Introduction
The olfactory nerve is the first cranial nerve and is instrumental in our sense of smell. The olfactory nerve contains only afferent sensory nerve fibers and, like all cranial nerves, is paired. The olfactory nerve is the shortest cranial nerve, and along with the optic nerve is one of the only two cranial nerves that do not converge with the brainstem. Embryologically, the olfactory nerve is a derivative of the forebrain and is therefore considered a component of the central nervous system. The olfactory nerve is not myelinated by Schwann cells but rather is ensheathed by olfactory ensheathing glia. The olfactory nerves originate from the cell bodies of bipolar olfactory neurons in the olfactory epithelium, a specialized epithelial tissue found in the posterosuperior portion of each nasal cavity. Olfactory neurons give off projections apically towards the outside world and basally towards the olfactory bulb, the central hub, and coordinator of olfactory transmission. From the olfactory bulbs, olfactory information reaches the primary olfactory cortex via the olfactory tract. The primary olfactory cortex interacts with a variety of cortical and limbic structures via sophisticated pathways that allow the smell to become integrated with memory, emotions, and taste.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Architecturally, the nose is designed in a way to facilitate the movement of inspired air toward the olfactory epithelium. The olfactory epithelium is specialized epithelial tissue covering the septum, the upper portion of the superior turbinate and the lateral surface of the posterosuperior portions of both nasal cavities. This specialized epithelium contains the cell bodies of bipolar olfactory neurons from which the olfactory nerve fibers originate and extend apically. In each nostril, human beings have 6 to 10 million olfactory sensory neurons distributed along a surface area of 2.5 cm^2. These cells continuously regenerate from stem cells within the basal portion of the epithelium and have a half-life of 30 to 40 days. The apical portion of these cells has dendrites that project into the epithelial surface, where they interact with odoriferous particles from the outside world via G protein-coupled receptors.
The basal projections of olfactory neurons ascend and traverse the cribriform plate of the ethmoid as unmyelinated axons grouped into small nerve bundles called fila olfactoria. Each of these small nerve bundles forms the olfactory nerves, and there are around 15 to 20 of them on each side of the nasal cavity. The junction at which the olfactory nerves traverse the bony cribriform plate is a potential area of harm, either through the form of infection or trauma. The foramina in the cribriform plate serve as easy entry points for pathogens to gain access to the intracranial space and also facilitate the shearing of olfactory nerves during trauma. After penetrating the cribriform plate and traversing the subarachnoid space, the fila olfactoria enter the olfactory bulbs ventrally. The dura mater ensheathing the intracranial surface of the cribriform plate runs continuously with the basal membrane of the olfactory epithelium emerging through the foramina.
The ventral surface of the olfactory bulb lies on top of the posterior third of the cribriform plate, while the dorsal surface sits beneath the inferior surface of the frontal lobes, specifically the orbital and rectus gyri. The olfactory bulbs serve as a relay station for all impulses transmitted between the olfactory epithelium and the primary olfactory cortex.
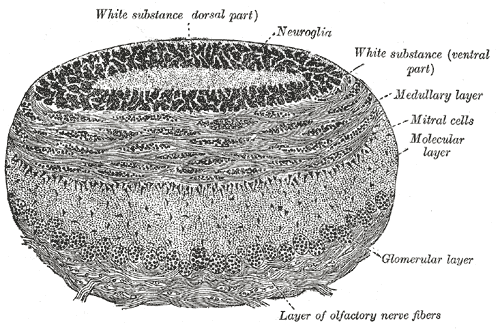
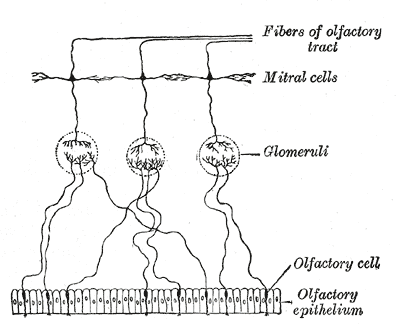
The olfactory bulb consists of a constellation of neurons and sophisticated synaptic fields distributed uniquely among five layers. There are a variety of neurons in the olfactory bulb, including the mitral cells, tufted cells, granule cells, and periglomerular neurons. The axons of olfactory nerves originating from cell bodies in the olfactory epithelium terminate at the olfactory bulb, where they converge with the dendrites of mitral and tufted cells in small clusters called glomeruli. These glomeruli form the first layer of the olfactory bulb that we come to know as the glomerular layer. Under high magnification, glomeruli are described as round, ball-like structures and receive inputs from the periglomerular cells, olfactory epithelium, mitral cells, and tufted cells. The periglomerular cells surround the entire glomeruli and maintain reciprocal dendrodendritic synapses with mitral and tufted cells. In conjunction with granule cells, periglomerular cells serve to modulate and fine-tune the processing of olfactory information. Both granule cells and periglomerular cells are considered interneurons.
After olfactory information is transmitted from olfactory receptor neurons to mitral and tufted cells in the glomeruli, the axonal projections of mitral and tufted cells form bundles that pass through the olfactory bulb and run dorsally, merging to form the olfactory tract. From the olfactory bulb, each olfactory tract runs posteriorly along the olfactory sulcus and ends in the olfactory trigone. The olfactory trigone is a triangular widening of the terminal olfactory tract located superior to the anterior clinoid process and directly rostral to the anterior perforated substance. At this unique anatomic landmark, the fibers of the tract diverge to form two main bundles, the lateral and medial olfactory stria.
The medial olfactory stria is responsible for autonomic responses associated with olfaction, such as an increase in salivation and gastric peristalsis/secretion in response to the smell of food. The medial olfactory stria sends projections to the ipsilateral anterior olfactory nucleus and through the anterior commissure to the contralateral olfactory bulb. These projections end in septal nuclei surrounding the para-terminal gyrus from which two fiber bundles split: the medullary stria and the olfacto-hypothalamic-tegmental bundle. The medullary stria pathway is responsible for salivation in response to the smell of food. It involves the activation of the superior and inferior salivatory nuclei via projections to the habenacular nuclei and the tegmentum. The olfacto-hypothalamic-tegmental bundle interacts with the dorsal vagal nucleus in the medulla and is responsible for increased peristalsis and gastric secretion in response to the smell of food.
The lateral olfactory stria contains the largest number of fibers in the olfactory tract and is responsible for the majority of functional olfactory transmission. The lateral olfactory stria carries the efferent projections of the olfactory bulb toward the limen of the insula, where it bends medially to enter the temporal lobe near the uncus, where the primary olfactory cortex is.
The primary olfactory cortex is the main site of olfactory information processing. The primary olfactory cortex interacts with a wide variety of cortical and limbic structures, and by definition, refers to the structures that receive axons from the olfactory bulb. These structures include the piriform cortex, amygdala, parahippocampal gyrus, olfactory tubercle, and anterior olfactory nucleus. These structures provide a multitude of functions that result in the integration of olfactory sensory information to encode, recognize, and contextualize scenarios.[1][2][3][4]
Embryology
Embryologically, the face develops from five swellings derived from the first and second pharyngeal arches called facial prominences that appear in the fourth week of development. These include the frontonasal prominence and the paired mandibular and maxillary prominences. Around the fourth week of embryonic development, an area of thickened ectoderm arises on each side of the frontonasal prominence called the olfactory placodes. The olfactory placodes continue to increase in size until the sixth week when the center of each placode invaginates to form the nasal pits. The nasal pits eventually give rise to the olfactory epithelium, from which the olfactory nerves originate, and divide into a medial and lateral nasal process. Subsequent formation of the nose, philtrum, and primary palate follows.[5]
Blood Supply and Lymphatics
The olfactory mucosa and olfactory neurons located in the posterosuperior portion of the nasal cavity receive blood from an abundant vascular supply formed by branches of the external and internal carotid arteries, the sphenopalatine artery and the anterior and posterior ethmoidal arteries respectively. The sphenopalatine artery emerges from the pterygopalatine segment of the maxillary artery, a branch of the external carotid artery. From the pterygopalatine fossa, the sphenopalatine artery courses through the sphenopalatine foramen where it reaches the upper nasal cavity posterosuperior to the middle nasal conchae. At this point, major branches called the posterior lateral nasal and nasal septal arteries form extensive networks and anastomosis.
The anterior and posterior ethmoidal arteries originate from the ophthalmic artery, a branch of the internal carotid artery. The posterior ethmoidal artery arises from a proximal segment of the ophthalmic artery and passes through the posterior ethmoidal foramen located near the optic canal. The anterior ethmoidal artery originates from a more distal portion of the ophthalmic artery and courses toward the medial wall of the orbit. The anterior ethmoidal artery eventually enters the anterior ethmoidal foramen located behind the orbital margin. In conjunction with the sphenopalatine artery, the anterior and posterior ethmoidal arteries form extensive anastomotic networks supplying the olfactory mucosa.
Intracranially, the olfactory nerve receives its blood supply from a branch of the anterior cerebral artery called the olfactory artery. The olfactory artery arises from the lateral aspect of the A2 segment of the anterior cerebral artery—the anterior cerebral artery is divided into five segments, A1-A5. The A2 segment extends from the anterior communicating artery to the bifurcation forming the pericallosal and callosomarginal arteries. Four branches arise from the A2 segment, and these include the recurrent artery of Heubner, the orbitofrontal artery, the frontopolar artery, and the olfactory artery. The olfactory artery typically arises immediately beyond the origin of the anterior communicating artery and between the origins of the orbitofrontal artery and the recurrent artery of Heubner.
From its branch point, the olfactory artery runs above the optic nerve, where it reaches the olfactory sulcus and adheres to the ventral surface of the olfactory tract by arachnoid. The olfactory artery has three terminal branches, and along its course within the olfactory sulcus supplies the entire olfactory tract and olfactory bulb.[6][7]
Physiologic Variants
The vast majority of studies involving physiologic variants and congenital abnormalities of the olfactory nerve involve the absence of the olfactory tract and olfactory bulb either unilaterally or bilaterally. Of these variations, the bilateral absence of the olfactory bulb and tract was the most common variation and appeared on MRI in patients with Kallman syndrome.[8][9]
Surgical Considerations
The pterional craniotomy, also known as a frontotemporal craniotomy, is a widely used neurosurgical approach. This approach allows access to critical intracranial spaces, including the anterior and middle fossa, suprasellar space, and cavernous sinus. The pterional approach is the standard approach for most lesions of the anterior and middle cranial fossa. Skull base tumors such as meningiomas, schwannomas, epidermoids, and surgeons commonly remove brain malignancies using the pterional approach. This approach requires some degree of frontal lobe retraction, which may result in olfactory damage because of nerve shearing or mechanical compression. Retractors used during surgery have the potential to damage the microvasculature on the dorsal surface of the olfactory nerve and should be carefully applied. Dissection near the olfactory artery and medial orbitofrontal arteries lying underneath the olfactory tract in the olfactory sulcus should be done with great care because damage to the terminal branches of these arteries may cause bleeding during surgery.[10][11]
Clinical Significance
Testing olfactory nerve function is intuitive and straightforward. Proper testing requires a conscious, cooperative patient with intact higher cognitive function, a patent airway, and unobstructed nostrils. The patient must close their eyes, and the examiner tests each nostril individually, while the other nostril is blocked. Anything with a characteristic smell is usable for the test. For example, coffee is a common choice. The patient is presented with the coffee to the open nostril and reports whether they recognize the scent or not. It is important to note that the primary olfactory cortex in each hemisphere receives input bilaterally, from both olfactory bulbs. Assessing olfactory symptoms in cases like stroke is clinically unhelpful because an isolated unilateral stroke in the primary olfactory cortex will not result in significant olfactory symptoms. Olfactory nerve dysfunction can arise from a variety of etiologies, some of which include genetic defects, trauma, neoplasms, and iatrogenic injury.
Congenital Anosmia:
Isolated congenital anosmia is a rare condition inherited in an autosomal dominant fashion that is characterized by a life long inability to smell, with no associated congenital structural or metabolic abnormalities. Although anosmia may occur alone, anosmia since birth may also be a symptom of another condition called Kallman syndrome. Kallman syndrome is a form of hypogonadotropic hypogonadism that is distinguished from other forms of hypogonadotropic hypogonadism by the additional and unique symptoms of anosmia. Kallman syndrome occurs as a result of impaired development of the olfactory system and disrupted embryonic migration of GnRH-synthesizing neurons from the olfactory epithelium to the hypothalamus. The failure to develop proper early olfactory neuron projections from the olfactory placode to the olfactory bulb results in aplasia or hypoplasia of the olfactory bulbs that are visible on MRI.[8][9][12]
Tumors and olfaction:
Tumors can affect the olfactory bulb and tract through compression and mass effect; meningiomas are most commonly the culprit. Tumors of the anterior skull base are known to affect olfactory sensation and can present insidiously with hyposmia, anosmia, or parosmia. Esthesioneuroblastoma is a malignant tumor arising from the neuroepithelium that lines the cribriform plate, superior aspect of the nasal septum, middle turbinate, and superior turbinate. These tumors involve the frontal skull base and are commonly seen growing along the cranial nerves as they exit and enter the skull base. These tumors commonly affect the olfactory nerve, olfactory bulb, and olfactory tract. Rare tumors like olfactory ensheathing cell tumors or primitive neuroectodermal tumors can also affect the olfactory system and are found almost exclusively in children.[12][13]
Post-Traumatic Anosmia:
Head trauma can occur through a multitude of mechanisms and is a common cause of olfactory dysfunction in the modern world. Regardless of the specific initiating incident, post-traumatic olfactory dysfunction occurs primarily through 3 mechanisms: Sinus or nasal tract disruption, shearing of olfactory nerve fibers at the cribriform plate, and localized hemorrhage/contusion within the olfactory bulb and primary olfactory cortex.[14]
Other Issues
According to some authors, the Zero nerve or N nerve exists, which would have many connections with the olfactory nerve, although it is still not recognized in the anatomy books. The zero nerve arises above the olfactory nerve.[15]
In the initial stages of idiopathic intracranial hypertension, the patient may undergo an alteration of the volume of the olfactory bulb (it decreases), with alteration of the sense of smell.[16]
The olfactory nerves are an essential way for the passage of cerebrospinal fluid, through the cribriform plate up to the mucous membranes of the nose, to reach the lymphatic system of the jaw and neck. The integrity of the nerve and its anatomical structures are fundamental for an adequate outflow of the CSF.[17]
Media
(Click Image to Enlarge)
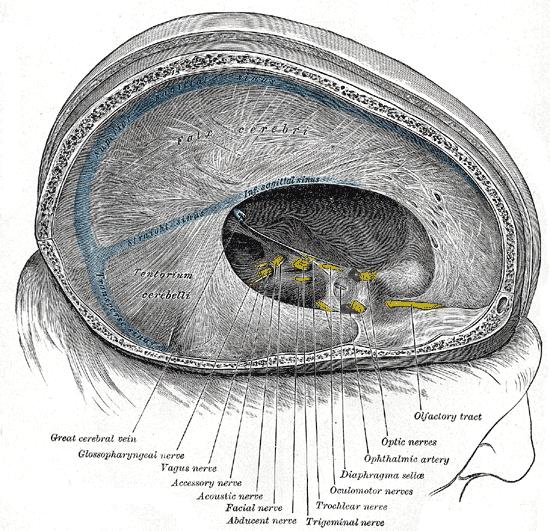
Dural Venous Sinuses. Dura mater, falx cerebri, tentorium cerebelli, transverse sinus, great cerebral vein, glossopharyngeal nerve, vagus nerve, accessory nerve, acoustic nerve, facial nerve, abducens nerve, trigeminal nerve, trochlear nerve, oculomotor nerves, diaphragma sellae, ophthalmic artery, optic nerves, and olfactory tract.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
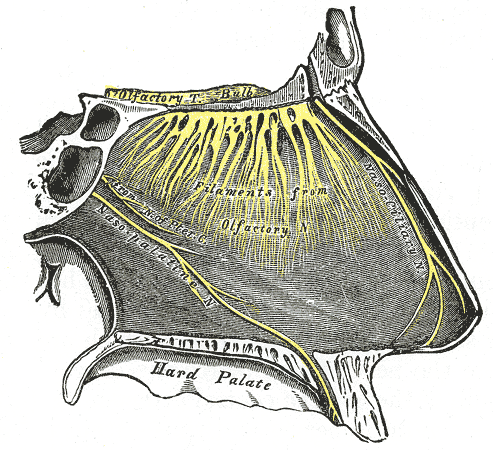
Nasopalatine Region Innervation. This image illustrates the innervation of the nasopalatine region, including the olfactory tract, bulb, and filaments. This image also highlights the nasociliary, nasopalatine, and pterygoid canal nerves. The nasopalatine nerve is shown entering the hard palate anteriorly.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clinical anatomy (New York, N.Y.). 2014 Jan:27(1):54-60. doi: 10.1002/ca.22338. Epub 2013 Nov 22 [PubMed PMID: 24272785]
Giessel AJ, Datta SR. Olfactory maps, circuits and computations. Current opinion in neurobiology. 2014 Feb:24(1):120-32. doi: 10.1016/j.conb.2013.09.010. Epub 2013 Oct 29 [PubMed PMID: 24492088]
Level 3 (low-level) evidenceShipley MT, Ennis M. Functional organization of olfactory system. Journal of neurobiology. 1996 May:30(1):123-76 [PubMed PMID: 8727988]
Level 3 (low-level) evidenceLópez-Elizalde R, Campero A, Sánchez-Delgadillo T, Lemus-Rodríguez Y, López-González MI, Godínez-Rubí M. Anatomy of the olfactory nerve: A comprehensive review with cadaveric dissection. Clinical anatomy (New York, N.Y.). 2018 Jan:31(1):109-117. doi: 10.1002/ca.23003. Epub 2017 Nov 10 [PubMed PMID: 29088516]
Suzuki J, Osumi N. Neural crest and placode contributions to olfactory development. Current topics in developmental biology. 2015:111():351-74. doi: 10.1016/bs.ctdb.2014.11.010. Epub 2015 Jan 20 [PubMed PMID: 25662265]
Level 3 (low-level) evidenceHendrix P, Griessenauer CJ, Foreman P, Shoja MM, Loukas M, Tubbs RS. Arterial supply of the upper cranial nerves: a comprehensive review. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1159-66. doi: 10.1002/ca.22415. Epub 2014 May 26 [PubMed PMID: 24863843]
Favre JJ, Chaffanjon P, Passagia JG, Chirossel JP. Blood supply of the olfactory nerve. Meningeal relationships and surgical relevance. Surgical and radiologic anatomy : SRA. 1995:17(2):133-8, 12-4 [PubMed PMID: 7482150]
Sailani MR, Jingga I, MirMazlomi SH, Bitarafan F, Bernstein JA, Snyder MP, Garshasbi M. Isolated Congenital Anosmia and CNGA2 Mutation. Scientific reports. 2017 Jun 1:7(1):2667. doi: 10.1038/s41598-017-02947-y. Epub 2017 Jun 1 [PubMed PMID: 28572688]
Zaghouani H, Slim I, Zina NB, Mallat N, Tajouri H, Kraiem C. Kallmann syndrome: MRI findings. Indian journal of endocrinology and metabolism. 2013 Oct:17(Suppl 1):S142-5. doi: 10.4103/2230-8210.119536. Epub [PubMed PMID: 24251137]
Cömert A, Uğur HC, Kahiloğullar G, Cömert E, Elhan A, Tekdemir I. Microsurgical anatomy for intraoperative preservation of the olfactory bulb and tract. The Journal of craniofacial surgery. 2011 May:22(3):1080-2. doi: 10.1097/SCS.0b013e3182139884. Epub [PubMed PMID: 21586949]
Wang SS, Zheng HP, Zhang X, Zhang FH, Jing JJ, Wang RM. Microanatomy and surgical relevance of the olfactory cistern. Microsurgery. 2008:28(1):65-70 [PubMed PMID: 18074374]
Abolmaali N, Gudziol V, Hummel T. Pathology of the olfactory nerve. Neuroimaging clinics of North America. 2008 May:18(2):233-42, preceding x. doi: 10.1016/j.nic.2007.10.002. Epub [PubMed PMID: 18466830]
Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: functional outcome in a series treated microsurgically. Acta neurochirurgica. 2007 Feb:149(2):109-21; discussion 121 [PubMed PMID: 17180303]
Level 2 (mid-level) evidenceHowell J, Costanzo RM, Reiter ER. Head trauma and olfactory function. World journal of otorhinolaryngology - head and neck surgery. 2018 Mar:4(1):39-45. doi: 10.1016/j.wjorl.2018.02.001. Epub 2018 Mar 14 [PubMed PMID: 30035260]
Bordoni B, Zanier E. Cranial nerves XIII and XIV: nerves in the shadows. Journal of multidisciplinary healthcare. 2013:6():87-91. doi: 10.2147/JMDH.S39132. Epub 2013 Mar 13 [PubMed PMID: 23516138]
Schmidt C, Wiener E, Hoffmann J, Klingebiel R, Schmidt F, Hofmann T, Harms L, Kunte H. Structural olfactory nerve changes in patients suffering from idiopathic intracranial hypertension. PloS one. 2012:7(4):e35221. doi: 10.1371/journal.pone.0035221. Epub 2012 Apr 6 [PubMed PMID: 22493741]
Level 3 (low-level) evidenceNorwood JN, Zhang Q, Card D, Craine A, Ryan TM, Drew PJ. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife. 2019 May 7:8():. doi: 10.7554/eLife.44278. Epub 2019 May 7 [PubMed PMID: 31063132]