Introduction
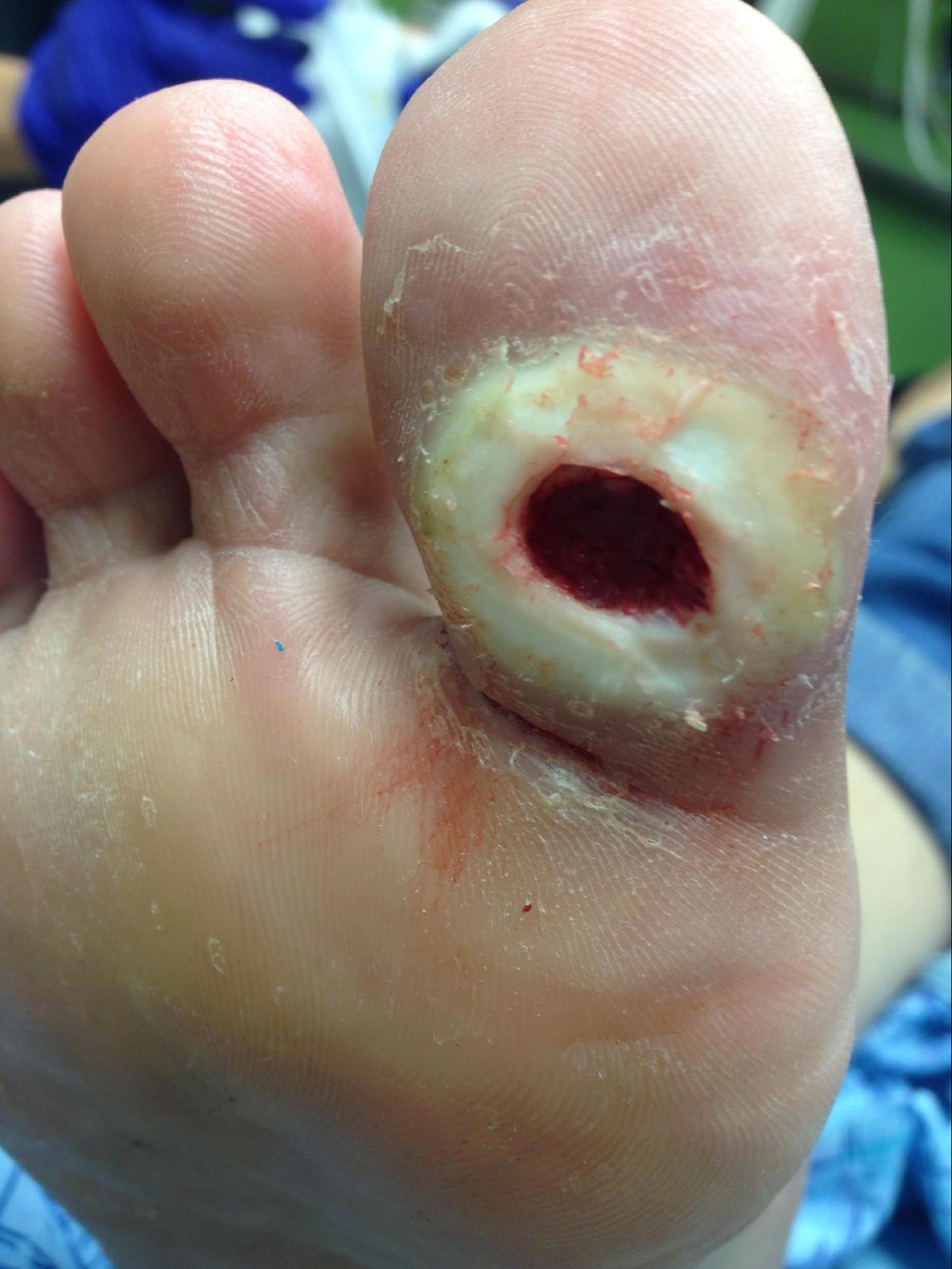
Neuropathy is a broad term that describes a lack of sensorium, movement, or autonomic function and feedback in a particular area. This can be a centralized neuropathy due to paralysis of extremities via the distribution of neurotomes or a peripheralized neuropathy. Most commonly seen in the extremities is a varying degree of peripheral neuropathy. Peripheral neuropathy usually affects only the extremities and can have multiple sources of causality, most likely being diabetic peripheral neuropathy (see Image. Neuropathic Ulcer). Other sources include shingles (post-herpetic neuralgia), B12 deficiency, alcoholism, toxins (such as chemotherapy), amyloid, hypothyroidism, autoimmune disorders, Lyme disease, syphilis, HIV, and hereditary conditions such as Charcot-Marie-Tooth and demyelinating polyneuropathy. Most neuropathic ulcerations occur on the lower extremity and affect prominent pedal surfaces such as the heel and metatarsal heads or areas of high friction prone to callus formation.[1]
The 3 types of peripheral nerves are motor, sensory, and autonomic. The motor nerves allow for the movement of muscles and tissues, and damage can lead to weakness and spasms. The sensory nerves send messages from the tissues to the brain from special sensors that allow us to identify sharp versus dull, rough versus smooth, hot versus cold, and damage can result in numbness, tingling, and pain. Autonomic nerves are involuntary to semi-voluntary systems that regulate homeostasis. Disrupting these pathways can lead to many issues, including nausea, vomiting, diarrhea, and the inability to regulate other bodily functions. Most commonly, there is some form of each of these in patients with progressed neuropathy. However, the progression of the type of neuropathy is based on the underlying etiology and is patient-specific.[2][3] Some trends are disorder-specific and often follow a certain progression of nerves affected.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Neuropathic ulcerations arise from prominences from the internal structure, causing pressure point abnormalities on the external surface of an insensate body part. This is most commonly seen in the foot when pedal prominences cause pressure during ambulation. Due to the lack of sensation in the area, the patient is much less likely to be able to feel any pain or abnormalities in sensation associated with the ulceration.[4]
Multiple known causes of peripheral neuropathy can be broken down into categories: anatomic, systemic, metabolic, and toxic. The anatomic causes of neuropathy are sciatic nerve compression, fibular nerve compression or entrapment, or nerve dissection from surgery or accidental injury. The systemic causes of peripheral neuropathy include but are not limited to HIV infection, carcinoma, paraneoplastic syndrome, monoclonal gammopathy, amyloidosis, sarcoidosis, Sjogren’s syndrome, and tick bites. The metabolic causes of neuropathy include diabetes mellitus, thyroid disease, renal disease, and chronic liver disease. The toxic causes of neuropathy include multiple vitamin deficiencies (B1, B6, B12), vitamin B6 excess, heavy metal poisoning, drug-induced neuropathy, organophosphate exposure, and alcohol use. Each of these has its way of causing an effect on the body.[4]
Peripheral neuropathy within the diabetic population is the most common. It is considered to be caused by cell death from inflammation and oxidative stress on the tissues, leading to nerve dysfunction. This is accentuated by hyperglycemia and insulin resistance, causing the inability to regulate many metabolic pathways within the body, increasing the reactive oxygen species (ROS) within the tissues. ROS leads to direct nerve injury and eventual peripheral polyneuropathy. This neuropathy progresses from unmyelinated fibers to eventual demyelination of myelinated nerve fibers, which is the reasoning behind sensory, autonomic, and motor neuropathies.[5]
A lack of blood flow and sensorium often leads to a disease progression called Charcot neuroarthropathy. Charcot neuroarthropathy is an inflammatory condition that leads to osseous subluxation, dislocation of joints, and bone fracture that leads to remodeling of the foot's structure. Charcot neuroarthropathy has multiple theories on etiology and can be challenging to manage as it has multiple stages with a variable timeline and different theories on the causality. It can often lead to neuropathic ulcerations and bone infections.[6]
Epidemiology
The prevalence of peripheral neuropathy affects up to 50% of patients with diabetes during their lifetime, with a prevalence noted to be between 6% and 51%, depending on multiple variables such as glycemic control, type of diabetes, and age.[7] The prevalence of patients with type 1 diabetes was between 30% to 34% and increased significantly with age. However, we see this often at a younger age in patients with type 1 diabetes compared to those with type 2 diabetes. Prevalence among those with type 2 diabetes has been reported as low as 6% and as high as 51% in some models, but most often, it is found to be somewhere between 35% and 45%.[8][9]
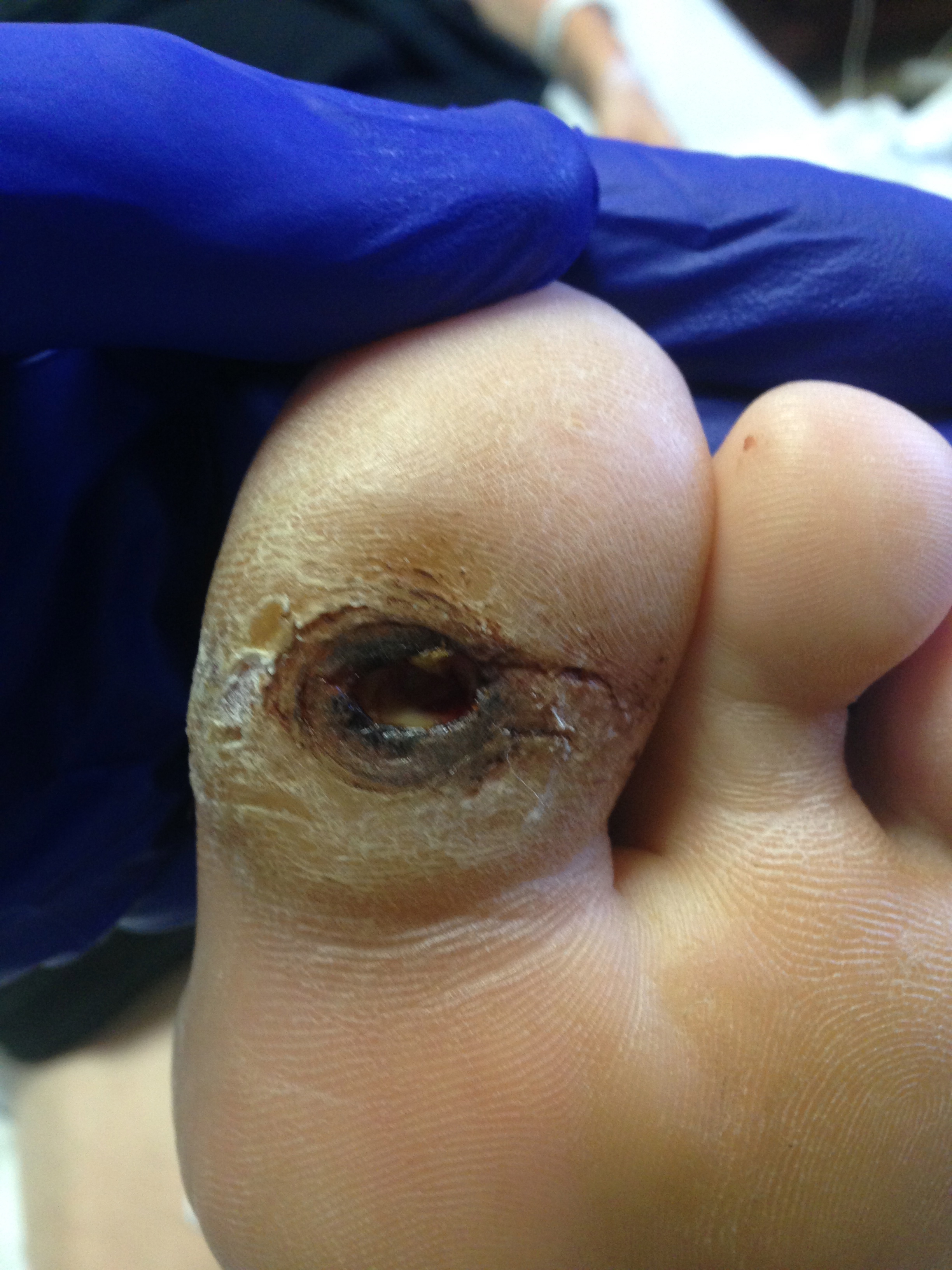
More frequently, in practice, we see peripheral neuropathy in males; however, it has the same potential to affect females within those with diabetes and neuropathy.[5] A study has shown that mortality increases from 3.1% to 17.4% with the patient’s first diabetic foot ulcer, with comorbidities of the duration of diabetes, nephropathy, and history of minor or major amputations playing a significant role in the mortality rate (see Image. Neuropathic Ulcerations in a Patient With Diabetes). This same study also indicates that up to 1/3 of patients diagnosed with a diabetic foot ulcer require an amputation at some point while being evaluated for up to 14 years.[10]
Pathophysiology
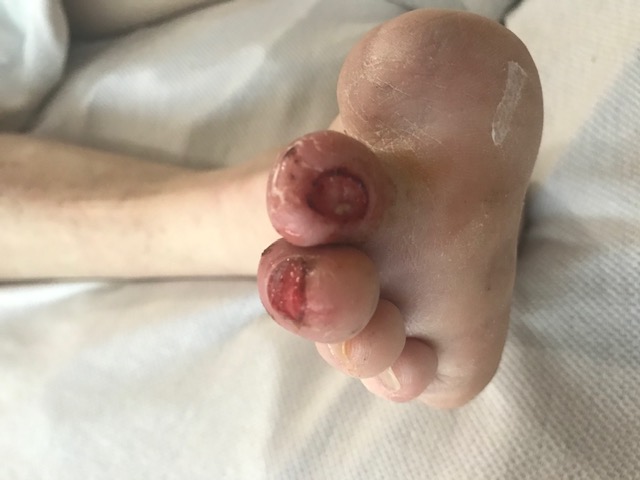
Neuropathy is described as a physiologic process that leads to decreased sensorium, motor weakness, and loss of autonomic function in an area. Neuropathic ulcers are caused by loss of sensorium, leading to the inability to withdraw the area from painful stimuli such as friction, shear forces, or traumatic processes leading to skin opening and ulceration. There are many common causes of neuropathy, including diabetes mellitus, central nervous system or peripheral nervous system trauma, alcoholism, and many other disease processes (see Image. Neuropathic Ulcers, Diabetes). The most common findings associated with neuropathic ulcerations are the inability of nerves to function properly, comorbidities leading to neuropathy, and some form of prominence leading to increased trauma or microtrauma to an area.
History and Physical
Patients frequently present with the initial complaint of finding blood in their socks or on their floors as they do not perform routine pedal examinations. For the ulceration itself, the history should be thorough and include the nature, location, duration, and onset, as well as the course, things that both aggravate the ulceration and alleviate the associated symptoms and any treatments or dressings used or pursued this issue.
A detailed past medical history is highly recommended. It is essential to establish the background of the disorder, including the length of time the patient has been neuropathic, if there is a diagnosis associated with the neuropathy, and all information pertinent to that disease. For instance, the patient often has diabetes, and it is vital to know the length of time they have had this disease, how their diabetes is controlled via medication and diet, and how their blood sugars have been. The laboratory value of the hemoglobin A1C of the patient can help provide insight into how well their diabetes is controlled and assess their healing potential. A history of prior ulcers, amputations, or foot surgery is also helpful. The patient's listed allergies and medications are also significant here and can help guide antibiotic therapy if needed.
Past surgical history should also include a history of pedal ulcerations, possible surgical interventions such as amputation, any history of vascular surgery, and how the patient's body reacted to anesthesia. This can also help guide the decision on whether the patient is a good surgical candidate.
Social history can provide insight into the patient's career and how often they are in a position that increases pressure on the area. Substance use history should be considered, as nicotine can decrease blood flow to an area, and alcohol can be a causality of neuropathy.
The history should be taken thoroughly and include a complete review of symptoms. Frequently, the ulcerations can present infected and are accompanied by nausea, fever, vomiting, or chills to some degree, as well as the potential for shortness of breath and chest pain if the patient is in systemic sepsis. The systemic inflammatory response syndrome (SIRS) criteria can help evaluate the possibility of sepsis; however, this is nonspecific. The SIRS criteria assess the body's temperature, heart rate, respiratory rate, and white blood cell count and correlate that with the clinical picture to evaluate the body's response to infection. The SIRS criteria have been refuted recently but are still used in most hospital systems' protocols.[11]
The physical exam should include all aspects of the ulceration, the patient's vascular status, neurological status, and any musculoskeletal abnormalities that could cause the ulceration. A dermatologic examination should include the appearance of the wound bed and edges if any undermining or tunneling is present, to what layer the wound is exposing (eg, fat, tendon, bone), if any malodor is associated with the wound, and whether or not the ulceration probes deep to the bone, and if there is any drainage, and what type of drainage is present.
The vascular examination should include palpation of pulses in the area, ultrasound doppler with an evaluation of waveforms and blood flow, and capillary refilling time both peri-wound and distal to the ulceration within the digits or distal foot if the digits are not present. Adequate blood flow and tissue perfusion increase healing potential and prognosis and can be used to assess for the concomitance of peripheral arterial disease and vascular insufficiency.
The neurological evaluation should include an assessment of all types of nerve fibers. This can be done by following multiple neurologic tests such as 2-point discrimination, sharp versus dull sensation, vibratory sensation, and pinpoint monofilament testing. Numerous other types of testing are available for further evaluation as well. They can include nerve conduction velocity testing, but a clinical evaluation without such advanced testing can usually provide information about the patient's sensorium. The evaluation should also include whether this is asymmetric or symmetric and the type of fibers the neuropathy affects (sensory, motor, autonomic).[4]
The musculoskeletal evaluation of the patient is important and often missed. The anatomical structure and biomechanical function should be fully assessed to evaluate the possibility of reducing deformity and decreasing pressure points' prominence. If lower extremity ulcerations are the issue, this should include a gait exam, shoe gear examination, and full biomechanical evaluation of the area.
Evaluation
Initially, the cause of the neuropathy should be identified and diagnosed (this is often done before the development of ulceration in an ideal world; however, oftentimes, this is not the case). The different types of neuropathy can be distinguished based on the physical exam in conjunction with electrodiagnostic testing and laboratory markers. The electrodiagnostic testing consists of electromyography (EMG) and nerve conduction studies/nerve conduction velocity (NCS/NCV). These tend to be performed at the initial presentation of symptoms and are often only used to evaluate peripheral neuropathy sources, not neuropathic ulcerations. EMG/NCV mainly evaluates the large myelinated fibers, while diabetes tends to affect the smaller unmyelinated fibers. Additionally, an epidermal nerve fiber density test can be performed to objectively measure and document small fiber peripheral neuropathy. Laboratory studies that can help evaluate sources of neuropathy include complete blood count, erythrocyte sedimentation rate, fasting blood glucose, hemoglobin A1c, thyroid studies, vitamin B12 levels, renal function studies, and any tests that can help evaluate autoimmune disorders, infectious processes, and toxic etiologies can be performed.[4]
All ulcerations should be evaluated with plain radiographs immediately. Plain radiographs help to assess multiple aspects of neuropathic ulcerations. The soft tissues can be evaluated for the possibility of soft tissue emphysema or gas-forming organisms infecting the foot. The bone structure can also be evaluated for any hypertrophy or deformity causing ulceration. Lastly, the quality of the bone can be assessed for signs of osteomyelitis, which requires further workup to confirm a diagnosis. Different signs of osteomyelitis may be seen as a sequestrum, a sclerotic area with a radiolucent rim, an involucrum of the thick bone surrounding a sequestrum, and cortical destruction. Plain radiographs are highly useful for ruling out many differentials.[12]
Vascular studies should also be ordered to help differentiate neuropathic ulcers from ulcers of vascular origin. This helps to evaluate blood flow, arterial efficacy, and perfusion to certain tissues. This can aid in adjunct surgical decisions and predict healing capabilities. The Society for Vascular Surgery developed a risk stratification system that considers healing potential in patients with diminished vascular status, including the likelihood of amputation. This shows the direct correlation between vascular insufficiency, the prognosis of ulceration, and the incidence and necessity of surgical intervention in patients with poor blood flow in a limb.[13]
The initial laboratory evaluation of a neuropathic ulcer should include a complete blood count with differential, comprehensive metabolic profile, c-reactive protein, erythrocyte sedimentation rate, and hemoglobin A1c (if recent value not known), with adjunct testing that can also be performed in certain situations. These help to evaluate markers of infection and inflammation. The white blood cell count gives further information on the body's attempt to fight infection, and the differential allows us to analyze which cells in the body are activated for this. The complete metabolic profile helps analyze the ability of the patient's body to maintain a proper electrolyte balance. The c-reactive protein, or CRP, is an enzyme released by the liver that quantifies the amount of inflammation occurring in the body. It has been shown that levels of 7.9 mg/dL associated with an erythrocyte sedimentation rate (ESR) of 60 mm/h are associated with an increased probability of osteomyelitis. ESR measures how fast the erythrocytes, or red blood cells, settle at the bottom of the test tube and is a nonspecific measurement of chronic inflammation. The ESR greater than 60mm/h has a sensitivity of 74% and a specificity of 56%, while the CRP levels of 7.9 mg/dL have a sensitivity of 49% and a specificity of 80%.[14] These tests can help us evaluate not only for infection but also for the severity of the patient's illness.
At the time of initial evaluation, performing any debridement of devitalized tissue, a cleansing of the wound with a sterile solution, and a wound culture for microbiological analysis is important. The culture and sensitivities that the laboratory can perform can help guide antibiotic treatment in cases of infection. The Infectious Diseases Society of America came out with clinical practice guidelines in 2012 for diabetic foot infections and have recommended criteria such as inflammation, purulent drainage, positive probe to bone test, chronic ulceration >30 days, previous amputation, a history of ulcerations and multiple other conditions to be of solid evidence for suspicion of infection; thus necessitating the need for cultures. This same paper also recommends that cultures be taken before initiating antibiotic therapy if possible and that the specimen should be taken from the deepest tissue after cleansing and debridement.[15]
Secondary evaluation should include advanced imaging if indicated. Indications include any suspicion of deep soft tissue abscess and bone infection, but it should be considered that any deep infection is possible in most instances. This can include computed tomography (CT), magnetic resonance imaging (MRI), 3-phase bone scintigraphy, and ultrasound. Each of these has its pitfalls; however, they can each provide insight into certain nuances of ulcerations for workup on soft tissue abscesses and osteomyelitis. A bone biopsy is the gold standard for evaluation if osteomyelitis is indicated. However, it has been shown that microbiological and pathological evaluation and interpretation are clinician-dependent and may not be as reliable as previously thought for diagnosing bone infection.
The preferred advanced imaging modality is MRI, which has a high sensitivity for infection diagnosis and can be used with contrast for further bone and soft tissue evaluation. Triple-phase bone scans are a viable alternative and can be combined with different radionuclides for further sensitivity and specificity. CT scan can also be a viable alternative as it has superior bone resolution and is much more readily available; however, evaluation of the soft tissue envelope is much poorer when compared to MRI.[12]
Treatment / Management
The treatment and management of neuropathic ulcers require a 2-fold approach: treating the underlying cause of the neuropathy and treating the ulceration itself. Both treatment strategies are multifactorial. To treat the underlying cause of neuropathy, testing, as discussed previously, can help dictate treatment guidelines depending on the patient's deficiency or disorder.
Ulceration treatment includes a multimodal approach. This is because after the ulceration heals (or before it begins), the efforts must be focused on preventative medicine. Understanding the biomechanical evaluation behind the structures at risk helps inform clinical practice guidelines, including orthotic prescriptions and modifications, as well as possible surgical intervention and reconstruction of underlying deformities to decrease plantar loading.[16]
The ulceration should be treated based on the physical exam, clinical evaluation, and laboratory findings. The ulceration should be evaluated for surgical intervention for proper irrigation and debridement. Debridement of necrotic or hyperkeratotic peri-wound tissue, proper cultures if an infection is suspected for microbiological analysis, and possible biopsy of underlying tissue (bone) for pathological evaluation should be done even if surgical intervention is not pursued. If an underlying infection is suspected, proper culture and sensitivity-directed antibiotic therapy should be included, as well as proper wound care techniques. If an underlying bone infection is suspected, the gold standard approach for evaluation is bone biopsy with both pathology and microbiology analysis for osteomyelitis; however, the sensitivity and specificity of the provider evaluating these remains debated.[15](A1)
All ulcerations should be offloaded to decrease the pressure and redistribute forces to the surrounding tissues. This has been evaluated in many studies and shown to help prevent the recurrence of neuropathic ulcers and promote healing in current ulcerations. Offloading treatment comes in many forms and is usually clinician-dependent. It can include total contact casting, felt offloading, controlled ankle motion (CAM), boot therapy with custom orthoses, forefoot casting, etc, and is increasingly important in plantar wounds.[17][18] There continues to be strong evidence supporting non-removable devices extending up to near the knee level as the primary therapy for offloading in both forefoot and midfoot ulcerations.[19] (A1)
A recent article published in May of 2019 of the guidelines for offloading the foot in patients with neuropathic ulcers recommends non-removable knee-high devices as first-line therapy for offloading, removable knee-high devices as second-line, removable ankle-high offloading as the third line, and felted foam or custom footwear as the fourth line for offloading ulcerations.[20] In adjunct to proper offloading, wound care therapy promotes wound healing. It should be based on the size, depth, and intrawound properties present, including the moisture and drainage level, pH of the wound, and type of tissue intrawound. Many different wound care products and grafts are available today, with diverse selections that expedite healing wounds with different characteristics.[15](A1)
Differential Diagnosis
Multiple separate subsets of ulcerations can and should be considered. Certain malignancies can also present similarly, and further evaluation should often be considered. Various types/conditions of ulcerations are:
- Venous stasis ulceration
- Arterial ulceration
- Traumatic ulcerations
- Decubitus ulceration
- Skin cancers
- Hypertensive ulceration
- Burns
- Cutaneous infectious diseases
- Chemotoxic or drug-induced ulceration
- Side effects of treatment
- Side effects of comorbidities (ie, Kaposi's sarcoma)
These must be evaluated for life or limb-threatening conditions such as gas gangrene and necrotizing fasciitis.
Staging
Multiple classification systems exist for diabetic foot wound evaluation (see Image. Diabetic Foot Ulcer). The most common systems are Meggitt-Wagner (often called Wagner), Texas University classification system, S(AD)SAD, PEDIS, and WIFI. Many studies evaluate the reliability and comparisons between the systems, and the general conclusion is that the Wagner classification system and Texas University remain reliable and simple to use for classifying diabetic foot ulcers, and WIFI provides the added ability to classify the ischemia with prognostic correlates.[21][22]
The Wagner Classification system is based on a grading system from 0-5. A grade 0 ulceration is considered a pre-ulcerative lesion with intact skin, with the potential of breakdown. A grade 1 ulceration presents with superficial skin or subcutaneous tissue breakdown. A grade 2 ulceration has an exposed tendon, bone, or capsule. A grade 3 ulceration is deep with osteomyelitis or an abscess present. A grade 4 ulceration is one with gangrene of the partial foot. A grade 5 ulceration is one with gangrene of the entirety of the foot. This system has been shown reliable; however, it does not address the ulceration's blood flow or infectious properties and is merely a grading system for the ulcer itself.[23]
The University of Texas Diabetic Foot Ulcer Classification System considers blood flow and infectious properties. It is a dual system with 4 separate grades and stages. The 4 grades from 0-3 are as follows grade 0- pre-ulcerative site or post-ulcerative site that has healed; grade 1- superficial wound not involving the tendon, capsule, or bone; grade 2- wound penetrating to tendon or capsule; grade 3- wound penetrating bone or joint. Within each of these wound grades, the staging of the ulceration is achieved by establishing the blood flow and infectious qualities of the ulceration. This is divided into 4 stages: A- clean wounds with adequate blood flow, B- non-ischemic wounds that are infected, C- noninfected wounds that are ischemic, and D- infected and ischemic wounds. This provides a more in-depth classification system for use in medicine; however, it lacks information on determining ischemia and infectious qualities.[24]
The Society for Vascular Surgery developed a risk stratification system based on wound, ischemic, and foot infection (WIFI), and details of the WIFI classification system can provide further insight into the analysis of vascular status and infection and how these correlate to the healing potential within the staging system. This system has 3 separate portions. The wound portion is from grades 0-3 and is as follows: grade 0- no ulcer and no gangrene with the possibility of ischemic rest pain; grade 1- minimal tissue loss and the ulcer has no exposed bone, grade 2- the ulcer extends to the tendon, joint or bone with gangrenous changes to the digits and grade 3- the ulcer involves extensive tissue loss of the forefoot or midfoot with or without hindfoot involvement with extensive gangrenous changes to the forefoot and midfoot. The ischemia aspect is broken down using ankle-brachial index (ABI) measurements, transcutaneous oximetry, pulse volume recording, and skin perfusion or toe pressure. It must be considered whether or not the patient has calcified vessels, which can alter many of these readings.
The ischemia portion is also broken down into 4 grades: grade 0- no ischemia with an ABI greater than or equal to 0.80 and toe pressure greater than or equal to 60 mmHg, grade 1- mild ischemia with an ABI between 0.6-0.79 with a toe pressure ranging from 40 to 59, grade 2- moderate ischemia with an ABI between 0.4 to 0.59 and a toe pressure between 30 to 39, and grade 3- severe ischemia with an ABI less than or equal to 0.39 and a toe pressure less than 30. The final portion of this describes the foot infection portion: grade 0- no infection present, grade 1- superficial infection with cellulitis/erythema that is localized and within 2 cm of the ulceration edge, grade 2- moderate infection with cellulitis/erythema greater than 2 cm from the wound edge with abscess present or infection extending into the bones or joints, and grade 3- severe infection with both local and systemic inflammatory response syndrome (SIRS). Upon completion of the grading system, each ulceration is staged from 1 to 5, with stage 1 being a low amputation risk and stage 5 being unsalvageable, with low, moderate, and high risk from stages 2 to 4.[13][21]
Prognosis
In a multicenter cohort prospective study, it was found that 17.4% of patients with diabetic foot ulcers die within 14 years, compared to 3.1% in patients without a pedal ulcer. In the diabetic foot ulcer group, the 5-year mortality rate was 22%, and the 10-year mortality rate was 71%. About 29% of all patients underwent some form of limb amputation. Other variables that predicted early mortality were the duration of diabetes greater than 10 years, a history of nephropathy, and both minor and major amputations. Overall, the presence of a diabetic foot ulcer increases the risk of amputation, complications, and long-term prognosis. This shows roughly one-third of patients diagnosed with a diabetic foot ulcer require amputation, which appears to increase with time.[10]
Complications
There are multiple complications associated with peripheral neuropathy, with the major complication being amputation. Amputations are due to ulcerations in a neuropathic foot associated with infection. In the United States, roughly 80% of nontraumatic amputations have been seen due to diabetes and peripheral neuropathy. With each ulceration and amputation, the morbidity and mortality rate increases, with the probability of contralateral amputation and death increasing with each ulceration and surgical intervention.[5] It has been previously estimated that anywhere between 14% and 24% of patients with a pedal ulcer require amputation of some portion or all of that limb.[25]
One study on predicting the lower extremity amputation rates in patients with diabetic foot ulcers found that roughly 35.4% of patients underwent amputation and that multiple factors such as soft tissue infection, osteomyelitis, duration of ulceration, and vascular insufficiency or peripheral arterial disease played a significant role in this prevalence.[26] This leads to the belief that prompt diagnosis and attention with proper interventions play a significant role in reducing the complications associated with peripheral neuropathy; however, the source of the neuropathy must be controlled as well. Many complications are discussed in the previous sections.
Consultations
An interprofessional approach should be used in all patients with neuropathic ulcerations. Consultations should be considered to help dictate certain types of treatment. Podiatry or general surgery should be consulted to evaluate the necessity of surgical intervention. Alongside the surgical team, multiple other specialties should be considered for inpatient and outpatient therapy in a patient. Most hospital systems also offer infectious disease specialists to help dictate antibiotic treatment and duration. Consultations for nerve conduction testing, vascular testing, and advanced imaging should be placed.
Deterrence and Patient Education
Clinical evaluation is provider and specialty-specific but operates on a generalized set of guidelines. If patients have a history of ulceration, have poor vascular status, or have a history of amputation, they should be seen by their foot and ankle specialist every few months, with regular primary care appointments. Suppose patients have any diagnosis related to peripheral neuropathy. In that case, their specialist should see them at least once per year, with primary care appointments more often for overall medical management. Patients should perform daily pedal examinations while at home. If they cannot evaluate the plantar aspect of their feet, a mirror placed on the floor may come in handy. Avoiding barefoot and wearing diabetic-approved shoes is essential. The best way to decrease the probability of neuropathic ulcerations is vigilance and proper treatment of the underlying causes of neuropathy.
Enhancing Healthcare Team Outcomes
Understanding proper pedal evaluation and which populations are at risk helps identify situations in which intervention is necessary. All practitioners must understand that although diabetes mellitus is the most commonly associated underlying disorder leading to plantar ulcers in neuropathic patients, increased age, obesity, improper healing of pedal injuries, and underlying neuropathic causes should all be evaluated. Early intervention and proper offloading are the keys to decreasing morbidity and mortality associated with neuropathic ulcers.[27] Proper patient and family education are vital to the long-term healthcare outcomes of peripheral neuropathy.
To that end, an interprofessional healthcare team approach to neuropathic ulcers is crucial. This team includes clinicians (MDs, DOs, PAs, NPs), specialists (including but not limited to endocrinologists, dermatologists, surgeons, etc), nurses, and other ancillary staff depending on the underlying neurological etiology (eg, diabetes educators). Clinicians are vital assets that coordinate communication between various clinicians, counsel patients, and answer patient questions about their condition and ongoing treatment. All team members must communicate openly with the rest of the team if they note changes in the patient's condition, and it is incumbent on all team members to maintain accurate and updated patient records so that everyone has access to the most current and accurate patient case data. This interprofessional approach yields the best results.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kernich CA. Patient and family fact sheet. Peripheral neuropathy. The neurologist. 2001 Sep:7(5):315-6 [PubMed PMID: 12803674]
Sidon E, Rogero R, McDonald E, Daecher A, Shakked R, Pedowitz DI, Fuchs D, Daniel JN, Raikin SM. Prevalence of Neuropathic Pain Symptoms in Foot and Ankle Patients. Foot & ankle international. 2019 Jun:40(6):629-633. doi: 10.1177/1071100719838302. Epub 2019 Mar 22 [PubMed PMID: 30902025]
He JK, Klavas DM, McKissack H, Ahuero JS, Shah A, Granberry WM, Schon LC. Don't Lose Your Nerve: Evaluation and Management of Neurogenic Pain in the Foot and Ankle. Instructional course lectures. 2020:69():509-522 [PubMed PMID: 32017749]
Mayans L, Mayans D. Causes of peripheral neuropathy: Diabetes and beyond. The Journal of family practice. 2015 Dec:64(12):774-83 [PubMed PMID: 26845001]
Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Current diabetes reports. 2019 Aug 27:19(10):86. doi: 10.1007/s11892-019-1212-8. Epub 2019 Aug 27 [PubMed PMID: 31456118]
Marmolejo VS, Arnold JF, Ponticello M, Anderson CA. Charcot Foot: Clinical Clues, Diagnostic Strategies, and Treatment Principles. American family physician. 2018 May 1:97(9):594-599 [PubMed PMID: 29763252]
Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, Ward JD, Boulton AJ. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabetic medicine : a journal of the British Diabetic Association. 1994 Jun:11(5):480-4 [PubMed PMID: 8088127]
Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O'Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I, ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet (London, England). 2010 Aug 7:376(9739):419-30. doi: 10.1016/S0140-6736(10)60576-4. Epub 2010 Jun 30 [PubMed PMID: 20594588]
Level 1 (high-level) evidenceDuckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009 Jan 8:360(2):129-39. doi: 10.1056/NEJMoa0808431. Epub 2008 Dec 17 [PubMed PMID: 19092145]
Level 1 (high-level) evidenceRastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, Kamath P, Jude EB, Armstrong DG, Bhansali A. Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: Epidemiology of diabetic foot complications study. Diabetes research and clinical practice. 2020 Apr:162():108113. doi: 10.1016/j.diabres.2020.108113. Epub 2020 Mar 9 [PubMed PMID: 32165163]
Haydar S, Spanier M, Weems P, Wood S, Strout T. Comparison of QSOFA score and SIRS criteria as screening mechanisms for emergency department sepsis. The American journal of emergency medicine. 2017 Nov:35(11):1730-1733. doi: 10.1016/j.ajem.2017.07.001. Epub 2017 Jul 6 [PubMed PMID: 28712645]
Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quantitative imaging in medicine and surgery. 2016 Apr:6(2):184-98. doi: 10.21037/qims.2016.04.01. Epub [PubMed PMID: 27190771]
Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G, Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). Journal of vascular surgery. 2014 Jan:59(1):220-34.e1-2. doi: 10.1016/j.jvs.2013.08.003. Epub 2013 Oct 12 [PubMed PMID: 24126108]
Lavery LA, Ahn J, Ryan EC, Bhavan K, Oz OK, La Fontaine J, Wukich DK. What are the Optimal Cutoff Values for ESR and CRP to Diagnose Osteomyelitis in Patients with Diabetes-related Foot Infections? Clinical orthopaedics and related research. 2019 Jul:477(7):1594-1602. doi: 10.1097/CORR.0000000000000718. Epub [PubMed PMID: 31268423]
Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E, Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Jun:54(12):e132-73. doi: 10.1093/cid/cis346. Epub [PubMed PMID: 22619242]
Level 1 (high-level) evidenceDiLiberto FE, Baumhauer JF, Nawoczenski DA. The prevention of diabetic foot ulceration: how biomechanical research informs clinical practice. Brazilian journal of physical therapy. 2016 Nov 16:20(5):375-383. doi: 10.1590/bjpt-rbf.2014.0195. Epub 2016 Nov 16 [PubMed PMID: 27849290]
Bus SA. The Role of Pressure Offloading on Diabetic Foot Ulcer Healing and Prevention of Recurrence. Plastic and reconstructive surgery. 2016 Sep:138(3 Suppl):179S-187S. doi: 10.1097/PRS.0000000000002686. Epub [PubMed PMID: 27556758]
Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR, International Working Group on the Diabetic Foot. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes/metabolism research and reviews. 2016 Jan:32 Suppl 1():99-118. doi: 10.1002/dmrr.2702. Epub [PubMed PMID: 26342178]
Level 1 (high-level) evidenceLazzarini PA, Jarl G, Gooday C, Viswanathan V, Caravaggi CF, Armstrong DG, Bus SA. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diabetes/metabolism research and reviews. 2020 Mar:36 Suppl 1(Suppl 1):e3275. doi: 10.1002/dmrr.3275. Epub [PubMed PMID: 32176438]
Level 1 (high-level) evidenceBus SA, Armstrong DG, Gooday C, Jarl G, Caravaggi C, Viswanathan V, Lazzarini PA, International Working Group on the Diabetic Foot (IWGDF). Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes/metabolism research and reviews. 2020 Mar:36 Suppl 1():e3274. doi: 10.1002/dmrr.3274. Epub [PubMed PMID: 32176441]
Monteiro-Soares M, Russell D, Boyko EJ, Jeffcoate W, Mills JL, Morbach S, Game F, International Working Group on the Diabetic Foot (IWGDF). Guidelines on the classification of diabetic foot ulcers (IWGDF 2019). Diabetes/metabolism research and reviews. 2020 Mar:36 Suppl 1():e3273. doi: 10.1002/dmrr.3273. Epub [PubMed PMID: 32176445]
Monteiro-Soares M, Martins-Mendes D, Vaz-Carneiro A, Sampaio S, Dinis-Ribeiro M. Classification systems for lower extremity amputation prediction in subjects with active diabetic foot ulcer: a systematic review and meta-analysis. Diabetes/metabolism research and reviews. 2014 Oct:30(7):610-22. doi: 10.1002/dmrr.2535. Epub [PubMed PMID: 24523130]
Level 1 (high-level) evidenceWagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot & ankle. 1981 Sep:2(2):64-122 [PubMed PMID: 7319435]
Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes care. 1998 May:21(5):855-9 [PubMed PMID: 9589255]
Level 1 (high-level) evidenceAmerican Diabetes Association. Consensus Development Conference on Diabetic Foot Wound Care: 7-8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes care. 1999 Aug:22(8):1354-60 [PubMed PMID: 10480782]
Level 3 (low-level) evidenceUgwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. Journal of foot and ankle research. 2019:12():34. doi: 10.1186/s13047-019-0345-y. Epub 2019 Jun 14 [PubMed PMID: 31223342]
Level 2 (mid-level) evidenceAndrews KL, Dyck PJ, Kavros SJ, Vella A, Kazamel M, Clark V, Litchy WJ, Dyck PJB, Lodermeier KA, Davies JL, Carter RE, Klein CJ. Plantar Ulcers and Neuropathic Arthropathies: Associated Diseases, Polyneuropathy Correlates, and Risk Covariates. Advances in skin & wound care. 2019 Apr:32(4):168-175. doi: 10.1097/01.ASW.0000550591.08674.98. Epub [PubMed PMID: 30624254]
Level 3 (low-level) evidence