Introduction
Osteosarcoma, or osteogenic sarcoma, is the most common primary malignant bone tumor, accounting for approximately 20% of all cases. Osteosarcoma demonstrates a bimodal age distribution and most commonly occurs in the extremities.[1] Approximately 75% of osteosarcoma diagnoses occur in patients less than 25 years of age; the average at diagnosis is 20 years. However, in patients 65 years and older, osteosarcoma often occurs secondary to irradiation or Paget's disease of bone.[2][3] No histopathological difference between primary and secondary osteosarcoma has been identified, but primary osteosarcoma originates within normal bone, and secondary osteosarcoma originates within bone affected by a pathologic disease process.
Osteosarcoma derived from primitive osteoid-producing mesenchymal cells manifests heterogeneously; the degree of differentiation, location within the bone, and histological variation determine each osteosarcoma subtype. Each subtype varies in demographic distribution, biological behavior, and radiological appearance. High-grade conventional intramedullary osteosarcoma is the most common subtype. This subtype is a biologically complex and aggressive tumor involving a long bone's metaphysis, usually adjacent to a physis with the most significant growth, such as the proximal humerus, distal femur, or proximal tibia.[4]
The most common presenting symptom of osteosarcoma is bone pain, initially with activity and then at rest. A reported history of a traumatic injury may or may not be present.[5] Lesions are typically identified on radiographs of the affected limb; magnetic resonance imaging (MRI) is then utilized to characterize a lesion further, and a biopsy is required for definitive diagnosis.
The treatment of osteosarcoma typically requires some combination of wide excision, chemotherapy, and radiotherapy. Localized high-grade osteosarcomas are treated with neoadjuvant chemotherapy, radical surgical resection, and adjuvant chemotherapy.[6] Approximately 10% to 20% of patients with osteosarcoma have evidence of metastases at their initial presentation; the most common metastatic site is the lungs.[7] With the routine use of chemotherapy, approximately two-thirds of children and adolescents with osteosarcoma will achieve long-term cures.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Primary Osteosarcoma
The etiology of osteosarcoma is currently unknown; studies traditionally have focused on multiple risk factors, including genetic predisposition, epidemiology, and the environment.[8] Osteosarcomatous lesions typically demonstrate complex karyotypic mutations; several genetic aberrations have been identified in primary osteosarcoma.[8][9] These mutations and their associated syndromes include:
- Hereditary retinoblastoma: Germline mutations in the RB1 gene are inherited in an autosomal dominant fashion and typically cause bilateral retinoblastoma by 1 year of age. This mutation imparts an increased risk of osteosarcoma later in life, especially in the setting of prior radiation treatment. Somatic RB1 mutations are associated with 30% to 75% of primary osteosarcoma cases.[10][8]
- Li-Fraumeni syndrome (LFS): Characterized by germline mutations in the TP53 tumor suppressor gene, LFS has been diagnosed in up to 5% of children with osteosarcoma. 12% of individuals with LFS will develop an osteosarcoma. Patients with Li-Fraumeni syndrome are also at increased risk of developing other types of cancer at a very early age.[8]
- Rothmund-Thompson syndrome: Mutations in the RECQL4 gene are inherited in an autosomal recessive fashion and predispose to osteosarcoma as well as a characteristic infantile rash, dysplastic osseous structures, alopecia, premature cataracts, and chronic gastrointestinal distress.[8][11]
- Bloom syndrome: The BLM gene maintains DNA stability during replication. When mutations in this gene are inherited in an autosomal recessive fashion, patients are predisposed to osteosarcoma, ultraviolet light-induced rashes, short stature, and sparse subcutaneous fat.[8]
- Werner syndrome: Also known as adult progeria, mutations in the WRN gene induce premature aging, bilateral cataracts, osteoporosis, short stature, scleroderma-like skin changes, and a propensity for osteosarcoma. Patients with Werner syndrome tend to develop osteosarcoma later in life, typically around 35 to 57 years old, and in atypical locations, eg, the feet.[8]
Secondary Osteosarcoma
Secondary osteosarcoma is associated most commonly with Paget disease of bone and radiation exposure.[12] Osteosarcoma has been induced in animal models with beryllium, alkylating agents, the Finkel-Biskis-Jinkins (FBJ) virus, and the Rous sarcoma virus.[13] Case reports correlating an osteosarcoma diagnosis with a history of electrical burns, trauma, and joint arthroplasty have also been documented.[13]
Epidemiology
The incidence of osteosarcoma is about 3.4 cases per million people per year.[14] Osteosarcomas constitute <1% of all newly diagnosed malignancies in adults and nearly 4% of all newly diagnosed malignancies in children.[4] Excluding hematological malignancies, osteosarcoma is the most commonly diagnosed malignancy in adolescents, with an incidence of 4.4 per million per year.[2] Osteosarcoma does have a slightly increased incidence in males versus females.[7] Approximately 20% of osteosarcoma are metastatic at presentation. Between 60% and 70% of metastatic disease is to the lungs; another 20% to 30% of metastatic lesions are skip or distant bony metastases.
Primary osteosarcoma is primarily a lesion of childhood and adolescence with a marked propensity for the knee.[1] Secondary osteosarcoma reflects the varied nature of the predisposing condition with a broader age distribution, primarily within adulthood. Secondary osteosarcoma is much more likely to occur in flat bones, including the pelvis; this is likely due to the causative effect of Paget's disease of bone, which shares a similar anatomical preference.[1]
Pathophysiology
Osteosarcoma frequently occurs near the metaphysis of the long bones of the appendicular skeleton. Rapid bone growth predisposes to the development of osteosarcoma; occurrence is most common during the pubertal growth spurt and near the growing physis.[15] High-grade intramedullary osteosarcoma comprises approximately 80% of all osteosarcomas; 42% occur in the femur, 19% in the tibia, and 10% in the humerus. Between 75% and 90% of these long-bone tumors occur near the distal femoral, proximal tibial, or proximal humeral physis. Other commonly encountered anatomical sites for osteosarcoma development include the skull, jaw, and pelvis, primarily the ilium.[1][3]
Osteosarcoma in adults is often secondary to another bone disorder. Approximately 1% of patients with Paget disease of bone develop osteosarcoma, although this risk may be decreasing as Paget disease is treated with bisphosphonates.[15][16] Ionizing radiation is implicated in 3% of osteosarcoma cases, 4 to 40 years after exposure.[15]
Histopathology
Osteosarcoma is a mesenchymal neoplasm that produces osteoid and woven bony matrix.[17] The definitive diagnostic feature of osteosarcoma is malignant osteoid matrix production; the quantity and quality of the matrix will vary with histologic subtypes.[8] Dense osteoid is characteristic of the sclerotic variant of osteoblastic osteosarcoma, while inconspicuous amounts of an osteoid matrix are seen in fibroblastic or small-cell osteosarcoma variants.[18] Normal osteoid is histologically glassy, densely eosinophilic, and homogenous. Osteoid deposition by malignant cells may be filigree or lacelike, sclerotic with dense confluent sheets of the matrix, or thick and trabecular.[19]
The 2020 World Health Organization Classification of Tumors of Bone recognizes the following subtypes of osteosarcoma:
- Low-grade central osteosarcoma
- Osteosarcoma not otherwise specified (NOS):
- Conventional osteosarcoma
- Telangiectatic osteosarcoma
- Small cell osteosarcoma
- Parosteal osteosarcoma
- Periosteal osteosarcoma
- High-grade surface osteosarcoma
- Secondary osteosarcoma [20]
Low-Grade Central Osteosarcoma
Low-grade central osteosarcoma comprises 1% to 2% of all osteosarcomas.[20] This tumor occurs predominately in the long-bone metaphyses of young adults and is characterized by low mitotic activity. Progression to a high-grade osteosarcoma has been documented in the setting of local recurrence.[21] Immunohistochemically, amplification of MDM2 and CDK4 can help differentiate low-grade osteosarcoma from other benign intramedullary bone tumors.[22]
Osteosarcoma Not Otherwise Specified (NOS)
Conventional osteosarcoma
Conventional osteosarcoma (COS) is typically a central or intramedullary high-grade sarcoma involving the metaphysis of long bones, accounting for 75% to 80% of all osteosarcomas.[23] Histologically, COS is composed of cells that are spindle to polyhedral in shape with variable nuclei and numerous mitotic figures.[24] The osteoid matrix produced by tumor cells must be identified somewhere in the lesion, even if only in a minuscule amount.[24] Historically, COS has been further subclassified histologically based on the predominant extracellular matrix as osteoblastic, chondroblastic, or fibroblastic.[24]
Telangiectatic osteosarcoma
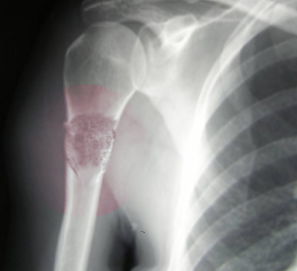
Telangiectatic osteosarcoma accounts for 2% to 12% of osteosarcomas.[23] Histologically, telangiectatic osteosarcoma is characterized by numerous blood-filled sinusoids with septations containing cells with nuclear pleomorphism and numerous mitotic figures.[24] The lesional tissue is also observed permeating into the surrounding marrow or cortex.[24] (Image.Telangiectactic Osteosarcoma).
Small cell osteosarcoma
Small cell osteosarcoma constitutes only 1% of osteosarcomas.[23] This condition is a histological combination of Ewing sarcoma and osteosarcoma and features numerous small round cells. Unlike Ewing sarcoma, spindling of tumor cells is seen with small-cell osteosarcoma.[24] A minuscule volume of an osteoid matrix also distinguishes this as a variant of osteosarcoma.[24]
Parosteal Osteosarcoma
Parosteal osteosarcoma accounts for <4% of osteosarcomas and occurs on the posterior distal femur in 75% to 80% of cases.[24]. A histopathological exam demonstrates “streamers of bone trabeculae” running in parallel and cellular fibrous tissue.[24] Immature osteoid can be found within the bone spicules. Parosteal osteosarcoma is a low grade sarcoma.[24] Immunohistochemical analysis shows both CDK4 and MDM2 amplification in a majority of parosteal osteosarcomas.[22]
Periosteal Osteosarcoma
Comprising <1% of osteosarcomas, this subtype is found between the cortex and the inner periosteal layer of the bone.[23] Histologically, periosteal osteosarcoma features ribbons of osseous trabeculae oriented in parallel, primarily composed of a chondroid matrix with a small amount of osteoid matrix.[24] Periosteal osteosarcoma is considered intermediate-grade histologically.[24]
High-Grade Surface Osteosarcoma
These are histologically identical to high-grade conventional and central variants and vary only in location.[24] High-grade surface osteosarcomas are, by definition, confined to the bone's surface. Some of these osteosarcomas may represent dedifferentiated parosteal osteosarcoma.[24]
Secondary Osteosarcoma
Secondary osteosarcomas arise in abnormal bone, most commonly in individuals older than 50 around the knee or hip joint.[23] Secondary osteosarcomas accounts for only about 4% of osteosarcomas. The 2020 WHO classification subdivided secondary osteosarcoma into 6 subtypes: osteosarcoma in Paget's disease of bone, radiation-associated osteosarcoma, infarct-related osteosarcoma, osteosarcoma dye to chronic osteomyelitis, implant-related osteosarcoma and osteosarcoma associated with disorders like fibrous dysplasia.[20] The histology varies between subtypes, but the distinguishing features of osteoid production, variable nuclei with pleomorphism, and numerous mitoses are present.
Extraskeletal Osteosarcoma
Extraskeletal osteosarcomas are divided into the following histological types:
- Low-grade: This type of osteogenic sarcoma is histologically identical to low-grade surface parosteal variants and low-grade central variants, varying only in geography, potentially appearing at any extraskeletal location in the body, including the soft tissues of the thigh, buttocks, upper extremities, or retroperitoneum.[24]
- High-grade: Histologically identical to high-grade conventional and central variants, differing only in geography, found at any extraskeletal location in the body.[24]
History and Physical
Clinical History of Osteogenic Sarcoma
Symptoms of osteosarcoma may be present for a significant amount of time, sometimes weeks to months, before patients seek evaluation. Most commonly, the presenting symptom is bone pain, particularly with activity. A history of traumatic musculoskeletal injury may or may not be reported.[5] Pain and swelling at the local site, usually at the growing ends of an extremity or long bones, are the most common presenting symptoms.[25]
Around 10% of patients present with pathological fractures due to primary tumors or bony metastases (ie, bone-to-bone spread).[26] Systemic symptoms seen in lymphoma (eg, fever and night sweats) are rare.[5] Respiratory symptoms (eg, frequent cough and hemoptysis) are also uncommon and, when present, indicate extensive lung involvement. Additional symptoms are unusual because metastases to other sites are extremely rare but typically pertain to the involved organ.[5][27]
Physical Examination Findings
Physical examination findings of osteogenic sarcoma are typically focused on the location of the primary tumor, including:
- A palpable, tender mass
- Decreased range of motion of adjacent joint, with possible effusion
- Pain on weight-bearing or inability to bear weight
- Local or regional lymphadenopathy (unusual)
- Respiratory findings with metastatic forms
The preoperative neurological deficit in vertebral osteosarcoma can be assessed according to the Frankel grading system.[28] The classification given by Tomita et al is based on the location and grade of spinal tumors to help determine prognosis and the optimal surgical approach.[28][29]
Evaluation
National Comprehensive Cancer Network 2020 Guidelines for Osteogenic Sarcoma Evaluation
The National Comprehensive Cancer Network (NCCN) recommends diagnostic laboratory, imaging, and histological studies following the initial clinical assessment.
Laboratory Analysis
Serum tests, including a complete blood count, alkaline phosphatase (ALP), and lactate dehydrogenases (LDH), are assessed in the initial workup because they provide evidence for diagnosis and prognosis. ALP levels will be high due to the increased osteoblastic activity associated with osteogenic sarcoma. Extremely high levels have been linked to heavy tumor burden and are generally considered a poor prognostic indicator. During initial evaluation, serum LDH is frequently higher in patients with metastatic disease than localized disease.
Furthermore, evaluating the levels of the biomarkers later in the treatment process is also essential, as levels may decrease with successful therapy or rise with residual disease or recurrence.[30] The NCCN also recommends that genetic evaluation and counseling be considered for patients diagnosed with osteogenic sarcoma with a family or personal history of bone sarcomas.[3]
Primary Tumor Site Diagnostic Imaging
The following imaging modalities are preferred for evaluation of osteosarcoma:
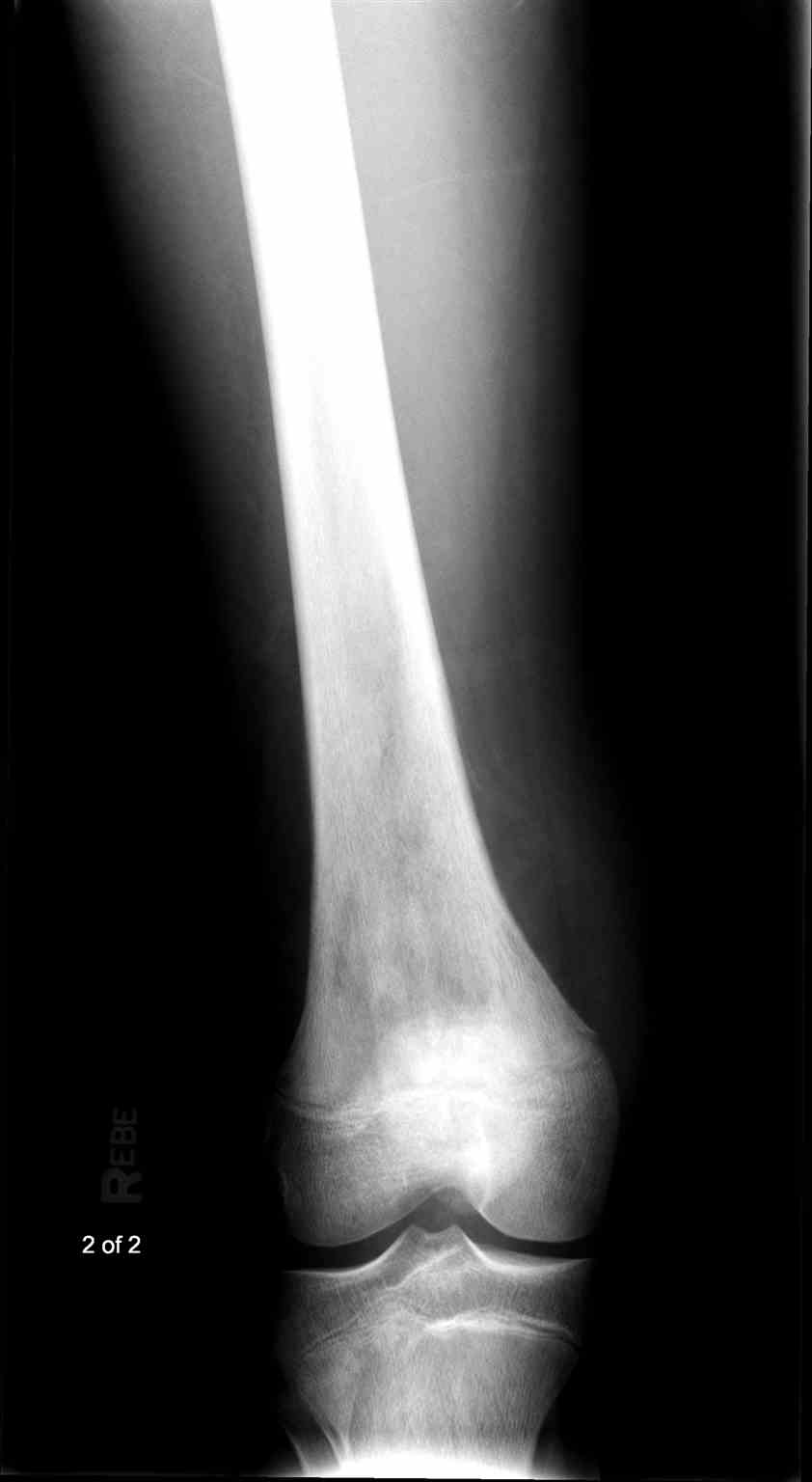
- Radiographs: Radiographs of the entire bone affected should be obtained. The radiographic findings vary depending on the type of osteosarcoma. A radiograph of conventional osteosarcoma usually demonstrates medullary and cortical bone destruction with mixed lytic and blastic appearance. High-grade osteosarcoma will often be described as having a permeative or moth-eaten appearance with a "sunburst" configuration due to aggressive periostitis or "codman triangle" configuration due to elevation of the periosteum away from the bone.[30] Telangiectatic osteosarcoma, on the other hand, is usually purely lytic, while low-grade intramedullary osteosarcoma may appear as a purely sclerotic lesion.[23] Parosteal osteosarcoma is a lobular sclerotic mass stuck onto the bone, while periosteal osteosarcoma is less sclerotic with cortical involvement and periosteal reaction.[23] (see Image. Osteogenic Sarcoma).
- Magnetic resonance imaging (MRI): MRI is an indispensable tool for defining the extent of a tumor inside and outside the bone. The entirety of the involved bone, as well as 1 joint above and 1 below the tumor, should be included in the study so that "skip" lesions are not missed. MRI can accurately and precisely delineate the degree of tumor in the adjacent soft tissues, joint involvement, whether or not the tumor crosses the physis, and proximity to the nearest neurovascular bundle. Nearly every aspect of treatment is assessable with MRI, from presurgical assessment for limb-sparing resection to the degree of chemotherapy response in the form of tumor necrosis, shrinkage, and improved capsulation.[30]
- Traditional sequences acquired in MRI of osteogenic sarcoma may demonstrate the following:[30]
- T1-weighted Images
- Non-ossified soft tissue component: intermediate signal intensity
- Osteoid components: low signal intensity
- Peritumoral edema: intermediate signal intensity
- Scattered foci of hemorrhage: variable signal intensity based on chronicity
- T2-weighted Images
- Non-ossified soft tissue component: high signal intensity
- Osteoid components: low signal intensity
- Peritumoral edema: high signal intensity
- Telangiectatic osteosarcoma: multi-cystic expansile lesion with fluid-fluid levels and septa that enhance with contrast.[23]
- Parosteal Osteosarcoma: lobular mass with a clear line between portions of the sarcoma and the underlying cortical bone.[23]
- Periosteal Osteosarcoma: areas of cortical erosion but rarely intramedullary involvement and no corticomedullary continuity.[23]
- Extraskeletal Osteosarcoma: Soft tissue mass with internal calcification.[23]
- Traditional sequences acquired in MRI of osteogenic sarcoma may demonstrate the following:[30]
Biopsy
A biopsy is necessary after the physical exam, laboratory analysis, and diagnostic imaging confirm the presence of a lesion consistent with osteosarcoma. The final surgical procedure must include resection of the biopsy tract, which can be tattooed for easy identification to avoid recurrence due to potential seeding of this tract with cancer cells. Ideally, the surgeon who undertakes the biopsy should be the same individual who completes the resection so they are familiar with the path and extent of the biopsy. An open approach to biopsy was previously considered the best option owing to a high accuracy rate.[14]
In recent years, however, research has determined that an open approach correlates with an increased risk of complications such as infection, improper wound healing, and seeding of the site by tumor cells, as previously discussed. As such, core biopsy has replaced the traditional open approach, mainly because of the reduced risk of contamination of the surgical bed with tumor cells but also due to lower cost and decreased recovery time. Attempting limb-sparing procedures in patients with the perceived potential to save local tissue safely is crucial.[14]
Core needle biopsy is achieved via a single deep stab with a needle through a trocar, which traverses a single tissue plane in a location that will be included in the final resection. Multiple cores are necessary from the representative region of the mass to the soft tissue portion in the lesion's periphery. The necrotic central region will yield little viable tissue, and the "Codman triangle" region will yield only reactive bone. Importantly, recent studies have shown that fine-needle aspiration is not an efficacious approach to biopsy because it does not yield an adequate tissue sample for an accurate diagnosis. Following the biopsy, pathologists should analyze tissue samples in fresh or frozen format for definitive diagnosis, grading, and histological subtyping, affecting medical and surgical treatment strategy.[14]
Tumor Staging
The following imaging modalities are preferred for osteosarcoma staging:
- Chest computed tomography (CT): Modality of choice for evaluating for pulmonary metastasis.[3]
- Nuclear imaging
- Positron emission tomography/CT: Positron emission tomography (PET)/CT is a nuclear medicine imaging modality that detects highly metabolic lesions. It is utilized to evaluate for metastatic disease, especially in the skeleton.[3]
- Radionuclide bone scan: Technetium 99 methylenediphosphonate (Tc99 MDP) bone scan is an effective and less expensive, though less sensitive, imaging modality for detecting bony metastasis.[3][31]
Pretreatment Chemotherapy Evaluation
Chemotherapy may result in auditory, renal, and cardiac toxicity, making baseline assessment of these parameters extremely important. Pure tone audiometry, baseline renal function testing, and echocardiographic evaluation might be warranted.[32] Additionally, because osteogenic sarcoma is most prevalent in children and young adults, fertility consultation with an interdisciplinary team, including reproductive specialists, is recommended, as chemotherapy and radiation therapy may affect fertility.
Treatment / Management
Osteogenic Sarcoma Management Guidelines
The NCCN advises clinicians to refer patients to a tertiary care center with osteogenic sarcoma specialists and interprofessional teams for optimal management of this condition. Furthermore, multimodality therapy consisting of neoadjuvant chemotherapy, followed by surgery and chemotherapy in the adjuvant setting, is considered the treatment of choice.[33][34] Generally, clinicians performing wide excision should expect to achieve histologically negative margins. The combination of chemotherapy with surgical procedures and advanced imaging modalities has increased limb salvage rates from 53% in the 1980s to greater than 90% in recent times.[14] Along with the advancement in psychological and cosmetic outcomes, limb salvage procedures have comparable overall survival and local recurrence rates to amputation.[35] The utility of neoadjuvant chemotherapy has been attributed to subclinical micrometastatic disease.[7] Enrollment in a clinical trial should be considered in all cases.(B2)
The National Cancer Institute (NCI) recommends the following management guidelines based on the grade and resectability of a tumor:[3](B3)
- Low-grade osteosarcoma without metastasis
- Intramedullary and surface
- Wide excision alone (ie, no neoadjuvant chemotherapy)
- If postsurgical pathology demonstrates low-grade features, then adjuvant chemotherapy is not recommended.
- If postsurgical pathology demonstrates high-grade features, consider adjuvant chemotherapy.
- Wide excision alone (ie, no neoadjuvant chemotherapy)
- Periosteal
- Consider neoadjuvant chemotherapy
- Wide excision
- If postsurgical pathology demonstrates is consistent with biopsy (low-grade features only), no adjuvant chemotherapy is recommended.
- If postsurgical pathology demonstrates high-grade features, adjuvant chemotherapy is recommended.
- Intramedullary and surface
- High-grade intramedullary or surface osteosarcoma without metastasis
- Neoadjuvant chemotherapy; then restage the lesion
- If restaging suggests the lesion is resectable, perform a limb-sparing wide excision.
- Positive margins
- If a favorable response to preoperative neoadjuvant chemotherapy (<10% viable tumor on postsurgical pathology) was seen, then continue the same neoadjuvant chemotherapy regimen and consider additional surgical resection with or without radiation therapy.
- If an inadequate response to preoperative neoadjuvant chemotherapy (eg, >10% viable tumor on postsurgical pathology) is noted, then continue the same neoadjuvant chemotherapy regimen or consider a new regimen and consider additional surgical resection with or without radiation therapy.
- Negative margins
- If a good response to preoperative neoadjuvant chemotherapy (ie, <10% viable tumor on postsurgical pathology) was observed, continue the same neoadjuvant chemotherapy regimen. No further resection is required.
- If an inadequate response to preoperative neoadjuvant chemotherapy (ie, ≥10% viable tumor on postsurgical pathology) is noted, continue the same neoadjuvant chemotherapy regimen or consider a new regimen. No further resection is required.
- Positive margins
- If restaging suggests the lesion is resectable, perform a limb-sparing wide excision.
- If restaging suggests the lesion is unresectable, then continue chemotherapy and consider radiation therapy.
- Neoadjuvant chemotherapy; then restage the lesion
- Any grade with metastasis at presentation, follow guidelines for high-grade osteosarcoma plus the following:
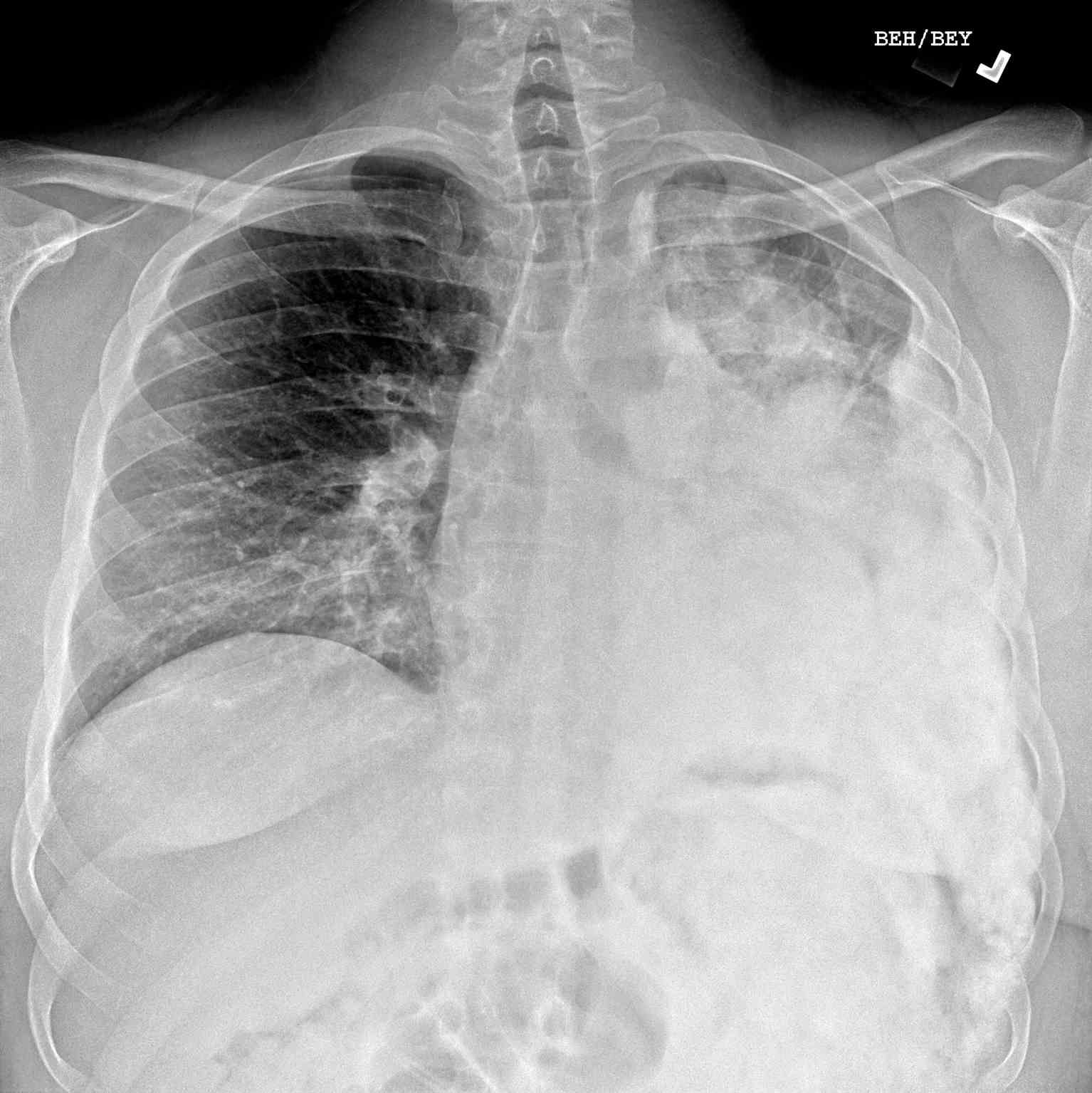
- If metastases are resectable (eg, pulmonary, visceral, or skeletal sites), a metastasectomy should be performed (see Image. Pulmonary Metastasis of Osteogenic Sarcoma).
- If metastases are unresectable, then consider chemotherapy and radiation therapy, after which the primary site requires reassessment for local control.
- Follow-up and surveillance
- Surveillance schedule
- Every 3 months for postoperative years 1 and 2
- Every 4 months in postoperative year 3
- Every 6 months in postoperative years 4 and 5
- Yearly for postoperative years 6 and beyond
- Surveillance visits should include:
- Physical exam with assessment of function
- Contrast-enhanced CT with or without MRI of the postoperative site and chest
- Consider PET/CT or bone scan
- Complete blood count with additional laboratory tests as clinically indicated (eg, alkaline phosphatase levels)
- If a relapse is detected, chemotherapy with resection, if possible, should be resumed in conjunction with the following guidelines:
- Evaluate tumor treatment by performing radiographs of the original tumor site, CT or MRI with contrast of the site of relapse, and CT of the chest to assess for pulmonary lesions
- In tumors responsive to treatment (ie, <10% viable tumor on postsurgical pathology), continue surveillance (ie, restart OSTEO-4 guidelines)
- In tumors with a poor response to treatment (ie, ≥10% viable tumor on postsurgical pathology) or continued progression of the disease, management strategies include:
(A1) - Surveillance schedule
Recurrent Osteogenic Sarcoma
In individuals with recurrent disease with or without metastasis, surgical resection is preferred if possible; primarily, adjuvant chemotherapy is given. A 5-year survival rate of 33% may be obtained in patients with a second surgical remission.[38][37] (Please refer to the Medical Oncology section for more information on chemotherapy regimens). In patients who are not candidates for surgery, chemotherapy with or without radiation is preferred. In those with metastasis, dismal 5-year survival rates of 20%, which has remained unchanged over the past 25 years, underline the need to explore newer approaches.(A1)
Supportive Management and Palliative Medicine
The management of chemotherapy-related complications such as nausea and vomiting, anemia, neutropenic fever, fatigue, neuropathy, and cardiotoxicity, provision of symptom-directed therapy, and counseling regarding goals of care discussions have shown improvement in the quality of life. The provision of continuity of care through home care and round-the-clock telephonic liaison might assume special significance, depending on environmental circumstances (eg, the COVID-19 pandemic). Hospice should be considered early.[39][40](A1)
Differential Diagnosis
The differential diagnosis for a meta-diaphyseal bone lesion in a pediatric patient includes benign entities, eg, osteoblastoma, osteoid osteoma, osteomyelitis, fracture callus, or Langerhans cell histiocytosis, and malignant conditions (eg, Ewing sarcoma and lymphoma).
Osteoblastoma and osteoid osteoma are usually well-circumscribed, with a peripheral rim of reactive bone consisting of a loose fibrous stroma with a vascular component, rimming of bone trabeculae, and absence of atypical mitoses.[19]
Callus formation is usually associated with a history of accompanying trauma, organized matrix deposition, presence of granulation tissue-like stroma, presence of hyaline cartilage with woven lamellar bone, and presence of transition area from immature osteoid to that of osteoblasts lined bony spicules.[19]
Langerhans cell histiocytosis of bone usually presents as a painful osteolytic lesion, and tissue biopsy demonstrates a clonal proliferation of cells that express CD1a, CD207, CD68, and S-100.[41]
Ewing sarcoma classically appears in the diaphysis of long bones but can occur in the metaphyseal region and have similar radiologic findings, eg, Codman triangles. Histologically, Ewing sarcoma has a clonal population of small round blue cells characterized by the EWS-FLI1 translocation.[42]
Among the differentials for morphologic variants, telangiectatic osteosarcoma must be differentiated from aneurysmal bone cyst (ABC). Both lesions can have surrounding periosteal reaction and fluid-fluid levels on MRI, but ABCs do not typically have a soft tissue mass associated with them. Histologically, the septa in aneurysmal bone cysts contain cells with significantly less pleomorphism and nuclear hyperchromasia than in telangiectatic osteosarcoma and lack lacey malignant osteoid. ABCs are also associated with a specific USP6 gene rearrangement not seen in osteosarcoma.[43] Low-grade parosteal osteosarcoma must be differentiated from osteochondroma, heterotropic ossification, and surface osteoma. Osteochondromas are characterized radiographically by corticomedullary continuity, which surface osteosarcoma lacks.
Surgical Oncology
Surgical Resection of Primary Site Osteosarcoma
The goal of surgical resection is a negative margin or R0 resection in which no tumor at the edges of the resection are present. This can be achieved through either limb salvage surgery or amputation, depending on the location and size of the sarcoma and patient preferences.[44]
Limb salvage surgery
Limb salvage surgery is completed in about 75% of osteosarcoma cases.[45][46] Because osteosarcoma is the most common primary osseous malignancy in the pediatric population, surgery presents a unique set of challenges. To achieve clear margins, excision may necessitate resection of the physis, leading to deformity or leg length discrepancy as the child matures. In the past, a tumor that traversed the growth plate was considered to be an indication for amputation because no means to restore function were available. With the advent of options that grow or expand with the patient, masses that cross the growth plate are no longer considered a contraindication to limb salvage.[14][6]
After resectioning the sarcoma, limb salvage surgery often requires reconstruction of the defect created. The purpose of reconstruction is the restoration of function to the affected limb. In the case of non-weight-bearing bones like the fibula or clavicle, reconstruction is unnecessary because the excision of these structures does not impart functional deficit. For reconstruction, the following options are available:
- Allograft or autograft bone reconstruction
- Allograft bone replacement utilizes bones collected from organ donors in the postmortem period. As with organ donation, potential donors undergo screening for infectious diseases. Once surgically grafted into the osteosarcoma patient, the native bone will grow into the allograft bone and heal. Rejection is rare because very few donor cells remain within the donated bone, and bones are relatively inert. The most serious complication that may arise with allograft reconstruction is the failure of fusion between the patient and allograft bone. Infection and fracture are also important complications requiring internal fixation or removal. A hybrid reconstructive option is an allograft prosthetic composite, which combines an allograft bone fragment with a metallic prosthesis. Allograft prosthetic composite arthroplasty is useful for reconstructing weight-bearing joints such as the knee or hip.[14][6]
- In centers without access to a donor bone bank, resected malignant bone can be irradiated or, less frequently, pasteurized or treated with liquid nitrogen and reimplanted, resulting in a perfect match for the defect at the surgical site. This process is known as autografting and can be very cost-effective. However, only limited indications for autografting have been established, as donor bone for allograft is relatively easy to procure.[14][6]
- Metallic prosthetics
- So-called "mega prostheses" replace large segments of the bone and the joint that connects them. When they were first utilized, most of these devices had to be custom-made, but today, "off-the-shelf" options are available for immediate implantation. Some prostheses are expandable "growing" implants that permit interval lengthening. These are particularly efficacious in skeletally immature individuals. Because the growth plates of the affected segment of bone often get resected, the prosthesis can be elongated by 1 to 2 cm at a time, so the length of the previously diseased limb correlates with the contralateral healthy limb.[14][6]
- Tissue regeneration
- Tissue regeneration for reconstruction following resection of osteosarcoma is a relatively new field. Generally, this process combines a patient's cells, purified intrinsic growth factors, and synthetic, scaffold-like matrix materials to induce autologous tissue regeneration. Until this emerging technology is more widely available, procedures (eg, the Ilizarov technique or spatial frame method) utilize external fixation devices to promote the growth of long bones of up to 1 mm per day or about 1 in each month.[14][6]
Limb amputation
Amputation, previously considered the gold standard for surgical management of osteosarcoma, is typically reserved only for nonresectable masses with contamination of myotendinous and neurovascular that make limb salvage impossible. Amputation may also be preferred in cases where the achievement of disease-free margins will lead to a nonfunctional limb or when the patient prefers a bioprosthesis with the potential to provide a greater degree of functionality over the cosmetic advantages that might accompany a limb salvage. Amputation may be performed as a standalone treatment or in conjunction with rotationplasty.[14][6]
Rotationplasty is a procedure utilized in distal femur or proximal tibia osteosarcoma. Resection of the sarcoma and the adjacent knee joint is followed by a 180-degree rotation of the lower extremity and fixation of the tibia to the femur, transforming the ankle into a "knee" joint. The foot plantar flexors (ie, soleus and gastrocnemius) are converted to knee extensors. The procedure has been shown to provide surprisingly favorable functionality.[14][6]
Outcomes of Limb Salvage Versus Amputation
Since the advent of chemotherapy, the overall survival in patients undergoing limb salvage surgery versus amputation is essentially the same. Limb salvage surgery is associated with a higher risk of local recurrence and the need for additional surgeries.[46][47]
Surgical Resection of Metastatic Osteosarcoma
The following resection approaches are recommended for metastatic osteosarcoma:
- Lung-only metastases: After neoadjuvant chemotherapy, if possible, lung nodules should be resected as long-term survival is improved if metastectomy is performed.[48][49]
- Bone metastases: If possible, resection of metastatic bone lesions should be performed, although ideal timing is not defined. While mortality is high in the setting of multiple bone metastasis, resection may prolong survival time.[50]
Radiation Oncology
Osteosarcoma cells are assumed to be relatively radioresistant, so radiotherapy is not routinely utilized to treat osteosarcoma. Radiotherapy may be used when margins are very close or positive after surgical resection, when surgery would be incredibly morbid, in the setting of metastatic disease or recurrent disease, and when a patient declines surgery.[51][52] The recommended dose of radiation is 56 to 76 Gy, based on surgical margins and site.[36][51] The NCCN specifically recommends 55 Gy if positive postoperative margins and 60 to 70 Gy for unresectable disease. (NCCN Guidelines Bone Cancer)
All modalities of radiation administration have been utilized with no clear benefit of one over the other. Modern modalities such as stereotactic body radiotherapy and high linear energy transfer carbon-ion radiotherapy may increase the efficacy of radiotherapy for advanced or unresectable osteosarcoma.[51] A phase 3 international randomized controlled trial is underway investigating the efficacy of stereotactic body radiotherapy in patients with oligometastatic osteosarcoma (Stereotactic Body Radiotherapy in Patients with Rare Oligometastatic Cancers, OligoRARE, NCT04498767). Radiation toxicity includes mild and moderate dermatitis and late fibrosis.[51]
Medical Oncology
Chemotherapy for Osteosarcoma
Chemotherapy is also the standard of care for high-grade osteogenic sarcoma, given in the neoadjuvant and adjuvant setting. However, the ideal timing of chemotherapy (ie, preoperative versus postoperative) is unclear.
Von Rosen was the first to introduce the concept of neoadjuvant chemotherapy in the management of osteosarcoma.[35][53] The objective was to provide ample time to manufacture custom prostheses and to decrease tumor burden. The advantages of neoadjuvant chemotherapy include improving the quality of life brought about by the amelioration of symptoms, treatment of micrometastatic disease, increased chances of complete resection, and assessment of response to chemotherapy (ie, the degree of necrosis).[54][55] The extent of the response to neoadjuvant chemotherapy has been used to predict survival.[56] Neoadjuvant chemotherapy has increased the proportion of patients qualifying for limb-salvage surgery; however, chemotherapy should not be considered a substitute for adequate surgery.
Primary Neoadjuvant Chemotherapy
Regimens recommended for primary neoadjuvant chemotherapy include:
- Three chemotherapeutic agents are superior to 2 [3]
- MAP (high-dose methotrexate (HD-MTX), doxorubicin, and cisplatin) +/- Ifosfamide
- If intolerant of HD-MTX: doxorubicin, cisplatin and ifosfamide [57][NCCN Guidelines Bone Cancer][57]
Relapsed or Refractory Disease Regimens
Regimens recommended for osteosarcoma relapse or refractory disease include:
- Etoposide plus ifosfamide (most commonly used second-line regimen)
- Alternative options
- Regorafenib
- High dose ifosfamide +/- etoposide
- Sorafenib +/- everolimus
- Cyclophosphamide and topotecan
- Docetaxel and gemcitabine
- Gemcitabine alone
- The following combinatorial regimens may be useful in specific circumstances:
- Cyclophosphamide and etoposide
- Ifosfamide, carboplatin, and etoposide
- HD-MTX, etoposide, and ifosfamide [57][NCCN Guidelines Bone Cancer][57]
Radiopharmaceuticals
Samarium-153 ethylene diamine tetramethylene phosphonate (153-Sm-EDTMP) is a bone-seeking radiopharmaceutical that may improve pain in patients with metastatic osteosarcoma who have failed second-line therapies.[58][59] Radium-223 acts like calcium, depositing within skeletal metastases and osteoblastic osteosarcoma target sites. Therefore, in combination with chemotherapeutic approaches, Radium-223 may be used in high-risk osteosarcoma.[60]
Bone Targeted Therapies
Osteosarcomas are unique in that bone-targeted therapies, including bisphosphonates and anti-RANK ligand monoclonal antibodies (ie, denosumab), have been evaluated in trials aimed at repositioning these approaches, which are typically supportive therapies, as curative. The use of bisphosphonates in osteosarcomas is only recommended in the setting of a clinical trial after a recent study evaluating the role of zoledronic acid in combination with chemotherapy failed to demonstrate an improvement in the overall survival, relapse-free survival, or the histological response.[61][62]
Staging
Two systems exist for the staging of bone tumors. The Musculoskeletal Tumor Society's Enneking system is used primarily by orthopedic surgeons because the tumor's anatomic location as either intra-compartmental, which is completely contained within the bone, or extra-compartmental, extending outside of the bone, is considered. The alternative system described by the American Joint Committee on Cancer (AJCC) does not consider anatomic location. Instead, the AJCC uses the tumor, node, metastasis (TNM) system, which considers the tumor's size and spread, which research has recognized as having significant predictive value for response to treatment and overall survival. Specifically, larger lesions tend to metastasize, so these patients may benefit from chemotherapeutic intervention, making the AJCC system more popular with oncologists.[63][38][37]
Musculoskeletal Tumor Society/Enneking System for Staging of Malignant Musculoskeletal Tumors
The Enneking system uses the following criteria to stage osteosarcomas:
- Stage IA: Low grade, intra-compartmental tumor location, no metastasis
- Stage IB: Low grade, extra-compartmental tumor location, no metastasis
- Stage IIA: High grade, intra-compartmental tumor location, no metastasis
- Stage IIB: High grade, extra-compartmental tumor location, no metastasis
- Stage III: Any grade, any location, metastasis present [30]
American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis System for Staging of Primary Bone Sarcomas
The AJCC recommends the following staging system for osteosarcomas:
- Stage IA: Low grade, <8 cm tumor size, no spread to regional lymph nodes, no distant metastasis
- Stage IB: Low grade, >8 cm tumor size or skip lesions, no spread to regional lymph nodes, no distant metastasis
- Stage IIA: High grade, >8 cm tumor size, no spread to regional lymph nodes, no distant metastasis
- Stage IIB: High grade, <8 cm tumor size, no spread to regional lymph nodes, no distant metastasis
- Stage III: High grade, discontinuous tumor involvement/"skip" lesions, no regional lymph nodes, no distant metastasis
- Stage IVA: Any grade, any size, no regional lymph node spread, lung metastasis
- Stage IVB: Any grade, any size, regional lymph node spread, lung or extrapulmonary metastasis [64]
Prognosis
High Grade Osteosarcoma
With the advent of chemotherapy, the 5-year overall survival rate for localized osteosarcomas went from 10% to 20% to about 70%.[14] The strongest predictors of overall survival are the presence or absence of metastatic disease and histopathological response to chemotherapy.
Prognostic Factors
Various factors can affect the prognosis of osteosarcoma, including:
- Location: Distal extremity tumors have a better prognosis than proximal extremity tumors. Pelvic osteosarcomas have a poorer prognosis than extremity osteosarcoma, with survival rates between 20% and 47%.[65][66] Craniofacial osteosarcomas that are resectable with negative margins have an overall survival rate of 74%, with mandible and maxillary tumors having a better prognosis than other sites.[67]
- Primary tumor size: Multiple studies associate larger tumors with poorer outcomes, with larger tumors being defined in a case series as greater than a third of the length of the bone in appendicular tumors.[68]
- Metastatic disease: Approximately 20% of patients have clinically detectable metastatic disease at diagnosis. Metastatic disease is associated with significantly lower 5-year survival rates than localized disease, 11% to 36% versus 58% to 78%.[68][69][70][71] Additionally, non-pulmonary metastases are associated with poorer outcomes than pulmonary metastases, especially resectable pulmonary metastases.[68][72]
- Surgical resection margin: Positive surgical margins increase the risk of local recurrence and decrease event-free and overall survival.[71][68]
- Chemotherapy response: Poor response to chemotherapy, defined as >10% viable tumor at resection, is associated with significantly decreased event-free and overall survival (55% versus 78%).[68][71]
- Age: Patients between 12 and 17 years old tend to do better overall than patients younger than 11 or older than 18.[69] Patients older than 40 years old may have a worse prognosis, but studies are discordant regarding the impact of age on prognosis.[73]
- De novo disease versus secondary disease: Overall, osteosarcomas secondary to underlying bone abnormality have poorer outcomes than de novo osteosarcomas. Postradiation-associated osteosarcoma treated with chemotherapy and surgery has outcomes approaching that of de novo osteosarcoma.[74] Pagetic osteosarcoma, however, has a survival rate of <15% despite aggressive therapy.[75]
- Sex: Male sex is associated with poorer prognosis in some studies.[71][69][70]
- Histological subtype: Telangiectatic and fibroblastic tumors have been shown to have a better prognosis and response to chemotherapy than chondroblastic and osteoblastic subtypes.[19][69]
- Pathological fracture: Pathologic fracture is not a statistically significant factor in event-free survival or overall survival in pediatric patients. Adults, on the other hand, do have poorer overall survival with pathological fractures.[76] Pathologic fracture is associated with an increased risk of metastatic disease.[77]
- Laboratory values: Elevated serum alkaline phosphatase and lactate dehydrogenase may be associated with a worse prognosis.[78]
Low-Grade and Parosteal Osteosarcomas
Outcomes of low-grade osteosarcoma are superior to high-grade tumors. Overall 5-year survival for patients with parosteal osteosarcoma is between 91.8% and 96%.[79][80] Local recurrence usually occurs in the setting of positive surgical margins and large tumors. When local recurrence happens, the recurrence is usually a high-grade sarcoma and predicts poor outcomes.[79]
Complications
Pathological Fracture
Pathologic fracture can be the presenting symptom of osteosarcoma or can occur during neoadjuvant chemotherapy. The incidence of pathological fracture is reported to be around 10%. Recent studies show no increased risk of local recurrence with pathologic fractures. Pathologic fracture does correlate with the presence of metastatic disease. One study reported that pathologic fracture is not a statistically significant factor in event-free survival or overall survival in pediatric patients. Adults, on the other hand, do have worse overall survival with pathological fractures.[76][26]
Treatment-Related Complications
Complications related to osteosarcoma treatment include:
- Secondary malignancy
- A combination of the chemotherapy administered, diagnostic irradiation, and radiotherapy, in addition to possible underlying genetic cancer predispositions, all likely contribute to the risk of a secondary malignancy.
- Secondary leukemias usually present in the first 10 years after osteosarcoma treatment, while secondary solid cancers are observed at any point.[81]
- Chemotherapy adverse effects
- Cisplatin: Platinum-based chemotherapies can cause both acute nephrotoxicity and chronic kidney disease. A retrospective case study, which included 147 patients, reported that 36% had CKD2 or higher at long-term follow-up (mean 8.1 years).[82] Cisplatin can also cause hearing loss that starts with high frequencies in a dose-dependent fashion, with younger patients at higher risk.[81] Older patients, on the other hand, are more susceptible to the peripheral neurotoxicity of cisplatin, which does improve with time.[81]
- High-dose methotrexate: Acutely, methotrexate can result in renal failure, which usually recovers.
- Doxorubicin: Acute, subacute, or chronic doxorubicin-induced cardiotoxicity can occur. Chronic DOX-induced cardiotoxicity can include congestive heart failure, coronary artery disease, or valvular disease.[83] Life-long surveillance with an echocardiogram at least every 5 years is recommended.[81]
- Ifosfamide: Permanent renal tubular toxicity can result from ifosfamide, especially in younger children.[81]
- Radiation adverse effects
- Radiation imparts superficial adverse effects, including skin dryness, itching, peeling, and, uncommonly, burns. Menstrual changes, erectile dysfunction, and infertility are all reported adverse events in cases of pelvic radiation. When the chest and abdomen are involved in radiation treatment, diarrhea, incontinence, rectal bleeding, nausea, vomiting, dry mouth, dysphagia, pneumonitis, and fibrosis are possible. Much like chemotherapy, a small risk of late development of a secondary malignancy is present.[84]
- Periprosthetic infection
- Prostheses-related infections are a relatively frequent complication occurring in approximately 20% of limb salvage surgeries, most often due to lengthy surgery time, repeated surgery at the same site, and immunosuppression secondary to chemotherapy.[85]
- First-line treatment of these periprosthetic infections typically involves several debridement procedures with local and systemic antibiotic therapy (systemic and local antibiotic cement beads).
- If ineffective, the implant must be removed, and soft tissue debridement must be performed with the subsequent placement of a cement spacer impregnated with antibiotics. If the infection can be eradicated, a new prosthesis may be implanted. Ultimately, amputation may be necessary for some of these patients.[6]
- Implant failure
- The most common reason for late reconstruction failure is the mechanical breakdown of the mega prosthesis.
- Mechanical failure necessitates the replacement of the prosthetic. The tibia is the most frequent site of mechanical failure.[6]
- Fracture or nonunion of allograft or autograft
- Fracture or nonunion of allograft or autograft reconstruction is a relatively infrequent complication, but it does occur.
- Chemotherapy, radiation, and extracorporeal treatment of autograft bone have been reported to increase the risk of these complications.
- Refractory cases may necessitate metallic implant placement or amputation.[6]
Consultations
Management of osteosarcoma requires an interprofessional team to improve outcomes, including:
- Musculoskeletal radiology
- Specialized surgical oncology based on the location of the tumor for biopsy, resection, limb salvage, prosthetics, and grafting
- Musculoskeletal pathology
- Medical oncology for preoperative and postoperative chemotherapy and neoadjuvant therapy
- Radiation oncology
- Physical and occupational therapy for postsurgical therapy
- Genetic counseling
Deterrence and Patient Education
Symptoms of bone pain, joint pain, and palpable mass warrant professional assessment. Patients and their families should be educated on these presenting symptoms as they may be related to an osseous neoplasm. Pain is the primary complaint in osteosarcoma; patients must be educated on pain management and available options. Because depression and anxiety are common, counseling of both the patient and the family is necessary. The patient and the family must be educated about the treatment options, pain management, and support services.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals with osteosarcoma requires a collaborative effort among healthcare professionals, including physicians, advanced practice practitioners, nurses, pharmacists, and others. Clinicians must possess the necessary clinical skills and expertise to diagnose, evaluate, and treat this condition effectively. This includes proficiency in interpreting radiological findings, recognizing potential complications, and understanding the nuances of treating osteosarcoma in the short and long term.
An interprofessional disease management group with expertise in sarcoma should be convened as soon as a diagnosis is made. Additionally, a strategic approach that involves evidence-based guidelines and individualized care plans tailored to each patient's unique circumstances is essential. Ethical considerations must be taken into account when determining treatment options and respecting patient autonomy in decision-making. Responsibilities within the interprofessional team should be clearly defined, with each member contributing their specialized knowledge and skills to optimize patient care. Effective communication among team members fosters a collaborative environment where information is freely shared, questions are encouraged, and concerns are addressed promptly.
Finally, care coordination is crucial for ensuring seamless and efficient patient care. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must work together to streamline the patient's journey from diagnosis through treatment and follow-up. This coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and a patient-centered approach that prioritizes the well-being and satisfaction of those affected by osteosarcoma.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer treatment and research. 2009:152():3-13. doi: 10.1007/978-1-4419-0284-9_1. Epub [PubMed PMID: 20213383]
Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009 Apr 1:115(7):1531-43. doi: 10.1002/cncr.24121. Epub [PubMed PMID: 19197972]
PDQ Pediatric Treatment Editorial Board. Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. 2002:(): [PubMed PMID: 26389179]
Level 3 (low-level) evidenceDurfee RA, Mohammed M, Luu HH. Review of Osteosarcoma and Current Management. Rheumatology and therapy. 2016 Dec:3(2):221-243 [PubMed PMID: 27761754]
Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. The Journal of bone and joint surgery. American volume. 2000 May:82(5):667-74 [PubMed PMID: 10819277]
Tiwari A. Current concepts in surgical treatment of osteosarcoma. Journal of clinical orthopaedics and trauma. 2012 Jun:3(1):4-9. doi: 10.1016/j.jcot.2012.04.004. Epub 2012 Jun 16 [PubMed PMID: 25983449]
Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2017 Jan-Mar:38(1):33-43. doi: 10.4103/0971-5851.203513. Epub [PubMed PMID: 28469335]
Hameed M, Mandelker D. Tumor Syndromes Predisposing to Osteosarcoma. Advances in anatomic pathology. 2018 Jul:25(4):217-222. doi: 10.1097/PAP.0000000000000190. Epub [PubMed PMID: 29668499]
Level 3 (low-level) evidenceLin YH, Jewell BE, Gingold J, Lu L, Zhao R, Wang LL, Lee DF. Osteosarcoma: Molecular Pathogenesis and iPSC Modeling. Trends in molecular medicine. 2017 Aug:23(8):737-755. doi: 10.1016/j.molmed.2017.06.004. Epub 2017 Jul 20 [PubMed PMID: 28735817]
Martin JW, Squire JA, Zielenska M. The genetics of osteosarcoma. Sarcoma. 2012:2012():627254. doi: 10.1155/2012/627254. Epub 2012 May 20 [PubMed PMID: 22685381]
Wang LL, Gannavarapu A, Kozinetz CA, Levy ML, Lewis RA, Chintagumpala MM, Ruiz-Maldanado R, Contreras-Ruiz J, Cunniff C, Erickson RP, Lev D, Rogers M, Zackai EH, Plon SE. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. Journal of the National Cancer Institute. 2003 May 7:95(9):669-74 [PubMed PMID: 12734318]
Savage SA,Mirabello L, Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011; [PubMed PMID: 21437228]
Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clinical orthopaedics and related research. 2002 Apr:(397):40-52 [PubMed PMID: 11953594]
Level 3 (low-level) evidenceMisaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT-J. 2018:4():12. doi: 10.1051/sicotj/2017028. Epub 2018 Apr 9 [PubMed PMID: 29629690]
Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer treatment and research. 2009:152():15-32. doi: 10.1007/978-1-4419-0284-9_2. Epub [PubMed PMID: 20213384]
Kar E, Ammanamanchi A, Yousif M, Geetha SD, Schwartz K, Mishra AS, Ling J, Nonyelu KN, Kannadath BS. From bimodal to unimodal: The transformed incidence of osteosarcoma in the United States. Journal of bone oncology. 2024 Aug:47():100613. doi: 10.1016/j.jbo.2024.100613. Epub 2024 Jun 6 [PubMed PMID: 38975333]
Yang Y, Yang R, Roth M, Piperdi S, Zhang W, Dorfman H, Rao P, Park A, Tripathi S, Freeman C, Zhang Y, Sowers R, Rosenblum J, Geller D, Hoang B, Gill J, Gorlick R. Genetically transforming human osteoblasts to sarcoma: development of an osteosarcoma model. Genes & cancer. 2017 Jan:8(1-2):484-494. doi: 10.18632/genesandcancer.133. Epub [PubMed PMID: 28435520]
Sarkar R. Pathological and clinical features of primary osseous tumours of the jaw. Journal of bone oncology. 2014 Nov:3(3-4):90-5. doi: 10.1016/j.jbo.2014.06.001. Epub 2014 Jul 11 [PubMed PMID: 26909304]
Gonzalez AL, Cates JM. Osteosarcoma: Differential Diagnostic Considerations. Surgical pathology clinics. 2012 Mar:5(1):117-46. doi: 10.1016/j.path.2011.07.011. Epub 2011 Dec 15 [PubMed PMID: 26837918]
Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Advances in anatomic pathology. 2021 May 1:28(3):119-138. doi: 10.1097/PAP.0000000000000293. Epub [PubMed PMID: 33480599]
Level 3 (low-level) evidenceKurt AM, Unni KK, McLeod RA, Pritchard DJ. Low-grade intraosseous osteosarcoma. Cancer. 1990 Mar 15:65(6):1418-28 [PubMed PMID: 2306687]
Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, Larousserie F, Muret Ad, Louis-Brennetot C, Aurias A, Coindre JM, Guillou L, Pedeutour F, Duval H, Collin C, de Pinieux G. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 May:24(5):624-37. doi: 10.1038/modpathol.2010.229. Epub 2011 Feb 18 [PubMed PMID: 21336260]
Crombé A, Simonetti M, Longhi A, Hauger O, Fadli D, Spinnato P. Imaging of Osteosarcoma: Presenting Findings, Metastatic Patterns, and Features Related to Prognosis. Journal of clinical medicine. 2024 Sep 25:13(19):. doi: 10.3390/jcm13195710. Epub 2024 Sep 25 [PubMed PMID: 39407770]
Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. American journal of clinical pathology. 2006 Apr:125(4):555-81 [PubMed PMID: 16627266]
Stitzlein RN, Wojcik J, Sebro RA, Balamuth NJ, Weber KL. Team Approach: Osteosarcoma of the Distal Part of the Femur in Adolescents. JBJS reviews. 2017 Dec:5(12):e5. doi: 10.2106/JBJS.RVW.17.00030. Epub [PubMed PMID: 29278618]
Zhou Y, Lu Q, Xu J, Yan R, Zhu J, Xu J, Jiang X, Li J, Wu F. The effect of pathological fractures on the prognosis of patients with osteosarcoma: a meta-analysis of 14 studies. Oncotarget. 2017 Sep 22:8(42):73037-73049. doi: 10.18632/oncotarget.20375. Epub 2017 Aug 21 [PubMed PMID: 29069847]
Level 1 (high-level) evidenceOng ZY, Chai HZ, How CH, Koh J, Low TB. A simplified approach to haemoptysis. Singapore medical journal. 2016 Aug:57(8):415-8. doi: 10.11622/smedj.2016130. Epub [PubMed PMID: 27549136]
Feng D, Yang X, Liu T, Xiao J, Wu Z, Huang Q, Ma J, Huang W, Zheng W, Cui Z, Xu H, Teng Y. Osteosarcoma of the spine: surgical treatment and outcomes. World journal of surgical oncology. 2013 Apr 18:11(1):89. doi: 10.1186/1477-7819-11-89. Epub 2013 Apr 18 [PubMed PMID: 23597053]
Level 2 (mid-level) evidenceChoi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, Melcher R, Tomita K, Global Spine Tumor Study Group. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2010 Feb:19(2):215-22. doi: 10.1007/s00586-009-1252-x. Epub 2009 Dec 29 [PubMed PMID: 20039084]
Level 3 (low-level) evidenceKundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian journal of orthopaedics. 2014 May:48(3):238-46. doi: 10.4103/0019-5413.132491. Epub [PubMed PMID: 24932027]
Byun BH, Kong CB, Lim I, Kim BI, Choi CW, Song WS, Cho WH, Jeon DG, Koh JS, Lee SY, Lim SM. Comparison of (18)F-FDG PET/CT and (99 m)Tc-MDP bone scintigraphy for detection of bone metastasis in osteosarcoma. Skeletal radiology. 2013 Dec:42(12):1673-81. doi: 10.1007/s00256-013-1714-4. Epub 2013 Aug 31 [PubMed PMID: 23995264]
Baguley DM, Prayuenyong P. Looking beyond the audiogram in ototoxicity associated with platinum-based chemotherapy. Cancer chemotherapy and pharmacology. 2020 Feb:85(2):245-250. doi: 10.1007/s00280-019-04012-z. Epub 2019 Dec 21 [PubMed PMID: 31865419]
Ando K, Heymann MF, Stresing V, Mori K, Rédini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers. 2013 May 24:5(2):591-616. doi: 10.3390/cancers5020591. Epub 2013 May 24 [PubMed PMID: 24216993]
Bielack SS, Hecker-Nolting S, Blattmann C, Kager L. Advances in the management of osteosarcoma. F1000Research. 2016:5():2767 [PubMed PMID: 27990273]
Level 3 (low-level) evidenceLamplot JD, Denduluri S, Qin J, Li R, Liu X, Zhang H, Chen X, Wang N, Pratt A, Shui W, Luo X, Nan G, Deng ZL, Luo J, Haydon RC, He TC, Luu HH. The Current and Future Therapies for Human Osteosarcoma. Current cancer therapy reviews. 2013 Feb:9(1):55-77 [PubMed PMID: 26834515]
Level 2 (mid-level) evidenceBiermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, Didwania A, Fabbri N, Hornicek FJ, Kuechle JB, Lindskog D, Mayerson J, McGarry SV, Million L, Morris CD, Movva S, O'Donnell RJ, Randall RL, Rose P, Santana VM, Satcher RL, Schwartz H, Siegel HJ, Thornton K, Villalobos V, Bergman MA, Scavone JL. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. Journal of the National Comprehensive Cancer Network : JNCCN. 2017 Feb:15(2):155-167 [PubMed PMID: 28188186]
Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, Bonvalot S, Boukovinas I, Bovee JVMG, Brennan B, Brodowicz T, Broto JM, Brugières L, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Dhooge C, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gaspar N, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hecker-Nolting S, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kager L, Kasper B, Kopeckova K, Krákorová DA, Ladenstein R, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Morland B, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Strauss SJ, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY, ESMO Guidelines Committee, PaedCan and ERN EURACAN. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2018 Oct 1:29(Suppl 4):iv79-iv95. doi: 10.1093/annonc/mdy310. Epub [PubMed PMID: 30285218]
Level 1 (high-level) evidenceGerrand C, Athanasou N, Brennan B, Grimer R, Judson I, Morland B, Peake D, Seddon B, Whelan J, British Sarcoma Group. UK guidelines for the management of bone sarcomas. Clinical sarcoma research. 2016:6():7. doi: 10.1186/s13569-016-0047-1. Epub 2016 May 4 [PubMed PMID: 27148438]
Shepperd S, Gonçalves-Bradley DC, Straus SE, Wee B. Hospital at home: home-based end-of-life care. The Cochrane database of systematic reviews. 2016 Feb 18:2(2):CD009231. doi: 10.1002/14651858.CD009231.pub2. Epub 2016 Feb 18 [PubMed PMID: 26887902]
Level 1 (high-level) evidenceBarnes H, McDonald J, Smallwood N, Manser R. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. The Cochrane database of systematic reviews. 2016 Mar 31:3(3):CD011008. doi: 10.1002/14651858.CD011008.pub2. Epub 2016 Mar 31 [PubMed PMID: 27030166]
Level 1 (high-level) evidenceMo JT, Darrow MA, Sharma JD. Langerhans cell histiocytosis with aneurysmal bone cyst-like changes: a case-based literature review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2023 Nov:39(11):3057-3064. doi: 10.1007/s00381-023-06108-7. Epub 2023 Jul 31 [PubMed PMID: 37522932]
Level 3 (low-level) evidenceZöllner SK, Amatruda JF, Bauer S, Collaud S, de Álava E, DuBois SG, Hardes J, Hartmann W, Kovar H, Metzler M, Shulman DS, Streitbürger A, Timmermann B, Toretsky JA, Uhlenbruch Y, Vieth V, Grünewald TGP, Dirksen U. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. Journal of clinical medicine. 2021 Apr 14:10(8):. doi: 10.3390/jcm10081685. Epub 2021 Apr 14 [PubMed PMID: 33919988]
Level 3 (low-level) evidenceRestrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: pathophysiology, histology, imaging and treatment. Pediatric radiology. 2022 Aug:52(9):1601-1614. doi: 10.1007/s00247-022-05396-6. Epub 2022 Aug 9 [PubMed PMID: 35941207]
Callan AK, Alexander JH, Montgomery NI, Lindberg AW, Scharschmidt TJ, Binitie O. Contemporary surgical management of osteosarcoma and Ewing sarcoma. Pediatric blood & cancer. 2024 Oct 15:():e31374. doi: 10.1002/pbc.31374. Epub 2024 Oct 15 [PubMed PMID: 39410791]
Tupper CJ, Reeson EA, Burdyny MR, Eaton VP, Silberstein PT. Extent of Surgery and Survival of Osteosarcoma: A Retrospective Population-Based Study. Cureus. 2024 Mar:16(3):e56030. doi: 10.7759/cureus.56030. Epub 2024 Mar 12 [PubMed PMID: 38606239]
Level 2 (mid-level) evidenceAbdelgawad MA, Parambi DGT, Ghoneim MM, Alotaibi NH, Alzarea AI, Hassan AH, Abdelrahim MEA. A meta-analysis comparing efficiency of limb-salvage surgery vs amputation on patients with osteosarcoma treated with neoadjuvant chemotherapy. International wound journal. 2022 Nov:19(7):1616-1624. doi: 10.1111/iwj.13758. Epub 2022 Feb 5 [PubMed PMID: 35122396]
Level 1 (high-level) evidenceJauregui JJ, Nadarajah V, Munn J, Pivec R, Kapadia BH, Lerman DM, Maheshwari AV. Limb Salvage Versus Amputation in Conventional Appendicular Osteosarcoma: a Systematic Review. Indian journal of surgical oncology. 2018 Jun:9(2):232-240. doi: 10.1007/s13193-018-0725-y. Epub 2018 Jan 20 [PubMed PMID: 29887707]
Level 1 (high-level) evidenceAljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Annals of oncology : official journal of the European Society for Medical Oncology. 2009 Jun:20(6):1136-41. doi: 10.1093/annonc/mdn731. Epub 2009 Jan 19 [PubMed PMID: 19153114]
Diemel KD, Klippe HJ, Branseheid D. Pulmonary metastasetomy for osteosarcoma: is it justified? Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2009:179():183-208 [PubMed PMID: 19230541]
Longhi A, Fabbri N, Donati D, Capanna R, Briccoli A, Biagini R, Bernini G, Ferrari S, Versari M, Bacci G. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. Journal of chemotherapy (Florence, Italy). 2001 Jun:13(3):324-30 [PubMed PMID: 11450892]
Level 3 (low-level) evidenceSpałek MJ, Poleszczuk J, Czarnecka AM, Dudzisz-Śledź M, Napieralska A, Matysiakiewicz J, Chojnacka M, Raciborska A, Sztuder A, Maciejczyk A, Szulc A, Skóra T, Cybulska-Stopa B, Winiecki T, Kaźmierska J, Tomasik B, Fijuth J, Rutkowski P. Radiotherapy in the Management of Pediatric and Adult Osteosarcomas: A Multi-Institutional Cohort Analysis. Cells. 2021 Feb 10:10(2):. doi: 10.3390/cells10020366. Epub 2021 Feb 10 [PubMed PMID: 33578676]
Tinkle CL, Lu J, Han Y, Li Y, McCarville BM, Neel MD, Bishop MW, Krasin MJ. Curative-intent radiotherapy for pediatric osteosarcoma: The St. Jude experience. Pediatric blood & cancer. 2019 Aug:66(8):e27763. doi: 10.1002/pbc.27763. Epub 2019 Apr 22 [PubMed PMID: 31012273]
Jaffe N, Puri A, Gelderblom H. Osteosarcoma: evolution of treatment paradigms. Sarcoma. 2013:2013():203531. doi: 10.1155/2013/203531. Epub 2013 May 27 [PubMed PMID: 23781130]
O'Kane GM, Cadoo KA, Walsh EM, Emerson R, Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, Eustace S, Carney DN. Perioperative chemotherapy in the treatment of osteosarcoma: a 26-year single institution review. Clinical sarcoma research. 2015:5():17. doi: 10.1186/s13569-015-0032-0. Epub 2015 Jul 14 [PubMed PMID: 26175892]
Bacci G, Forni C, Ferrari S, Longhi A, Bertoni F, Mercuri M, Donati D, Capanna R, Bernini G, Briccoli A, Setola E, Versari M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome. Journal of pediatric hematology/oncology. 2003 Nov:25(11):845-53 [PubMed PMID: 14608193]
Wu C, Wang Q, Li Y. Prediction and evaluation of neoadjuvant chemotherapy using the dual mechanisms of (99m)Tc-MIBI scintigraphy in patients with osteosarcoma. Journal of bone oncology. 2019 Aug:17():100250. doi: 10.1016/j.jbo.2019.100250. Epub 2019 Jul 9 [PubMed PMID: 31372331]
Strauss SJ, Frezza AM, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, de Álava E, Dei Tos AP, Garcia Del Muro X, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Gaspar N, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Gronchi A, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, López Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Ladenstein R, Casali PG, Stacchiotti S, ESMO Guidelines Committee, EURACAN, GENTURIS and ERN PaedCan. Electronic address: clinicalguidelines@esmo.org. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2021 Dec:32(12):1520-1536. doi: 10.1016/j.annonc.2021.08.1995. Epub 2021 Sep 6 [PubMed PMID: 34500044]
Level 1 (high-level) evidenceAnderson PM, Wiseman GA, Dispenzieri A, Arndt CA, Hartmann LC, Smithson WA, Mullan BP, Bruland OS. High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jan 1:20(1):189-96 [PubMed PMID: 11773169]
Anderson PM, Subbiah V, Rohren E. Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: samarium-153-EDTMP and radium-223. Advances in experimental medicine and biology. 2014:804():291-304. doi: 10.1007/978-3-319-04843-7_16. Epub [PubMed PMID: 24924181]
Level 3 (low-level) evidenceSubbiah V, Anderson PM, Kairemo K, Hess K, Huh WW, Ravi V, Daw NC, Somaiah N, Ludwig JA, Benjamin RS, Chawla S, Hong DS, Meric-Bernstam F, Ravizzini G, Kleinerman E, Macapinlac H, Rohren E. Alpha Particle Radium 223 Dichloride in High-risk Osteosarcoma: A Phase I Dose Escalation Trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019 Jul 1:25(13):3802-3810. doi: 10.1158/1078-0432.CCR-18-3964. Epub 2019 Feb 7 [PubMed PMID: 30733229]
Salmen J, Banys-Paluchowski M, Fehm T. Bone-Targeted Therapy. Geburtshilfe und Frauenheilkunde. 2015 Jun:75(6):584-587 [PubMed PMID: 26166839]
Farrell KB, Karpeisky A, Thamm DH, Zinnen S. Bisphosphonate conjugation for bone specific drug targeting. Bone reports. 2018 Dec:9():47-60. doi: 10.1016/j.bonr.2018.06.007. Epub 2018 Jul 3 [PubMed PMID: 29992180]
Jawad MU, Scully SP. In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system. Clinical orthopaedics and related research. 2010 Jul:468(7):2000-2. doi: 10.1007/s11999-010-1315-7. Epub [PubMed PMID: 20333492]
Tanaka K, Tsumura H. Eighth edition of the American Joint Committee on Cancer staging system for soft tissue sarcoma of the trunk and extremity: in search of a better staging system. Annals of translational medicine. 2019 Mar:7(Suppl 1):S11. doi: 10.21037/atm.2019.01.31. Epub [PubMed PMID: 31032292]
Isakoff MS, Barkauskas DA, Ebb D, Morris C, Letson GD. Poor survival for osteosarcoma of the pelvis: a report from the Children's Oncology Group. Clinical orthopaedics and related research. 2012 Jul:470(7):2007-13. doi: 10.1007/s11999-012-2284-9. Epub 2012 Feb 22 [PubMed PMID: 22354612]
Matsuo T, Sugita T, Sato K, Hotta T, Tsuchiya H, Shimose S, Kubo T, Ochi M. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005:68(4-6):375-81 [PubMed PMID: 16020966]
Level 2 (mid-level) evidenceJasnau S, Meyer U, Potratz J, Jundt G, Kevric M, Joos UK, Jürgens H, Bielack SS, Cooperative Osteosarcoma Study Group COSS. Craniofacial osteosarcoma Experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral oncology. 2008 Mar:44(3):286-94 [PubMed PMID: 17467326]
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Feb 1:20(3):776-90 [PubMed PMID: 11821461]
Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, Butterfass-Bahloul T, Calaminus G, Capra M, Dhooge C, Eriksson M, Flanagan AM, Friedel G, Gebhardt MC, Gelderblom H, Goldsby R, Grier HE, Grimer R, Hawkins DS, Hecker-Nolting S, Sundby Hall K, Isakoff MS, Jovic G, Kühne T, Kager L, von Kalle T, Kabickova E, Lang S, Lau CC, Leavey PJ, Lessnick SL, Mascarenhas L, Mayer-Steinacker R, Meyers PA, Nagarajan R, Randall RL, Reichardt P, Renard M, Rechnitzer C, Schwartz CL, Strauss S, Teot L, Timmermann B, Sydes MR, Marina N. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. European journal of cancer (Oxford, England : 1990). 2019 Mar:109():36-50. doi: 10.1016/j.ejca.2018.11.027. Epub 2019 Jan 25 [PubMed PMID: 30685685]
Level 2 (mid-level) evidencePapakonstantinou E, Athanasiadou KI, Markozannes G, Tzotzola V, Bouka E, Baka M, Moschovi M, Polychronopoulou S, Hatzipantelis E, Galani V, Stefanaki K, Strantzia K, Vousvouki M, Kourou P, Magkou E, Nikita M, Zambakides C, Michelarakis J, Alexopoulou A, Gavra M, Malama A, Ntzani EE, Petridou ET. Prognostic factors in high-grade pediatric osteosarcoma among children and young adults: Greek Nationwide Registry for Childhood Hematological Malignancies and Solid Tumors (NARECHEM-ST) data along with a systematic review and meta-analysis. Cancer epidemiology. 2024 Jun:90():102551. doi: 10.1016/j.canep.2024.102551. Epub 2024 Mar 5 [PubMed PMID: 38447251]
Level 1 (high-level) evidenceJaneway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R, Marina N. Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children's Oncology Group. Cancer. 2012 Sep 15:118(18):4597-605. doi: 10.1002/cncr.27414. Epub 2012 Jan 17 [PubMed PMID: 22252521]
Slade AD, Warneke CL, Hughes DP, Lally PA, Lally KP, Hayes-Jordan AA, Austin MT. Effect of concurrent metastatic disease on survival in children and adolescents undergoing lung resection for metastatic osteosarcoma. Journal of pediatric surgery. 2015 Jan:50(1):157-60; discussion 160. doi: 10.1016/j.jpedsurg.2014.10.038. Epub 2014 Oct 28 [PubMed PMID: 25598115]
Kim C, Davis LE, Albert CM, Samuels B, Roberts JL, Wagner MJ. Osteosarcoma in Pediatric and Adult Populations: Are Adults Just Big Kids? Cancers. 2023 Oct 19:15(20):. doi: 10.3390/cancers15205044. Epub 2023 Oct 19 [PubMed PMID: 37894411]
Bacci G, Longhi A, Forni C, Fabbri N, Briccoli A, Barbieri E, Mercuri M, Balladelli A, Ferrari S, Picci P. Neoadjuvant chemotherapy for radioinduced osteosarcoma of the extremity: The Rizzoli experience in 20 cases. International journal of radiation oncology, biology, physics. 2007 Feb 1:67(2):505-11 [PubMed PMID: 17118571]
Level 3 (low-level) evidenceRuggieri P, Calabrò T, Montalti M, Mercuri M. The role of surgery and adjuvants to survival in Pagetic osteosarcoma. Clinical orthopaedics and related research. 2010 Nov:468(11):2962-8. doi: 10.1007/s11999-010-1473-7. Epub [PubMed PMID: 20652460]
Kelley LM, Schlegel M, Hecker-Nolting S, Kevric M, Haller B, Rössig C, Reichardt P, Kager L, Kühne T, Gosheger G, Windhager R, Specht K, Rechl H, Tunn PU, Baumhoer D, Wirth T, Werner M, von Kalle T, Nathrath M, Burdach S, Bielack S, von Lüttichau I. Pathological Fracture and Prognosis of High-Grade Osteosarcoma of the Extremities: An Analysis of 2,847 Consecutive Cooperative Osteosarcoma Study Group (COSS) Patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Mar 10:38(8):823-833. doi: 10.1200/JCO.19.00827. Epub 2020 Jan 13 [PubMed PMID: 31928458]
Gonzalez MR, Bedi A, Karczewski D, Lozano-Calderon SA. Are Pathologic Fractures in Patients With Osteosarcoma Associated With Worse Survival Outcomes? A Systematic Review and Meta-analysis. Clinical orthopaedics and related research. 2023 Dec 1:481(12):2433-2443. doi: 10.1097/CORR.0000000000002687. Epub 2023 May 12 [PubMed PMID: 37184541]
Level 1 (high-level) evidenceMin D, Lin F, Shen Z, Zheng S, Tan L, Yu W, Yao Y. Analysis of prognostic factors in 333 Chinese patients with high-grade osteosarcoma treated by multidisciplinary combined therapy. Asia-Pacific journal of clinical oncology. 2013 Mar:9(1):71-9. doi: 10.1111/j.1743-7563.2012.01560.x. Epub 2012 Jul 23 [PubMed PMID: 22897971]
Laitinen M, Parry M, Albergo JI, Jeys L, Abudu A, Carter S, Sumathi V, Grimer R. The prognostic and therapeutic factors which influence the oncological outcome of parosteal osteosarcoma. The bone & joint journal. 2015 Dec:97-B(12):1698-703. doi: 10.1302/0301-620X.97B12.35749. Epub [PubMed PMID: 26637687]
Alpan B, Aycan OE, Bayram S, Özmen E, Valiyev N, Özger H, Eralp L. Prognostic Factors Influencing Survival and Oncological Outcome in Patients with Parosteal Osteosarcoma. Indian journal of surgical oncology. 2024 Mar:15(Suppl 1):29-37. doi: 10.1007/s13193-022-01670-z. Epub 2022 Oct 25 [PubMed PMID: 38545575]
Hecker-Nolting S, Langer T, Blattmann C, Kager L, Bielack SS. Current Insights into the Management of Late Chemotherapy Toxicities in Pediatric Osteosarcoma Patients. Cancer management and research. 2021:13():8989-8998. doi: 10.2147/CMAR.S287908. Epub 2021 Dec 1 [PubMed PMID: 34880679]
Kan A, Marokakis S, Ward JH, Kim S, Gabriel M, Durkan AM. Long-term renal outcomes of children with cancers treated with platinum-based chemotherapy: A retrospective chart analysis. Pediatric blood & cancer. 2025 Jan:72(1):e31404. doi: 10.1002/pbc.31404. Epub 2024 Nov 6 [PubMed PMID: 39503057]
Level 2 (mid-level) evidenceMancilla TR, Iskra B, Aune GJ. Doxorubicin-Induced Cardiomyopathy in Children. Comprehensive Physiology. 2019 Jun 12:9(3):905-931. doi: 10.1002/cphy.c180017. Epub 2019 Jun 12 [PubMed PMID: 31187890]
Longhi A, Ferrari S, Tamburini A, Luksch R, Fagioli F, Bacci G, Ferrari C. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: the Italian Sarcoma Group Experience (1983-2006). Cancer. 2012 Oct 15:118(20):5050-9. doi: 10.1002/cncr.27493. Epub 2012 Mar 13 [PubMed PMID: 22415578]
Cianni L, Taccari F, Bocchi MB, Micheli G, Sangiorgi F, Ziranu A, Fantoni M, Maccauro G, Vitiello R. Characteristics and Epidemiology of Megaprostheses Infections: A Systematic Review. Healthcare (Basel, Switzerland). 2024 Jun 27:12(13):. doi: 10.3390/healthcare12131283. Epub 2024 Jun 27 [PubMed PMID: 38998818]
Level 1 (high-level) evidence