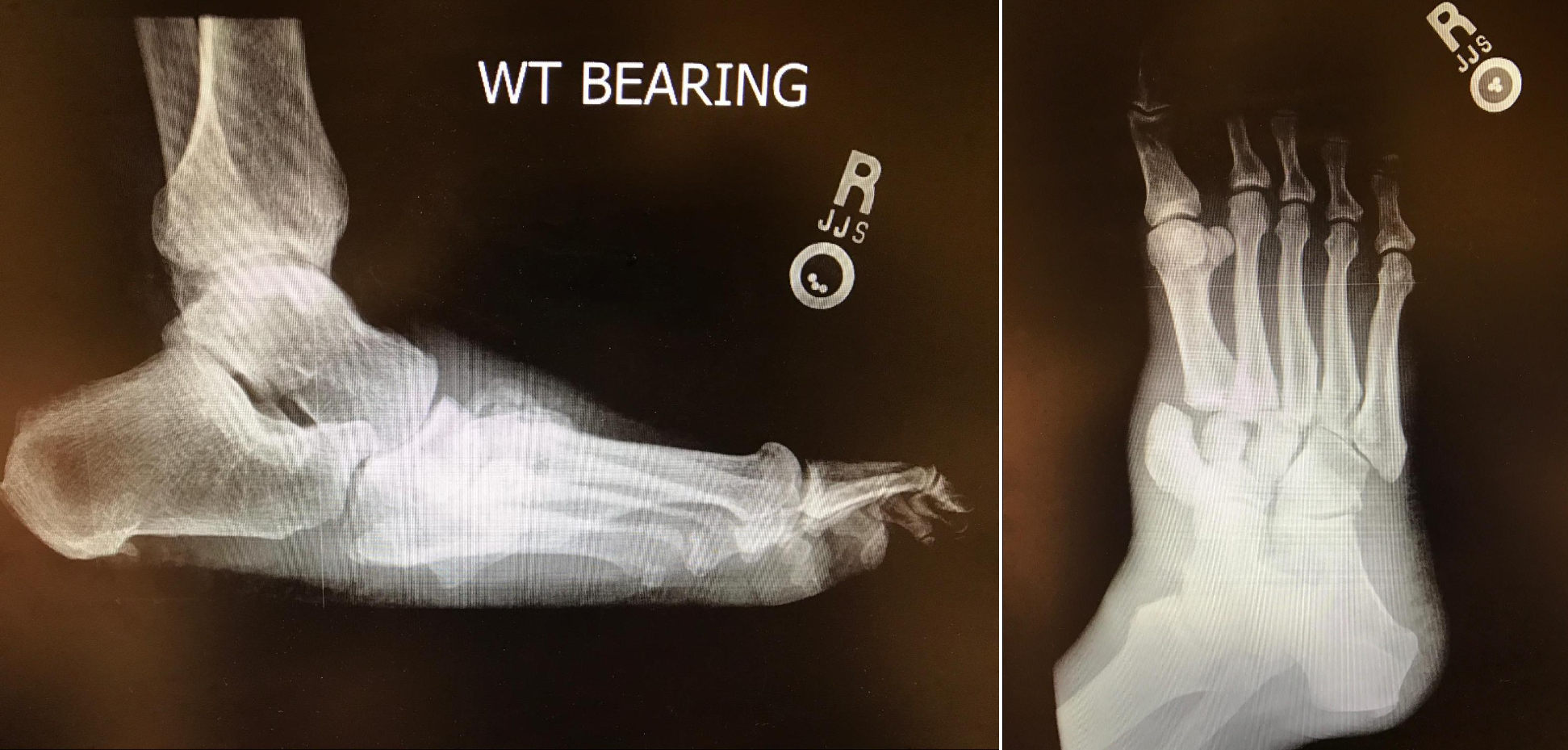
Introduction
Charcot neuropathic osteoarthropathy is a destructive joint disorder initiated by trauma to a neuropathic extremity. It can lead to dislocations and fractures of the foot. Correct diagnosis and treatment of acute Charcot are imperative to decrease permanent foot deformity and allow for a stable and plantigrade foot that is suitable for ambulation. This review article of Charcot discusses the etiology and pathogenesis of the disease, presentation, and treatment options.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Charcot neuropathic osteoarthropathy is seen in the lower extremity and is characterized by bone and joint fragmentation of the foot and ankle in individuals with various peripheral neuropathies. Diabetes, neuropathy, trauma, and metabolic abnormalities of the bone result in an acute localized inflammatory condition. The inflammatory response can permanently disrupt the bony architecture of the foot resulting in abnormal plantar pressures that are at risk for ulceration, osteomyelitis, and amputation.[4][5][6][7]
Charcot is a debilitating condition affecting the lower extremity of patients with established peripheral neuropathy caused by many complicated etiologies; however, diabetic neuropathy has become the most common etiology. Other etiologies include spinal cord injury, poliomyelitis, leprosy, syphilis, syringomyelia, or chronic alcoholism.
Epidemiology
It has been reported that Charcot affects between 0.1% to 0.9% of people with diabetes. [8] [9] An estimated 63% of patients with Charcot neuropathic osteoarthropathy will develop a foot ulceration. McEwen et al. found a significant association between elevated body mass index and Charcot arthropathy.[10]
Pathophysiology
There are two commonly accepted theories used to describe the pathogenesis of Charcot: neuro-traumatic and neurovascular. The neuro-traumatic theory proposes neuropathy, and repeated micro-trauma produces joint destruction. The neurovascular theory suggests the increased peripheral blood flow results in osteolysis and demineralization.
Pathophysiologically, key inflammatory markers have been identified that contribute to the development of an acute Charcot foot. Calcitonin gene-related peptide (CGRP) acts typically at the nerve terminal to antagonist the synthesis of nuclear factor-kB ligand (RANKL), a cytokine involved in the inflammatory process. However, in the neuropathic foot, the CGRP is not functioning, and therefore RANKL is synthesized without inhibition. RANKL is a very important marker in the development of Charcot as it is responsible for the proliferation of osteoclastogenesis. The RANKL osteoclastic relationship is normally moderated by osteoprotegerin (OPG) by acting as a decoy receptor to RANKL binding. In the Charcot foot, this relationship between RANKL and OPG is disrupted. The unregulated synthesis of RANKL accounts for the excessive bony turnover and accumulation that is seen in the Charcot limb. Additionally, CGRP may have a further role in Charcot in that it is involved in upholding the integrity of the joint capsule. Therefore, in its absence, it is presumed to lead to joint destruction and dislocation that is characteristic of the Charcot foot.
Histopathology
Histologically, specimens will appear similar to degenerative joint disease including ghost chondrocytes, subchondral cysts, sclerotic bone, fragmented and irregular cartilage thinning. [11] Histology and bone biopsies are important to differentiate acute Charcot joint versus osteomyelitis. Histologically, features of osteomyelitis include plasma cells, lymphocytes, and neutrophils with reactive new bone formation and bone necrosis. Capillary fibrosis and proliferation may also be present. [12]
History and Physical
High suspicion for Charcot needs to be present to diagnose the condition accurately. Charcot presents as an erythematous foot with edema and calor. Often, it is unilateral with a sudden onset of symptoms that may be precipitated by macro-trauma (ankle sprain) or repetitive micro-trauma (walking). Charcot may be initially misdiagnosed as a deep venous thrombosis or cellulitis. Charcot can also be confused with osteomyelitis due to the similar clinical appearance of a red, hot swollen foot with skeletal lysis on radiographs and often unilateral presentation. Although distinct and separate processes, these entities can occur simultaneously. Additionally, there is evidence that osteomyelitis can cause Charcot. This is due to the inflammatory cascade of cytokine release osteomyelitis triggers in the body. This risk of osteomyelitis evolving into Charcot is further escalated if surgical intervention was performed to remove the infected bone. This is because both osteomyelitis and surgery are forms of trauma that promote an inflammatory response which triggers the opportunity for Charcot to manifest.
In addition to the trauma caused by surgery, the changes to the bony architecture of the foot post-surgery can also lead to inflammatory triggering events due to biomechanical and gait alterations in plantar pressures. For example, amputations and other surgical interventions of the foot can lead to altered pressure points for which the foot is unable to compensate. These changes in plantar pressure, even if mild, can cause microtrauma which initiates the inflammatory events that lead to an acute Charcot.[13][14][15][16]
Evaluation
Stages of Charcot
Common classifications that map the phases of Charcot include the Eichenholtz classification and the Sanders and Frykberg classification. Eichenholtz classifications describe the three stages of disease progression based on clinical and radiographic findings. The Sanders/Frykberg classification is used to type and class the five common anatomical locations of Charcot in the foot.
Eichenholtz [17]
Stage 0 Pre-Charcot/Prodromal
- Clinically: red, hot, swollen foot. No deformity.
- Radiographically: no changes yet are seen. Normal radiograph
Stage I Development/Destruction
- Clinically: Erythema, foot edema, elevated temperature, no pain
- Radiographically: Boney debris at joints, fragmentation of subchondral bone, joint subluxation, and/or fracture-dislocation
Stage II Coalescence
- Clinically: Decreased signs of inflammation
- Radiographically: Worsening of stage 1 features. Absorption of boney debris with new bone formation. Coalescence of large fragments with sclerosis of bone ends. Some increased stability
Stage III Consolidation
- Clinically: Resolution of inflammation. Changes in overall foot architecture due to underlying final bony remodeling that can lead to new pressure points which are at risk of ulceration
- Radiographically: Remodeling of affected bones and joints
Sanders and Frykberg [18]
- Metatarsophalangeal to interphalangeal joints: 15%
- Tarsometatarsal joints: 40%
- Naviculocuneiform joint, navicular-cuneiform, talonavicular and calcaneocuboid joints: 30%
- Ankle and subtalar joints: 10%
- Calcaneus: 5%
Treatment / Management
Treatments of Charcot aim to decrease permanent foot deformity and ultimately allow for a stable and plantigrade foot that is stable and suitable for ambulation. In the acute phase, it is imperative to immobilize the foot and restrict weight bearing to prevent permanent deformity. Non-operatively, this is achieved through off weight-bearing via total non-weight bearing or protective weight-bearing devices. Assistive devices such as crutches and wheelchairs can aid with non-weight bearing. Total contact casts (TCC) with a controlled ankle motion (CAM) walker can provide protected weight-bearing. TCC redistribute and reduce pressures on the plantar foot while allowing ambulation. The hyperemic phase of the Eichenholtz classification can last for weeks. By using these types of protective measures, the foot may heal the fractures in a stable position if the stress does not exceed the rate of healing.
Pharmacological treatments also exist to control the osteoclastic activity including bisphosphonates and calcitonin supplements. Bisphosphonates may help the acute phase of Charcot as they inhibit osteoclastic reabsorption. Calcitonin also serves as an antiresorptive agent. Alternative agents include pamidronate or zoledronic acid which act on new hydroxyapatite crystal by blocking osteoclast precursors in the newly formed bone matrix.
Surgery is also a treatment option and it remains controversial if one should intervene in the acute or chronic Charcot phase. During the acute phase, there is a firestorm of inflammatory modulators and cytokines (tumor necrosis factor-alpha, interleukin 1, and interleukin 6) that promote edema and bone resorption/fragmentation. [19] The main rationale behind Charcot reconstruction is surgical off-loading to prevent ulceration and deformity progression. A surgeon can achieve this goal via multiple techniques including exostectomy, tenotomy, isolated, or multiple fusions with external and/or internal fixation. Due to softer bone, surgeons tend to "double-up" on hardware to create a solid "super-construct" to prevent breakdown. Minimally invasive intramedullary techniques are also used based on surgeon experience.
Medical optimization including smoking cessation, HbA1c control under 7-8%, and recognizing micro or macro-vascular disease can lessen potential complications post-operatively. [20](B2)
Differential Diagnosis
The most important differential diagnosis of Charcot is osteomyelitis as both can appear clinically and radiographically similar. Cellulitis, septic arthritis, gout, pseudogout, foot/ankle sprain or fracture, and deep vein thrombosis are also important differentials. Charcot is misdiagnosed 25% of the time which can cause a 7-month delay in the true diagnosis.[6][21]
Pertinent Studies and Ongoing Trials
Currently, there are 7 ongoing trials, including:
- Investigating the Use of Prolia (Denosumab) in the Treatment of Acute Charcot Neuroarthropathy
- Characterization of the Charcot Foot
- Characterizing and Diagnosis's of the Charcot Foot (Charcot Osteoarthropathy) in Diabetic Patients
- Efficacy of Teriparatide in Diabetic Inactive Charcot Neuroarthropathy of Foot
- Assessment of Surgical Correction of Deformity in Diabetic Charcot Arthropathy of the Foot and Ankle
- Zoledronic Acid or Methylprednisolone for Active Charcot's Neuroarthropathy of Foot in Patients With Diabetes Mellitus
- Genetic Contribution to the Pathophysiology of the Charcot Foot in Qatari Patients With Diabetes
Treatment Planning
Diagnostic imaging including initial radiographs is key to making and confirming a clinically suspected Charcot foot. [22] Serial foot or ankle radiographs are then used to monitor the disease progression through the Eichenholtz stages. Surgically, a CT may be warranted to visualize the extent of the bone destruction for pre-operative planning. When trying to differentiate Charcot versus osteomyelitis, labeled white blood cell nuclear imaging has high sensitivity and specificity. [23]
Prognosis
It has been shown that the resolution time of acute Charcot to progress through all stages around 8 months. [24] [25][26] In a study by Jansen et. al., 67% of Charcot patients developed complications such as ulcerations. Additionally, non-adherence to the treatment was noted to significantly worsen the prognosis.
Complications
- Foot deformities such as flatfoot, rocker-bottom foot, hammertoes, ankle equinus contracture.
- Boney prominences which can lead to ulceration, infection, and in some cases loss of limb (amputation) or life.
- Recurrence of Charcot joint
- From the first diagnosis of acute Charcot, the 5-year mortality rate is 13%, which is similar to diabetics without Charcot. [24]
Consultations
- Podiatry and/or Foot & Ankle Surgery
- Endocrine and/or Internal Medicine
- Certified wound physician
- Infectious Disease if underlying bone infection is present
- Physical and Occupational Therapy
- Home health service
- Orthotist and/or pedorthist
Deterrence and Patient Education
Patients must understand the magnitude of their condition and the possible life-altering complication if strict adherence to the treatment plan is not followed. Charcot joint can lead to multiple staged reconstructive surgeries of a rigid and deformed foot. Alternatively, Charcot joint can lead to a rocker bottom deformity which easily progresses to a neuropathic ulceration with an underlying bone infection that can result in an amputation. The presence of a rocker-bottom foot increases the risk of a major lower extremity amputation by 15 to 40 fold. [21] A major amputation in a diabetic has a 5-year mortality rate ranging from 30 -80%. [27][28][29] One and five-year mortality rates for diabetics after a major amputation surpass breast and colon cancer mortality rates. Daily self-foot checks, ensuring proper glucose control, and avoiding injury are basic prevention guidelines.
Pearls and Other Issues
Charcot is a rare but serious complication of peripheral neuropathy that can mimic many other disease processes common in the foot of a patient with diabetes. Correctly diagnosing Charcot osteoarthropathy is imperative due to the risk of permanent foot deformity that can be at risk for ulceration and amputation. For the acute phase, treatment endorses non-weight bearing with immobilization of the affected limb to prevent permanent deformity as the disease progresses through the Eichenholtz stages. However, total non-weight bearing and immobilization cannot always be achieved. Common reasons for nonadherence include the length of time required for non-weight bearing, lack of physical ability, and employment restrictions. Alternative treatment options to total non-weight bearing include the administration of offloading ambulating devices. Many offloading ambulating devices exist, such as the controlled ankle motion walker with an offloading insole, total contact cast, or assistive devices like knee scooters.
Enhancing Healthcare Team Outcomes
The management of Charcot joint is an interprofessional; any error in the diagnosis or failure to treat it can lead to the progression of damage and amputation. The aim of treatment is to prevent progression of the neuropathic joint and avoid amputation. Acutely, immobilization may help. Later the patient may be asked to limit weight bearing. Most of these patients may benefit from an ambulatory device. Total contact casts (TCC) with a controlled ankle motion (CAM) walker can provide protected weight bearing. TCC redistribute and reduce pressures on the plantar foot while allowing ambulation. The hyperemic phase of the Eichenholtz classification can last for weeks. By using these types of protective measures, the foot may heal the fractures in a stable position if the stress does not exceed the rate of healing.
Pharmacological treatments also exist to control the osteoclastic activity including bisphosphonates and calcitonin supplements. [21] Finally, patients must be educated on foot safety and wear proper shoe-wear both inside and outside the home. Unfortunately, once Charcot joint has developed, the risk of amputation is always present.[3] (Level V)
Media
(Click Image to Enlarge)
References
Kampen WU, Westphal F, Van den Wyngaert T, Strobel K, Kuwert T, Van der Bruggen W, Gnanasegaran G, Jens JH, Paycha F. SPECT/CT in Postoperative Foot and Ankle Pain. Seminars in nuclear medicine. 2018 Sep:48(5):454-468. doi: 10.1053/j.semnuclmed.2018.03.003. Epub 2018 Apr 18 [PubMed PMID: 30193651]
Figueiredo A, Ferreira R, Alegre C, Fonseca F. Charcot osteoarthropathy of the knee secondary to neurosyphilis: a rare condition managed by a challenging arthrodesis. BMJ case reports. 2018 Aug 20:2018():. pii: bcr-2018-225337. doi: 10.1136/bcr-2018-225337. Epub 2018 Aug 20 [PubMed PMID: 30131415]
Level 3 (low-level) evidenceDixon J, Coulter J, Garrett M, Cutfield R. A retrospective audit of the characteristics and treatment outcomes in patients with diabetes-related charcot neuropathic osteoarthropathy. The New Zealand medical journal. 2017 Dec 15:130(1467):62-67 [PubMed PMID: 29240741]
Level 2 (mid-level) evidenceZhao HM, Diao JY, Liang XJ, Zhang F, Hao DJ. Pathogenesis and potential relative risk factors of diabetic neuropathic osteoarthropathy. Journal of orthopaedic surgery and research. 2017 Oct 2:12(1):142. doi: 10.1186/s13018-017-0634-8. Epub 2017 Oct 2 [PubMed PMID: 28969714]
Sinacore DR, Bohnert KL, Smith KE, Hastings MK, Commean PK, Gutekunst DJ, Johnson JE, Prior FW. Persistent inflammation with pedal osteolysis 1year after Charcot neuropathic osteoarthropathy. Journal of diabetes and its complications. 2017 Jun:31(6):1014-1020. doi: 10.1016/j.jdiacomp.2017.02.005. Epub 2017 Feb 14 [PubMed PMID: 28254346]
Trieb K. The Charcot foot: pathophysiology, diagnosis and classification. The bone & joint journal. 2016 Sep:98-B(9):1155-9. doi: 10.1302/0301-620X.98B9.37038. Epub [PubMed PMID: 27587513]
Miller RJ. Neuropathic Minimally Invasive Surgeries (NEMESIS):: Percutaneous Diabetic Foot Surgery and Reconstruction. Foot and ankle clinics. 2016 Sep:21(3):595-627. doi: 10.1016/j.fcl.2016.04.012. Epub [PubMed PMID: 27524708]
McInnes AD. Diabetic foot disease in the United Kingdom: about time to put feet first. Journal of foot and ankle research. 2012 Oct 11:5(1):26. doi: 10.1186/1757-1146-5-26. Epub 2012 Oct 11 [PubMed PMID: 23050905]
Kaynak G, Birsel O, Güven MF, Oğüt T. An overview of the Charcot foot pathophysiology. Diabetic foot & ankle. 2013:4():. doi: 10.3402/dfa.v4i0.21117. Epub 2013 Aug 2 [PubMed PMID: 23919113]
Level 3 (low-level) evidenceMcEwen LN, Ylitalo KR, Herman WH, Wrobel JS. Prevalence and risk factors for diabetes-related foot complications in Translating Research Into Action for Diabetes (TRIAD). Journal of diabetes and its complications. 2013 Nov-Dec:27(6):588-92. doi: 10.1016/j.jdiacomp.2013.08.003. Epub 2013 Sep 10 [PubMed PMID: 24035357]
O'Connell JX, Nielsen GP, Rosenberg AE. Subchondral acute inflammation in severe arthritis: a sterile osteomyelitis? The American journal of surgical pathology. 1999 Feb:23(2):192-7 [PubMed PMID: 9989846]
Sybenga AB, Jupiter DC, Speights VO, Rao A. Diagnosing Osteomyelitis: A Histology Guide for Pathologists. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2020 Jan-Feb:59(1):75-85. doi: 10.1053/j.jfas.2019.06.007. Epub 2019 Nov 19 [PubMed PMID: 31753572]
Płaza M, Nowakowska-Płaza A, Walentowska-Janowicz M, Chojnowski M, Sudoł-Szopińska I. Charcot arthropathy in ultrasound examination - a case report. Journal of ultrasonography. 2016 Jun:16(65):210-5. doi: 10.15557/JoU.2016.0022. Epub 2016 Jun 29 [PubMed PMID: 27446605]
Level 3 (low-level) evidenceFosbøl M, Reving S, Petersen EH, Rossing P, Lajer M, Zerahn B. Three-phase bone scintigraphy for diagnosis of Charcot neuropathic osteoarthropathy in the diabetic foot - does quantitative data improve diagnostic value? Clinical physiology and functional imaging. 2017 Jan:37(1):30-36. doi: 10.1111/cpf.12264. Epub 2015 Jul 3 [PubMed PMID: 26147681]
Mautone M, Naidoo P. What the radiologist needs to know about Charcot foot. Journal of medical imaging and radiation oncology. 2015 Aug:59(4):395-402. doi: 10.1111/1754-9485.12325. Epub 2015 Jun 3 [PubMed PMID: 26041322]
Hofstaetter SG, Trieb K. [Diagnostic for Charcot foot]. Der Orthopade. 2015 Jan:44(1):45-9. doi: 10.1007/s00132-014-3052-1. Epub [PubMed PMID: 25510223]
Rosenbaum AJ, DiPreta JA. Classifications in brief: Eichenholtz classification of Charcot arthropathy. Clinical orthopaedics and related research. 2015 Mar:473(3):1168-71. doi: 10.1007/s11999-014-4059-y. Epub 2014 Nov 21 [PubMed PMID: 25413713]
Rosskopf AB, Loupatatzis C, Pfirrmann CWA, Böni T, Berli MC. The Charcot foot: a pictorial review. Insights into imaging. 2019 Aug 5:10(1):77. doi: 10.1186/s13244-019-0768-9. Epub 2019 Aug 5 [PubMed PMID: 31385060]
Baumhauer JF, O'Keefe RJ, Schon LC, Pinzur MS. Cytokine-induced osteoclastic bone resorption in charcot arthropathy: an immunohistochemical study. Foot & ankle international. 2006 Oct:27(10):797-800 [PubMed PMID: 17054880]
Wukich DK, Crim BE, Frykberg RG, Rosario BL. Neuropathy and poorly controlled diabetes increase the rate of surgical site infection after foot and ankle surgery. The Journal of bone and joint surgery. American volume. 2014 May 21:96(10):832-9. doi: 10.2106/JBJS.L.01302. Epub [PubMed PMID: 24875024]
Level 2 (mid-level) evidenceMarmolejo VS, Arnold JF, Ponticello M, Anderson CA. Charcot Foot: Clinical Clues, Diagnostic Strategies, and Treatment Principles. American family physician. 2018 May 1:97(9):594-599 [PubMed PMID: 29763252]
Ertugrul BM, Lipsky BA, Savk O. Osteomyelitis or Charcot neuro-osteoarthropathy? Differentiating these disorders in diabetic patients with a foot problem. Diabetic foot & ankle. 2013 Nov 5:4():. doi: 10.3402/dfa.v4i0.21855. Epub 2013 Nov 5 [PubMed PMID: 24205433]
Capriotti G, Chianelli M, Signore A. Nuclear medicine imaging of diabetic foot infection: results of meta-analysis. Nuclear medicine communications. 2006 Oct:27(10):757-64 [PubMed PMID: 16969256]
Level 1 (high-level) evidenceJansen RB, Jørgensen B, Holstein PE, Møller KK, Svendsen OL. Mortality and complications after treatment of acute diabetic Charcot foot. Journal of diabetes and its complications. 2018 Dec:32(12):1141-1147. doi: 10.1016/j.jdiacomp.2018.09.013. Epub 2018 Sep 29 [PubMed PMID: 30301593]
Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabetic medicine : a journal of the British Diabetic Association. 1997 May:14(5):357-63 [PubMed PMID: 9171250]
Level 2 (mid-level) evidenceGame FL, Catlow R, Jones GR, Edmonds ME, Jude EB, Rayman G, Jeffcoate WJ. Audit of acute Charcot's disease in the UK: the CDUK study. Diabetologia. 2012 Jan:55(1):32-5. doi: 10.1007/s00125-011-2354-7. Epub 2011 Nov 8 [PubMed PMID: 22065087]
Level 2 (mid-level) evidenceJupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. International wound journal. 2016 Oct:13(5):892-903. doi: 10.1111/iwj.12404. Epub 2015 Jan 20 [PubMed PMID: 25601358]
Level 1 (high-level) evidenceThorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality After Nontraumatic Major Amputation Among Patients With Diabetes and Peripheral Vascular Disease: A Systematic Review. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2016 May-Jun:55(3):591-9. doi: 10.1053/j.jfas.2016.01.012. Epub 2016 Feb 19 [PubMed PMID: 26898398]
Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, Campbell DR, Scovell SD, LoGerfo FW, Pomposelli FB Jr. Major lower extremity amputation: outcome of a modern series. Archives of surgery (Chicago, Ill. : 1960). 2004 Apr:139(4):395-9; discussion 399 [PubMed PMID: 15078707]
Level 2 (mid-level) evidence