing increased morbidity due to the need for more surgical procedures and extended antibiotic courses required for treatment. Various measures used to prevent postoperative infections include good preoperative preparation where possible and the use of surgical rooms with laminar airflow. Recommended also is the use of prophylactic preoperative antibiotic treatment administered parenterally 30 minutes before skin incision with first-generation (cefazolin) or second-generation cephalosporins (cefuroxime). All these measures have been shown to decrease the rate of postoperative infections from 0.5% to 2%, thereby improving patient outcomes.[2]
Complications
Early treatment, including antibiotic therapy, is necessary to prevent the development of complications. Some complications which may arise with untreated or inadequately treated osteomyelitis are:
- Septic arthritis
- Pathological fractures
- Squamous cell carcinoma
- Sinus tract formation
- Amyloidosis (rare)
- Abscess
- Bone deformity
- Systemic infection
- Contiguous soft tissue infection
Deterrence and Patient Education
Patient education about the prolonged nature of therapy and the need for compliance with treatment recommendations to ensure adequate wound healing thereby reducing the risk for recurrence is an essential part of the care in these patients.
Enhancing Healthcare Team Outcomes
Osteomyelitis is a complicated infection to treat. In most cases, management involves a multifaceted, interprofessional approach, including the primary care provider, radiologist, surgeons (orthopedic, vascular), a podiatrist, an infectious disease specialist, pharmacist, nurse wound care team, and sometimes a plastic surgeon, a pain specialist or interventional radiologist. The primary care provider often plays a vital role in the initial diagnosis and coordination of care across these medical and surgical specialties.
The therapeutic approach to management is guided sometimes by the site of infection and the presence of vascular insufficiency. Minimally invasive CT or fluoroscopically guided aspiration of the disc space (usually by interventional radiology) for microbiological and histopathological testing is recommended for the diagnosis of suspected NVO if clinical, imaging and other laboratory data suggest osteomyelitis in the absence of positive blood cultures with an organism known to cause osteomyelitis (S. aureus, Staphylococcus lugdunensis, Brucella).[7] However, in patients with suspected subacute NVO and strongly positive Brucella serology or those with suspected NVO based on available clinical, radiological, laboratory data and positive blood cultures involving an organism known to cause osteomyelitis, image-guided aspiration biopsy is not recommended. A 6-week course of parenteral antibiotics is reserved for cases with neurological complications or failure of medical therapy.[7]
Once again, a strong collaboration between the surgical and vascular teams is essential for the effective management of osteomyelitis due to vascular insufficiency, especially if associated with diabetes mellitus. Revascularization of the affected limb is critical for proper wound healing. Procedures to restore adequate blood flow, including therapeutic angiogram by either interventional radiology or vascular surgery and even femoral to popliteal by-pass, are used before amputation depending on the severity of vascular insufficiency. Surgical revascularization procedures that use local pedicle muscle flaps and myocutaneous flaps maintain vascular supply to the surgical site, fill the space left by surgical debridement, and also fight infection.[2]
Following surgical debridement, the need for close follow-up with prolonged antibiotics and meticulous wound care cannot be overemphasized. Infectious disease specialists involved in the patient's care often monitor response to therapy and adjust antibiotics regimens where needed. Many patients can continue with parental antibiotics outpatient via peripherally inserted intravenous access to complete their antibiotic course while others can transition to highly bioavailable oral antibiotic therapy such as quinolones when feasible based on microbiological results. Qualified home-bound patients benefit from home health wound nurse visits several times a week, while those who are ambulatory are encouraged to use wound care clinics if available. Family members and caregivers are also an integral part of the care team, providing support and care between healthcare provider visits. Only through such an approach can the morbidity of osteomyelitis be decreased.[11] [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
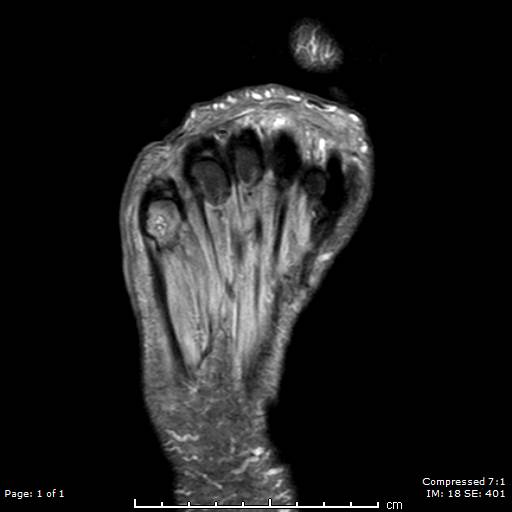
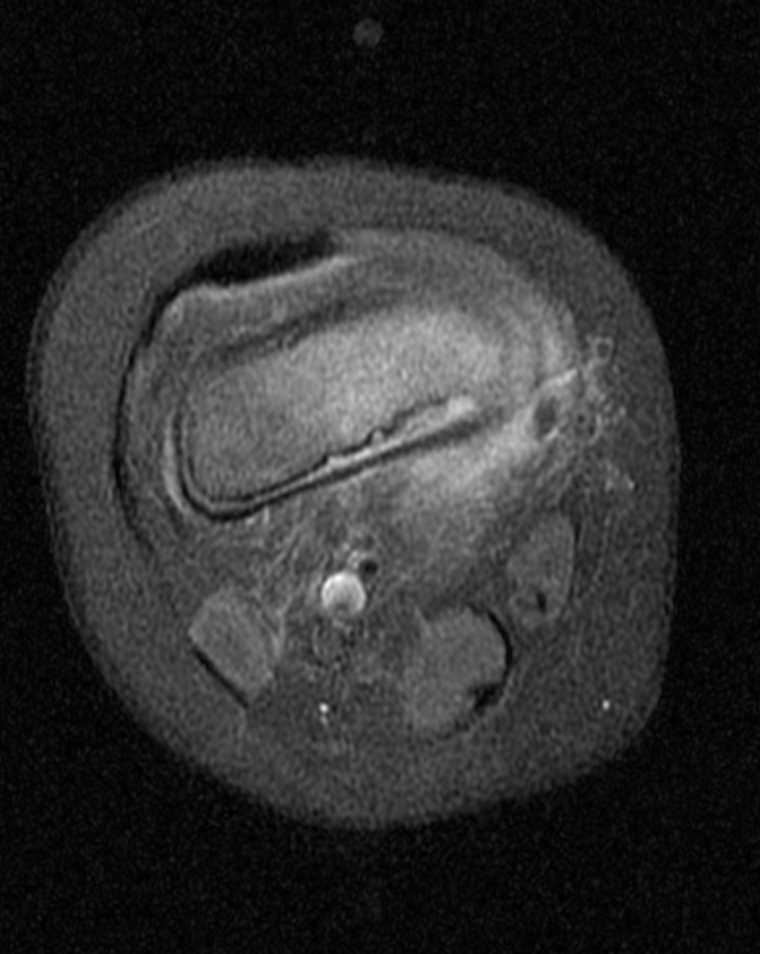
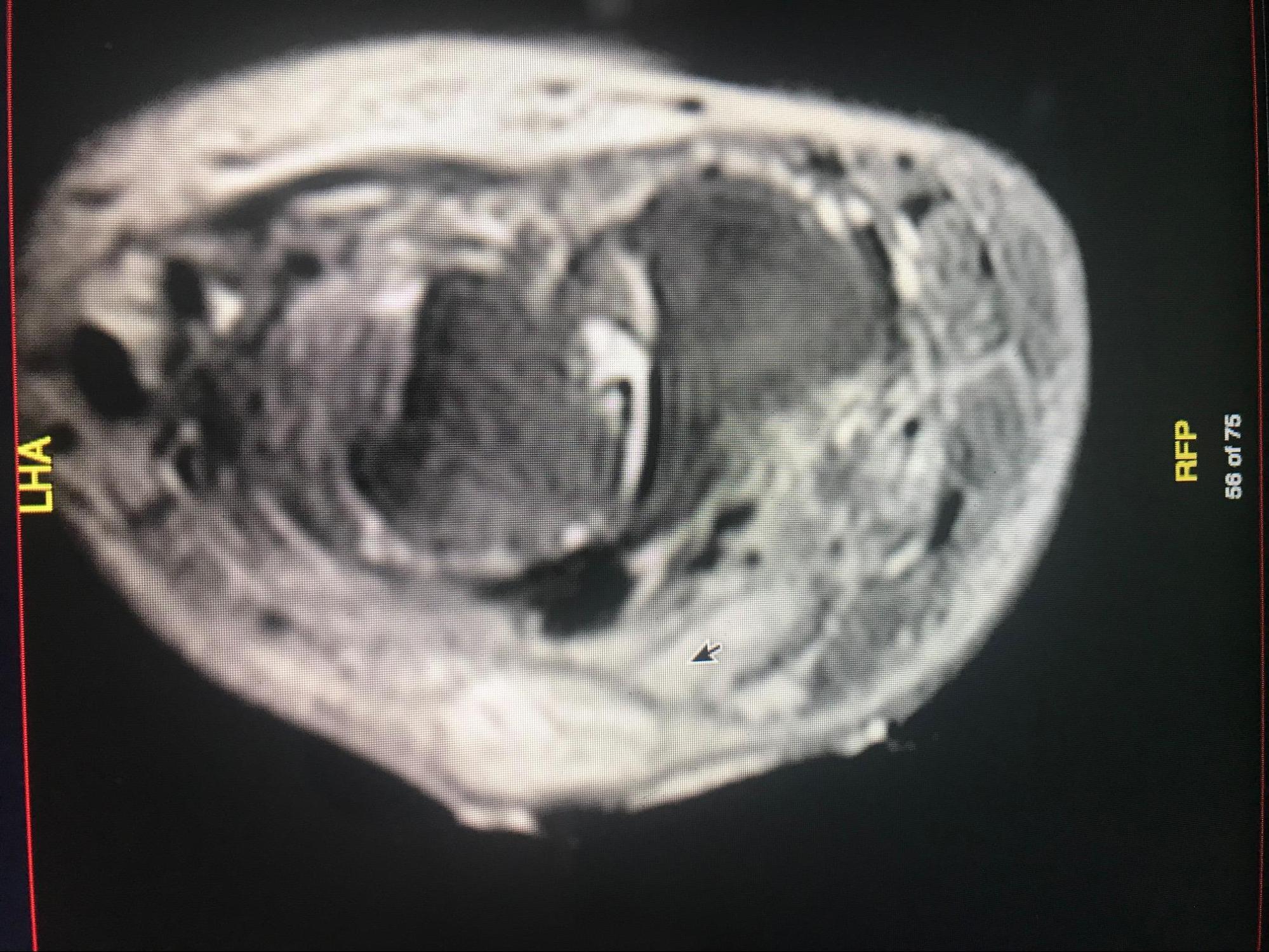
T2-weighted MRI image of a patient's right foot. Patient had history of uncontrolled diabetes, peripheral neuropathy and a long-standing ulceration under the 5th metatarsal head resulting in osteomyelitis of the 5th metatarsal head and shaft. Patient subsequently underwent a partial 5th ray resection with long term IV antibiotics for treatment and healed uneventfully. Contributed by Aaron R. Chambers, DPM, FACFAS
References
Schmitt SK. Osteomyelitis. Infectious disease clinics of North America. 2017 Jun:31(2):325-338. doi: 10.1016/j.idc.2017.01.010. Epub [PubMed PMID: 28483044]
Lew DP, Waldvogel FA. Osteomyelitis. The New England journal of medicine. 1997 Apr 3:336(14):999-1007 [PubMed PMID: 9077380]
Rubin RJ, Harrington CA, Poon A, Dietrich K, Greene JA, Moiduddin A. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerging infectious diseases. 1999 Jan-Feb:5(1):9-17 [PubMed PMID: 10081667]
Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ 3rd, Huddleston PM 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. The Journal of bone and joint surgery. American volume. 2015 May 20:97(10):837-45. doi: 10.2106/JBJS.N.01350. Epub [PubMed PMID: 25995495]
Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. American family physician. 2011 Nov 1:84(9):1027-33 [PubMed PMID: 22046943]
Zimmerli W. Clinical practice. Vertebral osteomyelitis. The New England journal of medicine. 2010 Mar 18:362(11):1022-9. doi: 10.1056/NEJMcp0910753. Epub [PubMed PMID: 20237348]
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM 3rd, Petermann GW, Osmon DR, Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Sep 15:61(6):e26-46. doi: 10.1093/cid/civ482. Epub 2015 Jul 29 [PubMed PMID: 26229122]
Level 1 (high-level) evidenceLam K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic Accuracy of Probe to Bone to Detect Osteomyelitis in the Diabetic Foot: A Systematic Review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Oct 1:63(7):944-8. doi: 10.1093/cid/ciw445. Epub 2016 Jul 1 [PubMed PMID: 27369321]
Level 1 (high-level) evidenceLima AL, Oliveira PR, Carvalho VC, Cimerman S, Savio E, Diretrizes Panamericanas para el Tratamiento de las Osteomielitis e Infecciones de Tejidos Blandos Group. Recommendations for the treatment of osteomyelitis. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2014 Sep-Oct:18(5):526-34. doi: 10.1016/j.bjid.2013.12.005. Epub 2014 Apr 1 [PubMed PMID: 24698709]
Calhoun JH, Manring MM. Adult osteomyelitis. Infectious disease clinics of North America. 2005 Dec:19(4):765-86 [PubMed PMID: 16297731]
Dutra LMA,Melo MC,Moura MC,Leme LAP,De Carvalho MR,Mascarenhas AN,Novaes MRCG, Prognosis of the outcome of severe diabetic foot ulcers with multidisciplinary care. Journal of multidisciplinary healthcare. 2019; [PubMed PMID: 31118658]