Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that causes chronic inflammation and damage to multiple organs. This condition is diagnosed clinically and serologically with the presence of autoantibodies. Hippocrates first documented a case of lupus in 400 BCE, and in the 18th and 19th centuries, SLE was believed to be associated with tuberculosis or syphilis. Over time, the understanding of lupus evolved from being seen as solely a dermatologic manifestation to being recognized as a comprehensive multisystemic disease.
A common and severe manifestation of SLE that requires evaluation is kidney involvement, referred to as "lupus nephritis." Monitoring kidney function in patients with SLE is crucial, as early detection and management of renal impairment can significantly improve outcomes. Lupus nephritis typically develops 3 to 5 years after the onset of SLE. Histological evidence of lupus nephritis is present in most SLE patients, even when clinical signs of renal disease are not apparent.
Monitoring for the development of lupus nephritis involves serial assessments of creatinine levels, urine protein-to-creatinine ratio, and urinalysis. These tests help detect increases in serum creatinine and the presence of proteinuria, which is commonly observed in lupus nephritis. Given the high risk of increased morbidity, timely treatment is crucial to prevent progression to end-stage renal disease (ESRD).[1][2][3][4] The primary objective of treatment is to normalize kidney function or, at the very least, prevent further decline. Treatment options vary significantly based on the underlying pathological lesions.[5][6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The pathogenesis of lupus nephritis involves a combination of genetic, environmental, and immune system factors. The condition is primarily driven by a type III hypersensitivity reaction, which leads to the formation of immune complexes. Anti-double-stranded deoxyribonucleic acid (anti-dsDNA) antibodies bind to DNA, which forms anti-dsDNA immune complexes. These immune complexes deposit in the mesangium, subendothelial, or subepithelial spaces near the glomerular basement membrane, triggering an inflammatory response. This activates the complement pathway, resulting in the influx of neutrophils and other inflammatory cells, which contribute to the development of lupus nephritis.
Genetic Factors
As with many other autoimmune diseases, genetic predilection has a significant role in the development of SLE and lupus nephritis. The loss of self-tolerance is considered a polygenic phenomenon, although this is incompletely understood. Over 50 genetic polymorphisms have been linked to the development of lupus nephritis, including those involving platelet-derived growth factor receptor-alpha, apolipoprotein L1, and hyaluronan synthase 2. Human leukocytic antigen (HLA) alleles are also associated with lupus nephritis. Specifically, HLA-DR3 and HLA-DR15 increase the risk of lupus nephritis, particularly in patients of European descent, whereas HLA-DR4 and HLA-DR11 appear to offer protective effects.[7]
SLE is more commonly observed in first-degree relatives of affected individuals, with a reported familial prevalence of 10% to 12%. Monozygotic twins exhibit higher concordance rates (25%-57%) than dizygotic twins (2%-9%). These concordance rates support the significant role of genetics in the development of SLE. However, the fact that monozygotic twins do not have a 100% concordance rate suggests that environmental factors also have an important role in the development of the disease.[8][9]
Several gene variations contribute to the development of lupus nephritis, as mentioned below.
- IFIH1: This gene encodes melanoma differentiation-associated protein-5 (MDA5)—a sensor for double-stranded ribonucleic acid (dsRNA). This gene variation enhances RNA binding and leads to stronger baseline and ligand-induced type I interferon (IFN) responses. Patients with SLE who carry IFIH1 risk variants exhibit heightened type-I IFN responses and a greater likelihood of developing anti-dsDNA antibodies, which may contribute to the development of lupus nephritis.[10]
- ITGAM: This gene codes for CD11b-integrin (alpha-M), which is a subunit of the alpha-M beta-2 integrin complex, also known as complement receptor 3 (Mac-1). This protein is expressed in dendritic cells, macrophages, and granulocytes.[8]
- FCGR: This gene encodes Fc gamma receptors, which have a crucial role in the removal of immune complexes.[8][9]
- APOL1 and FcγRIIa: These genetic variants are associated with an increased risk of lupus nephritis in Black individuals.[7]
Environmental Factors
Up to 80% of patients with SLE exhibit sensitivity to UV light, with skin exposure often triggering symptoms and lupus flares. UV light promotes neutrophilic infiltration in the skin.[11] Neutrophils are crucial in mediating both local and systemic lupus symptoms. A study observed that neutrophils migrated to the kidney tubulointerstitial space upon light exposure, intensifying the inflammatory response. This interaction between the skin and kidneys has led some researchers to suggest a potential link between air pollution and the development or worsening of SLE.[12][13]
Dysregulation of the gut microbiome may contribute to SLE, as increased intestinal membrane permeability can allow bacterial translocation into the systemic circulation. A potential marker for this dysregulation is the Bacteroides/Firmicutes ratio.[14] A proposed etiologic theory for SLE suggests that translocated bacteria may trigger autoimmunity through molecular mimicry. Additionally, some evidence indicates that food antigens could influence autoimmune responses. Further research is needed to validate these hypotheses.[15][16]
Viral infections, particularly Epstein-Barr virus, parvovirus B19, and human endogenous retroviruses, have been associated with SLE flares and lupus nephritis.[17] SARS-CoV-2 has recently been associated with de novo SLE diagnoses, with molecular mimicry suggested as the underlying mechanism.[18]
Immune System Dysregulation
Immune system dysregulation in SLE has been well-understood for many decades. Lupus nephritis is a type III hypersensitivity reaction that results from the formation of immune complexes. Autoimmunity has a crucial role in the development of lupus nephritis, leading to the production of autoantibodies directed against nuclear elements.[9][19] These autoantibodies form immune complexes within blood vessels, which are then deposited in the glomeruli. Additionally, autoantibodies may bind to glomerular basement membrane antigens, forming immune complexes in situ. These immune complexes trigger an inflammatory response by activating the complement system and recruiting inflammatory cells. Glomerular thrombosis is another pathogenic phenomenon in lupus nephritis, especially in patients with antiphospholipid syndrome. This may result from the interaction between antibodies and negatively charged phospholipid proteins.[20]
Lupus nephritis can progress in severity over time through a phenomenon called "epitope spreading," where autoantibodies initially recognize one epitope, then expand to recognize additional epitopes on the same molecule, followed by recognition of epitopes on other molecules. This process involves both intramolecular and intermolecular epitope spreading. Initially, deposits form in the mesangium (class I/II), but as epitope spreading occurs, additional antibodies are produced, leading to deposits in the subendothelial and subepithelial compartments (class III/IV).[20]
Anti-dsDNA antibodies are a hallmark of SLE, with apoptotic cells providing the extracellular DNA against which these antibodies are directed. These autoantibodies form immune complexes with nucleosomes that are deposited in the glomeruli and interstitial space. Additionally, anti-dsDNA antibodies activate the complement system and immune cells, particularly B lymphocytes and dendritic cells. However, anti-enolase-1 and anti-histone-2 antibodies may correlate more strongly with lupus nephritis than anti-dsDNA antibodies.[20][21] Anti-C1q, anti-nucleosome, anti-alpha actinin, and anticardiolipin antibodies also have a role in the pathogenesis of SLE. These antibodies can cross-react; for example, anti-alpha actinin interacts with anti-dsDNA to form a subset of higher affinity anti-dsDNA.
Anticardiolipin antibodies are believed to induce mesangial cell apoptosis, while anti-dsDNA antibodies are thought to activate neutrophil extracellular traps (NETs).[21] Both marrow and extramedullary production of neutrophils and NETs have been observed in SLE. Evidence also suggests that macrophages shift from phagocytic cells to antigen-presenting cells, impairing the clearance of apoptotic cell debris. Additionally, dendritic cells secrete cytokines, particularly IFN-1, which have a crucial role in activating T lymphocytes and initiating fibrosis.[9] T-follicular helper (Tfh) cells are expanded, and the ratio of Tfh to T-regulatory cells (Treg) cells increases in active SLE, particularly in classes III and IV. These circulating immune cells interact with kidney cells, including glomerular epithelial cells, mesangial cells, podocytes, and tubular epithelial cells, to initiate autoimmunity.[18][22] Tubular epithelial cells secrete B-cell activating factor, which promotes the formation of tertiary lymphoid structures and has been investigated as a potential therapeutic target.[23]
Uncontrolled complement activation also contributes to the pathogenesis of SLE, involving all 3 complement pathways. Low C3 levels are more strongly correlated with lupus nephritis than low C4 levels, highlighting the significance of the alternative complement pathway in this process. Vitamin D has immunomodulatory properties, and low 25-hydroxyvitamin D3 (25(OH)D) levels are associated with lupus nephritis; an inverse correlation has been observed between 25-D3 levels and SLE activity. However, determining cause and effect is challenging, as patients with SLE are often advised to limit sun exposure.[18][24]
Epidemiology
SLE can affect individuals of any age, gender, or ethnicity, but it is most common in women in their 30s and 40s, particularly in high-income countries. Lupus nephritis affects approximately 40% of patients with SLE and is the most prevalent form of secondary glomerulonephritis. Around 10% to 30% of patients with lupus nephritis will progress to ESRD within 10 years.[18]
Age-Related
Lupus nephritis typically develops early in the disease course, most commonly in women between the ages of 20 and 40. Children with SLE are at a higher risk for renal involvement than adults.[25][26]
Gender-Related
SLE has a higher prevalence in women, with a female-to-male ratio of 9:1. Similarly, lupus nephritis is more common in women; however, clinically evident renal disease with a worse prognosis is more frequently observed in men with SLE.[27]
Ethnicity-Related
SLE is more prevalent in Hispanic, Black, and Asian populations than White populations, with the highest prevalence observed in Caribbean populations. Lupus nephritis is more common in Asian individuals with SLE than in their White counterparts, but the 10-year outcome and survival rates tend to be better in Asians.[18][28] Black and Hispanic patients with SLE generally present with higher creatinine levels and more proteinuria at the time of diagnosis than White patients.[7]
Pathophysiology
Lupus nephritis results from glomerular, tubulointerstitial, and vascular lesions. This condition typically affects 40% of patients with SLE, often within 5 years of diagnosis. Approximately 10% to 30% of patients with lupus nephritis will progress to ESRD within 10 years.[18] Lupus nephritis can be asymptomatic or present with urinary abnormalities, and over time, the clinical symptoms generally decrease in severity.[29][30]
Renal biopsy is fundamental to accurately diagnosing lupus nephritis. A biopsy is usually performed in cases of a urine protein-to-creatinine ratio exceeding 500 mg/24 h, persistent renal dysfunction, or active urinary sediment. The current standardized classification system for lupus nephritis is based on recommendations from the World Health Organization (WHO) and the International Society of Nephrology/Renal Pathology Society. This classification system categorizes lupus nephritis based on glomerular morphological changes observed under light microscopy, immune deposits identified through immunofluorescence, and findings from electron microscopy (see Table 1).
- Class I, minimal mesangial lupus nephritis: Glomeruli appear normal on light microscopy. Immunofluorescence shows immune complex deposits in the mesangial space.
- Class II, proliferative mesangial lupus nephritis: Light microscopy reveals mesangial proliferation, distinguishing it from class I. Immunofluorescence shows immune complex deposits in the mesangial space similar to class I.
- Class III, focal lupus nephritis (<50% glomerular involvement): Immune complex deposits may be visualized in the mesangial, subendothelial, or subepithelial space using immunofluorescence imaging.
- Class IV, diffuse lupus nephritis (≥50% glomerular involvement): Immune complex deposits may occur in the mesangial, subendothelial, or subepithelial space. Lesions may be segmental, involving less than 50% of the glomeruli, or global, involving more than 50% of the glomeruli.
- Class V, membranous lupus nephritis: Immune complex deposits are present in the mesangial and subepithelial spaces. Capillary loops appear thickened due to subepithelial immune complex deposits. In this class, nephrotic-range proteinuria occurs. Class V may also exist with class III or IV pathology.
- Class VI, advanced sclerosing lupus nephritis (≥90% glomerular involvement): Most glomeruli are sclerosed. However, immune complex deposits are not visualized on immunofluorescence, as more than 90% of the glomeruli are scarred.
Table 1. Standardized Classification System for Lupus Nephritis
| Class | Prevalence on Biopsy | Characteristics | Progression |
| I | 0.9%-4.2% | Possible nephrotic syndrome | Low risk of progression |
| II | 9.3%-21.5% | Isolated hematuria, low-grade proteinuria, and normal renal function | Progression to focal or diffuse disease is possible |
| III | 11.2%-24.2% | Second most likely to have active lesions | High risk of progression to ESRD |
| IV | 27.8%-47.7% | Most likely to have active lesions | Highest risk of progression to ESRD |
| V | 12.1%-20.3% | Proteinuria, mildly elevated creatinine | Low rate of progression to ESRD, but complications from nephrotic syndrome are common |
| VI | 1.3%-4.7% | Variable proteinuria, impaired renal function | Biopsy numbers may be low due to ESRD [29] |
Results from multiple lupus nephritis studies indicate that class IV is the most common form and is associated with the worst prognosis.[29][31] A meta-analysis revealed that 15% to 30% of patients with class IV disease fail to achieve remission, and of those who do, 15% to 30% experience relapse.[29] Additionally, patients with lupus nephritis face a higher risk of atherosclerosis, with coronary artery disease (CAD) being the leading cause of mortality in individuals who have had SLE for more than 5 years. The increased CAD risk is strongly linked to lupus nephritis, with underlying mechanisms including atherosclerosis, vasculitis, thrombosis, embolization, and vasospasm. The rate of fatal myocardial infarction in these patients is reported to be 3 times higher than in age-matched controls.[32]
Histopathology
The histological type of lupus nephritis that develops in patients with SLE is influenced by various factors, including the properties of the autoantibodies, such as antigen specificity, and the type of inflammatory response determined by other host factors. In more severe forms of lupus nephritis, the proliferation of endothelial, mesangial, and epithelial cells, along with the production of matrix proteins, leads to fibrosis. Lupus nephritis can affect various kidney compartments, including the glomeruli, interstitium, tubules, and capillary loops. In addition to anti-dsDNA immune complex deposits, immunoglobulins G (IgG), A (IgA), and M (IgM), as well as complement components (C1, C3, and properdin), are commonly found as mesangial, subendothelial, and subepithelial deposits. Leukocytes may also be present.
The current standardized classification system for lupus nephritis is based on recommendations from the WHO and the International Society of Nephrology/Renal Pathology Society. This system classifies lupus nephritis according to glomerular morphological changes observed under microscopy, immune deposits detected through immunofluorescence, and findings from electron microscopy. In 2018, the International Society of Nephrology/Renal Pathology Society introduced more quantitative recommendations, emphasizing the assessment of active and chronic lesions.[30][33]
- Class I, minimal mesangial lupus nephritis: Glomeruli appear normal on light microscopy. Immunofluorescence reveals immune complex deposits in the mesangial space. Podocyte foot process effacement may be present.
- Class II, proliferative mesangial lupus nephritis: Similar to class I, immunofluorescence reveals immune complex deposits in the mesangial space. At least 4 nuclei are fully surrounded by the matrix in the mesangial area (excluding the hilar region). Subepithelial and subendothelial immune complexes are absent (see Image. Lupus Nephritis, Class II).
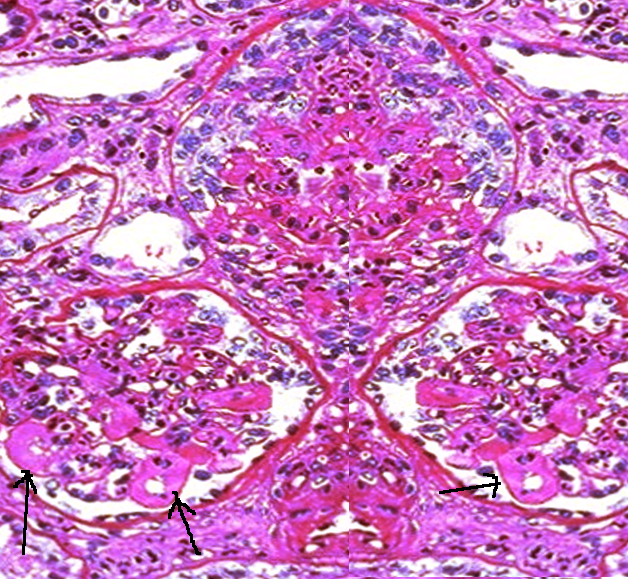
- Class III, focal lupus nephritis: Segmental or global hypercellularity involves fewer than 50% of glomeruli. Immune complex deposits may be observed in the mesangial, subendothelial, or subepithelial space (see Image. Lupus Nephritis, Class III).
- Class IV, diffuse lupus nephritis: Segmental or global hypercellularity affects more than 50% of glomeruli. Immune complex deposits may be present in the mesangial, subendothelial, or subepithelial space. Endocapillary hypercellularity, typically characterized by infiltration by immune cells, is commonly observed (see Image. Lupus Nephritis, Class IV).
- Class V, membranous lupus nephritis: Immune complex deposits are present in the mesangial and subepithelial spaces. Capillary loops become thickened due to subepithelial immune complex deposits. Nephrotic-range proteinuria is observed in this class, and class V may also exhibit features of classes III and IV pathology.
- Class VI, advanced sclerosing lupus nephritis: Most glomeruli are sclerosed, and immune complex deposits are not detectable on immunofluorescence, as more than 90% of the glomeruli are scarred.
The below-mentioned definitions also apply, particularly to classes III and IV.
- Crescent: Extracapillary hypercellularity involving 10% or more of the circumference of the Bowman capsule, composed of a mixture of cells, with the possible presence of fibrin and fibrous matrix.
- Cellular crescent: More than 75% cells and fibrin, with less than 25% fibrous matrix.
- Fibrous crescent: More than 75% fibrous matrix, with less than 25% cells and fibrin.
- Fibrocellular crescent: Between 25% and 75% cells and fibrin, with the remainder consisting of a fibrous matrix.
- Adhesion: This refers to an area of continuous extracellular matrix material between the glomerular tuft and the Bowman capsule, even when the underlying segment does not show overt sclerosis.
- Fibrinoid necrosis: Fibrin is associated with disruption of the glomerular basement membrane or lysis of the mesangial matrix. This lesion does not require karyorrhexis and is similar to those seen in antineutrophil cytoplasmic antibody-associated vasculitis.
- Tubulointerstitial inflammation: This should be specified as occurring with or without accompanying fibrosis.[33]
Active and chronic lesions are also defined in the 2018 guidelines. Activity is scored from 0 to 24, while chronicity is scored from 0 to 12 (see Table 2).[33] Multiple study results have shown that a high chronicity index is directly associated with poor kidney prognosis and inversely related to treatment response.[34][35] Wire loops are a common indicator of renal activity (see Image. Lupus Nephritis With Wire Loop Lesions).
Table 2. Active Versus Chronic Lesional Activity in Lupus Nephritis
| Activity | Lesion | Score |
| Endocapillary hypercellularity | Endocapillary hypercellularity in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Neutrophils or karyorrhexis | Neutrophils and/or karyorrhexis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrinoid necrosis | Fibrinoid necrosis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | (0–3)×2 |
| Hyaline deposits | Wire loop lesions or hyaline thrombi in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Cellular or fibrocellular crescents | Cellular or fibrocellular crescents in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | (0–3)×2 |
| Interstitial Inflammation | Interstitial leukocytes in <25% (1+), 25%–50% (2+), or >50% (3+) in the cortex | 0–3 |
| Total Score | 0-24 | |
| Chronicity | ||
| Total glomerulosclerosis score | Global or segmental sclerosis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrous crescents | Fibrous crescents in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Tubular atrophy | Tubular atrophy in <25% (1+), 25%–50% (2+), or >50% (3+) of the cortical tubules | 0–3 |
| Interstitial fibrosis | Interstitial fibrosis in <25% (1+), 25%–50% (2+), or >50% (3+) in the cortex | 0–3 |
| Total Score | 0–12 |
History and Physical
Patients with lupus nephritis typically exhibit various clinical manifestations of SLE, including malar or discoid rash, fatigue, fever, photosensitivity, serositis, oral ulcers, nonerosive arthritis, seizures, psychosis, or hematologic disorders. In the early stages of lupus nephritis, patients are often asymptomatic. Some may develop symptoms such as polyuria, nocturia, foamy urine, hypertension, and edema. Early indications of proteinuria, reflecting tubular or glomerular dysfunction, include foamy urine or nocturia. When proteinuria meets the nephrotic syndrome threshold of more than 3.5 g/d, peripheral edema may occur due to hypoalbuminemia. Microscopic hematuria may also be present.
Some patients may be asymptomatic at presentation; however, regular follow-up often reveals laboratory abnormalities indicative of active lupus nephritis, such as elevated serum creatinine levels, hypoalbuminemia, proteinuria, or active urinary sediment. These findings are commonly associated with mesangial or membranous lupus nephritis. In diffuse lupus nephritis, symptoms related to hypertension are more frequent and may include dizziness, headache, visual disturbances, and signs of cardiac compromise.
The physical examination in focal and diffuse immune-complex-mediated lupus nephritis (classes III and IV) may reveal generalized SLE features, such as oral or nasal ulcers, rash, synovitis, or serositis. Signs of active nephritis are also frequently observed, including peripheral edema caused by hypertension or hypoalbuminemia. Significant peripheral edema is more common in patients with diffuse or membranous lupus nephritis, as these renal lesions are often associated with heavy proteinuria.
Signs of isolated nephrotic syndrome are commonly seen in membranous lupus nephritis and isolated podocytopathy (more common in children), including peripheral edema, ascites, and pericardial and pleural effusions without hypertension.[27][36] Collapsing glomerulopathy and thrombotic microangiopathy may present with severe hypertension and rapid loss of kidney function; however, these lesions are relatively rare. A kidney biopsy is necessary to differentiate these pathologies from the more common immune-complex-mediated types.[27]
Evaluation
Laboratory
In active SLE, serum C3 and C4 levels are typically low, and anti-dsDNA autoantibodies are often positive. Serum creatinine levels may be elevated or normal in the presence of proteinuria. Urinalysis commonly reveals proteinuria, microscopic hematuria, or red blood cell casts, with proteinuria indicating glomerular damage. Proteinuria exceeding 3.5 g/d is classified as nephrotic range. If significant proteinuria is present, a comprehensive metabolic panel may show hypoalbuminemia following a prolonged period of active disease. In patients with active SLE, screening for proteinuria and hematuria is recommended every 3 months.
Proteinuria and creatinine are the most extensively studied markers for evaluating lupus nephritis activity. However, these markers are relatively insensitive and may reflect longstanding disease when abnormal. The urinary soluble cluster of differentiation (CD163), a cleavage product of a macrophage receptor, has emerged as a novel marker for detecting early damage in lupus nephritis. Studies have demonstrated a strong correlation between urinary CD163 levels and active disease, with decreases below a specific threshold preceding improvements in proteinuria and creatinine. Additionally, urinary CD163 has shown greater sensitivity and specificity for lupus flares than traditional antibody and complement measurements, making it a promising prognostic indicator for various inflammatory renal diseases.[37][38][39]
Radiographic
A bilateral kidney ultrasound is recommended to exclude hydronephrosis or obstruction.
Biopsy
Renal biopsy is a cornerstone in diagnosing lupus nephritis, providing critical information about the histological form and stage of the disease, including activity and chronicity. These findings guide prognosis and treatment strategies. However, a biopsy is not always performed, as lupus nephritis can sometimes be diagnosed clinically. Biopsy complications are possible, particularly in patients with thrombocytopenia, abnormal coagulation profiles, or small kidneys. In such cases, empiric treatment is often initiated without a biopsy. Studies have shown that clinical symptoms do not consistently align with biopsy findings, thereby complicating the prediction of disease course. The decision to perform a renal biopsy varies across institutions.
A renal biopsy is recommended when the results are likely to influence the management of lupus nephritis. For patients with other severe SLE manifestations, such as central nervous system or hematological involvement, who are already planned for treatment with cyclophosphamide, a biopsy may not be strictly necessary. However, a biopsy should still be considered, as it may provide valuable prognostic information regarding renal outcomes. Sampling errors can occur during a renal biopsy, making it essential to interpret biopsy results in the context of the patient's clinical presentation and laboratory findings. The expertise of pathologists in evaluating lupus nephritis biopsy specimens varies significantly. Studies indicate that larger medical centers, which treat many SLE patients, tend to provide more consistent and reliable biopsy interpretations.
Multiple studies have demonstrated discrepancies between clinical and histological responses in lupus nephritis. Even with an activity index of 0 at 6 to 8 months of treatment, many patients may have histologically active renal disease despite achieving clinical remission, which is typically defined as creatinine levels near baseline and proteinuria below 500 mg/d or reduced by half. Some studies have shown a correlation between ongoing histological activity, lupus nephritis flares, and poor renal outcomes.[40][41][42] In some patients, clinical remission may not be achievable due to chronic damage. For these patients, a repeat biopsy can be useful—if the activity index is near 0, immunosuppression may be discontinued.[27][43] Complete histological remission can take months or even up to 10 years in certain cases.[27]
A trial in Argentina prospectively followed patients with proliferative lupus nephritis, withdrawing immunosuppression only if the activity index was 0 on repeat biopsy. The cohort was followed for a median of 8 years, with 9.2% developing subsequent lupus flares—significantly lower than the average flare rates observed in similar cohorts, which ranged from 1.7 to 68 times more frequent, depending on the study.[44]
Treatment / Management
The treatment of lupus nephritis is primarily guided by histopathological class. All patients should initiate therapy with hydroxychloroquine at baseline unless contraindicated, with regular ophthalmological exams to assess for retinal toxicity. A recent trial showed that individuals receiving hydroxychloroquine experienced fewer flares compared to those who were not treated.[45][46][47] Generally, classes I and II may require monitoring and may not need treatment, particularly if proteinuria is under 500 mg/d. Immunosuppressive therapy and steroids are necessary for classes III and IV, while renal replacement therapy is considered for class VI, where most glomeruli are sclerotic. Active disease generally predicts a better response to treatment than chronic disease.(B3)
Treating risk factors that may contribute to the progression of CKD or ESRD is crucial for patients with lupus nephritis. Statin therapy should be initiated to manage lipid levels, as both lupus nephritis and CKD increase cardiovascular morbidity and mortality. Antihypertensive treatment with either angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is recommended for patients with proteinuria or hypertension. Vitamin D or E supplementation, along with omega-3 fatty acids, has shown improvements in inflammatory markers, endothelial function, and energy levels in SLE. Additionally, curcumin has demonstrated promising anti-inflammatory, antioxidant, and anti-proteinuric effects in lupus nephritis.[15][48]
The treatment of lupus nephritis involves induction and maintenance phases, utilizing both immunosuppressive and non-immunosuppressive therapies. The induction phase focuses on achieving a renal response through immunosuppressive agents and anti-inflammatory medications. Once a renal response is achieved, maintenance therapy is implemented for a prolonged period with both immunosuppressive and non-immunosuppressive agents to prevent relapse, requiring regular monitoring.
European Alliance of Associations for Rheumatology (EULAR), European Renal Association (ERA), and Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend maintenance therapy with low-dose mycophenolate mofetil (MMF) or azathioprine, with or without glucocorticoids (<7.5 mg), for at least 3 years after achieving stable clinical remission (12 months per KDIGO).[45] During induction therapy, prophylaxis against pneumocystis pneumonia should be administered. Chronic glucocorticoid use raises concerns about bone density loss, making it crucial to take preventive measures, including supplementation and a baseline dual-energy x-ray absorptiometry scan.[49][50]
Induction Therapy for Classes III and IV Lupus Nephritis
The 2019 EULAR guidelines recommend initiating treatment with MMF (2-3 g/d or equivalent mycophenolic acid) or cyclophosphamide (500 mg for 6 biweekly doses), combined with 3 days of intravenous glucocorticoids, followed by a prednisone taper to the lowest dose. Pulse steroids with each cyclophosphamide dose are linked to improved outcomes and reduced oral glucocorticoid use in patients with classes III, IV, and V SLE. MMF with a calcineurin inhibitor or high-dose cyclophosphamide may be alternatives for patients with nephrotic-range proteinuria or poor prognostic factors.[45][51] The median time to induction for complete remission in responders is 4.3 months (range 2-6 months).[40]
Cyclophosphamide is the preferred treatment for life-threatening complications of SLE, such as pulmonary involvement or rapidly progressive lupus nephritis.[18] MMF has shown superiority over cyclophosphamide for induction therapy in proliferative lupus nephritis, particularly in Black, Hispanic, and Chinese populations.[18][52][53] Evidence suggests that MMF specifically targets dendritic cells, which may explain its effectiveness in these cases.[9](A1)
Sirolimus (a mammalian target of rapamycin inhibitor), tacrolimus, cyclosporine, and methotrexate have been used in patients who cannot tolerate other medications.[9] The Chinese Systemic Lupus Erythematosus Treatment and Research Group found sirolimus to be superior to tacrolimus in reducing glucocorticoid use and improving the serological profile.[54] If improvement is observed after 6 months, a lower dose of MMF or azathioprine can be continued as maintenance therapy. A randomized controlled trial demonstrated that MMF is superior to azathioprine for maintenance therapy.[55][56] (A1)
If there is no improvement or sufficient renal response after 6 months, therapy is often switched to an alternative agent, or rituximab is administered.[45] Although rituximab is frequently used in the treatment of lupus nephritis—possibly due to the perception of fewer adverse effects—the LUNAR trial did not demonstrate a statistically significant benefit when rituximab was added to standard MMF therapy in patients with active proliferative lupus nephritis, although a nonsignificant trend toward improvement was observed.[18][57]
Induction Therapy for Class V Lupus Nephritis
Immunosuppressive therapy should be considered alongside hydroxychloroquine for patients with proteinuria exceeding 1 g/d.[7] Initial treatment involves MMF combined with prednisone for a duration of 6 months. If clinical improvement is achieved, maintenance therapy is initiated with either a reduced dose of MMF or azathioprine. For cases without improvement, cyclophosphamide with pulse-dose glucocorticoids may be administered for an additional 6 months. When class V lupus nephritis is accompanied by class III or IV features, treatment should follow the protocol for class III or IV disease.[30] Cyclophosphamide, calcineurin inhibitors, or azathioprine with steroids can also be considered.[7]
Renal Replacement Therapy
Patients with ESRD due to lupus nephritis who initiate dialysis experience outcomes similar to those of dialysis patients without lupus nephritis. Patients with lupus nephritis should receive education regarding the potential for renal transplantation should be provided when the glomerular filtration rate falls below 20 mL/min, as with other patients. The risk of recurrent lupus pathology in the renal allograft is estimated at 2% to 11%, with a median follow-up of 4 years. Previously, it was believed that patients with lupus nephritis should remain on hemodialysis for a period to ensure disease quiescence; however, studies have not supported this practice. Patients who underwent preemptive renal transplantation demonstrated better allograft function without higher rates of recurrent lupus nephritis. Additionally, patients with lupus nephritis have allograft outcomes comparable to those of transplant recipients with other etiologies of ESRD.[7][58]
Pregnancy
SLE and lupus nephritis predominantly affect women of childbearing age, making pregnancy management and fertility preservation key concerns. Rituximab is often preferred over cyclophosphamide for women prioritizing fertility preservation. Before pregnancy, women with SLE should undergo testing for antiphospholipid antibodies, including lupus anticoagulant, anticardiolipin, and β-2 glycoprotein, to assess the risk of pregnancy-related complications. Anti-Ro (SSA) and anti-La (SSB) antibodies should also be assessed, as they are associated with an increased risk of complete fetal heart block.[59] The treatment of class III, IV, and V lupus nephritis during pregnancy differs from standard approaches. Glucocorticoids, such as prednisone, dexamethasone, or betamethasone, are commonly used to manage active lupus nephritis. Azathioprine may be added to minimize glucocorticoid dosage and its associated risks.
Hydroxychloroquine is the primary treatment for mild lupus nephritis during pregnancy, and prednisone is the preferred therapy for clinically active lupus nephritis. Azathioprine is considered safe during pregnancy, and belimumab can be used until the second trimester.[9][18] Ideally, patients should achieve remission for at least 6 months before attempting pregnancy. Additionally, pregnant individuals should begin low-dose aspirin around 12 weeks of gestation to reduce the risk of thrombosis. Please see StatPearls' companion resource, "Glomerulonephritis in Pregnancy," for more information.
Antiphospholipid Syndrome
Patients with SLE who test positive for antiphospholipid antibody syndrome are at an increased risk of thrombotic events. Hydroxychloroquine is recommended for these patients due to its thromboprotective properties. Low-dose aspirin may also be considered as part of the treatment.[60] Please see StatPearls' companion resource, "Antiphospholipid Syndrome," for more information.
Lupus anticoagulant significantly increases the risk of arterial thrombosis. Patients with SLE and positive antiphospholipid antibodies should avoid using combined oral contraceptives, patches, or rings due to the heightened risk of thrombosis. Additionally, patients with lupus nephritis and antiphospholipid antibody syndrome face a higher risk of renal allograft loss following a transplant, and this factor should be carefully evaluated during the transplant workup.[61][62](B2)
New Therapies
In the past 4 years, several new agents have been studied or approved for use in SLE and lupus nephritis, paving the way for a multitargeted approach that combines standard therapies with novel treatments addressing different mechanisms of action. We are likely entering an era where targeted therapies will enable improved personalization based on laboratory, demographic, and biopsy-specific factors. In addition to standard treatment, the 2 drugs approved for use in lupus nephritis include belimumab and voclosporin.
Belimumab is a soluble B-cell activating factor (BAFF) antibody that was approved in 2011 for treating active SLE, and it was also approved in 2020 for induction therapy in lupus nephritis, in addition to standard MMF/cyclophosphamide/steroid protocols. Belimumab is a fully human monoclonal IgG1λ antibody that neutralizes BAFF and has demonstrated improved outcomes when used with standard protocols. The Belimumab International Study in Lupus Nephritis (BLISS-LN) study trial demonstrated its effectiveness in lupus nephritis, leading to its approval for use.[46][63] (A1)
A theory suggests that while rituximab depletes B lymphocytes, elevated BAFF levels following treatment may trigger lupus flares. To investigate this, the BEAT LUPUS trial compared belimumab to placebo in patients with refractory disease who had previously received rituximab. The trial found significantly lower anti-dsDNA antibody levels and a reduction in lupus nephritis flares in patients treated with belimumab.[64] Additionally, belimumab may be particularly beneficial for patients with high disease activity and those with cutaneous, musculoskeletal, or serologic manifestations of lupus.[61](A1)
Voclosporin is a calcineurin inhibitor that inhibits interleukin 2, decreasing T-cell activation. This drug is more potent than tacrolimus or cyclosporine and is a more stable molecule, so monitoring levels is unnecessary.[65][66] Belimumab appears less nephrotoxic than voclosporin and can be used with impaired renal function. On the other hand, voclosporin may be more effective in patients with proteinuria greater than 3.0 g/d.[67][68] Both voclosporin and belimumab have been associated with more rapid tapering of glucocorticoids.[18](A1)
Phase-3 trials
Several medications currently undergoing phase-3 trials show promising results.
- Anifrolumab, an IFN receptor antagonist, has demonstrated improved parameters when used alongside standard therapy protocols. The drug blocks the downstream activation of B- and T lymphocytes, dendritic cells, and epithelial cells. Anifrolumab was approved in 2021 for the treatment of moderate-to-severe SLE.[9]
- Atacicept is a fusion protein that combines the transmembrane activator calcium modulator and cyclophilin ligand interactor (TACI) receptor, a BAFF receptor, with the Fc region of IgG. This molecule can bind and neutralize both BAFF and APRIL, which may offer increased efficacy compared to treatments targeting BAFF alone. Telitacicept shares a similar mechanism of action.
- Obinutuzumab, ocrelizumab, and epratuzumab are antibodies that target surface receptors on CD20 and CD22 B cells.[9]
- Ianalumab is a dual-action human monoclonal antibody that targets B lymphocytes expressing BAFF and has shown effectiveness in Sjögren syndrome.
- Deucravacitinib is a Janus kinase inhibitor that also modulates type-I IFN-associated gene expression and is believed to be more specific and targeted in its actions.
- Sodium-glucose cotransporter-2 inhibitors have proven effective in reducing proteinuria and mortality across various renal diseases. A large cohort study found that this class of medications reduces the risk of developing lupus nephritis in patients with SLE.[18][46]
- Povetacicept is a dual BAFF/APRIL antagonist that has recently been tested in humans and shown to be well-tolerated. This is being studied for its potential in treating antibody-related autoimmune diseases, including lupus nephritis and IgA nephropathy. Preliminary results indicate a reduction in specific antibody types.[69] (A1)
Differential Diagnosis
The differential diagnoses for lupus nephritis include other causes of nephrotic syndrome, reflux nephropathy, hydronephrosis, acute kidney injury due to medication, and acute tubulointerstitial nephritis. Other autoimmune disorders to consider include:
- IgA nephropathy
- IgA vasculitis
- Antineutrophil cytoplasmic antibody-associated vasculitis
- Membranous glomerulonephritis
- Polyarteritis nodosa
- Sjögren syndrome
Prognosis
Although lupus nephritis is associated with morbidity and mortality, the prognosis largely depends on the WHO histopathology classification. Classes I (minimal) and II (proliferative mesangial) generally have a favorable long-term prognosis. However, as lupus nephritis progresses to higher classes, the prognosis worsens. Class III has a poor prognosis, while class IV has the worst outcome. Early initiation of therapy is crucial—starting treatment earlier in the disease course typically results in better outcomes.
Over the past 4 decades, treatment advancements for lupus nephritis have significantly improved renal function and overall survival. In the 1950s, the 5-year survival rate for patients with lupus nephritis was nearly 0%. However, the introduction of immunosuppressive agents, such as MMF and cyclophosphamide, has improved survival rates for patients with biopsy-proven lupus nephritis, with current 5-, 10-, and 20-year survival rates of 94%, 86%, and 71%, respectively.[29]
Mortality associated with lupus nephritis in patients with ESRD has significantly declined in recent decades. The mortality rate per 100 patient-years decreased from 11.1 between 1995 and 1999 to 6.7 between 2010 and 2014. The 2 common causes of mortality are cardiovascular disease and infection; deaths related to cardiovascular disease declined by 44%, while those due to infection decreased by 63% during this period.[29][70]
Complications
Clinicians should be mindful of the following important complications:
- Hypertension (sometimes refractory)
- Excessive edema
- Chronic kidney disease
- End-stage renal disease
- Increased thrombotic risk for patients with nephrotic syndrome
Deterrence and Patient Education
Healthcare providers should educate patients on the signs and symptoms of lupus nephritis and regularly monitor their renal function. If renal replacement therapy becomes necessary, patients and their families should receive detailed information and support regarding hemodialysis and early referral for kidney transplantation. Patients with SLE should understand that early detection of complications, such as lupus nephritis, enables prompt treatment and improves long-term outcomes. Pharmacists should inform patients about commonly prescribed medications and the potential adverse events associated with them.
Enhancing Healthcare Team Outcomes
Patients with SLE are at an increased risk of developing lupus nephritis, making early identification and management essential for reducing morbidity and mortality. Effectively caring for these patients requires a collaborative approach among healthcare professionals, ensuring patient-centered care and improving overall outcomes. Nephrologists, rheumatologists, pathologists, dermatologists, advanced clinicians, nurses, pharmacists, and other healthcare providers involved in patient care must possess the essential clinical skills and knowledge to accurately diagnose and manage lupus nephritis. This includes expertise in recognizing its diverse clinical presentations and understanding diagnostic techniques such as electroencephalography and neuroimaging.
Interdisciplinary collaboration has been shown to improve the progression of CKD. Additionally, educating patients and caregivers about the signs and symptoms of worsening renal function is vital for timely intervention. A strategic approach is equally important, incorporating evidence-based strategies to optimize treatment plans while minimizing adverse effects. Ethical considerations should guide decision-making, ensuring informed consent and respecting patient autonomy in treatment choices. Each healthcare professional must understand their responsibilities and contribute their unique expertise to the patient’s care plan, promoting a collaborative, multidisciplinary approach.
Effective interprofessional communication is essential for seamless information exchange and collaborative decision-making among healthcare team members. Care coordination is crucial in ensuring that the patient’s journey from diagnosis to treatment and follow-up is well-managed, minimizing errors and enhancing patient safety. By embracing principles of skill, strategy, ethics, responsibility, communication, and coordination, healthcare professionals can deliver patient-centered care, improving patient outcomes and enhancing team performance in managing lupus nephritis.
Media
(Click Image to Enlarge)

Lupus Nephritis, Class II. Hematoxylin and eosin staining at ×400 magnification, highlighting mesangial proliferation (A). Silver staining reveals the absence of segmental sclerosis or membrane thickening (B).
Hashmi AA, Ali J, Rahman M, Rehan Taseer A, Kumar J, Irfan M. Spectrum of morphologic features of lupus nephritis according to Nephrology/Renal Pathology Society (ISN/RPS) classification. Cureus. 2020;12(9);e10520. doi: 10.7759/cureus.10520.
(Click Image to Enlarge)

Lupus Nephritis, Class III. Hematoxylin and eosin–stained sections at ×400 magnification, demonstrating segmental sclerosing lesions in glomeruli (A and B). Trichrome staining highlighting segmental sclerosis (C). Silver staining shows segmental sclerosis in a glomerulus (D).
Hashmi AA, Ali J, Rahman M, Rehan Taseer A, Kumar J, Irfan M. Spectrum of morphologic features of lupus nephritis according to Nephrology/Renal Pathology Society (ISN/RPS) classification. Cureus. 2020;12(9);e10520. doi: 10.7759/cureus.10520.
(Click Image to Enlarge)

Lupus Nephritis, Class IV. Hematoxylin and eosin staining at ×400 magnification, highlighting endocapillary proliferation (A and B). Periodic acid-schiff (PAS) staining demonstrates endocapillary proliferation with no membrane thickening (C). Silver staining shows the absence of membrane thickening (D).
Hashmi AA, Ali J, Rahman M, Rehan Taseer A, Kumar J, Irfan M. Spectrum of morphologic features of lupus nephritis according to Nephrology/Renal Pathology Society (ISN/RPS) classification. Cureus. 2020;12(9);e10520. doi: 10.7759/cureus.10520.
References
Liu G, Wang H, Le J, Lan L, Xu Y, Yang Y, Chen J, Han F. Early-stage predictors for treatment responses in patients with active lupus nephritis. Lupus. 2019 Mar:28(3):283-289. doi: 10.1177/0961203319826703. Epub 2019 Jan 25 [PubMed PMID: 30682900]
Slight-Webb S, Guthridge JM, Chakravarty EF, Chen H, Lu R, Macwana S, Bean K, Maecker HT, Utz PJ, James JA. Mycophenolate mofetil reduces STAT3 phosphorylation in systemic lupus erythematosus patients. JCI insight. 2019 Jan 24:4(2):. doi: 10.1172/jci.insight.124575. Epub 2019 Jan 24 [PubMed PMID: 30674728]
Wang ZR, Ren LM, Li R, Guan X, Han QM, Liu ML, Shao M, Zhang X, Chen S, Li ZG. [Analysis of 20-year survival rate and prognostic indicators of systemic lupus erythematosus]. Zhonghua yi xue za zhi. 2019 Jan 15:99(3):178-182. doi: 10.3760/cma.j.issn.0376-2491.2019.03.005. Epub [PubMed PMID: 30669759]
Wilson HR, Medjeral-Thomas NR, Gilmore AC, Trivedi P, Seyb K, Farzaneh-Far R, Gunnarsson I, Zickert A, Cairns TD, Lightstone L, Cook HT, Pickering MC. Glomerular membrane attack complex is not a reliable marker of ongoing C5 activation in lupus nephritis. Kidney international. 2019 Mar:95(3):655-665. doi: 10.1016/j.kint.2018.09.027. Epub 2019 Jan 14 [PubMed PMID: 30655025]
Tamirou F, Houssiau FA. Management of Lupus Nephritis. Journal of clinical medicine. 2021 Feb 9:10(4):. doi: 10.3390/jcm10040670. Epub 2021 Feb 9 [PubMed PMID: 33572385]
Houssiau FA, Ginzler EM. Current treatment of lupus nephritis. Lupus. 2008 May:17(5):426-30. doi: 10.1177/0961203308090029. Epub [PubMed PMID: 18490421]
Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on Lupus Nephritis: Core Curriculum 2020. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2020 Aug:76(2):265-281. doi: 10.1053/j.ajkd.2019.10.017. Epub 2020 Mar 24 [PubMed PMID: 32220510]
Iwamoto T, Niewold TB. Genetics of human lupus nephritis. Clinical immunology (Orlando, Fla.). 2017 Dec:185():32-39. doi: 10.1016/j.clim.2016.09.012. Epub 2016 Sep 28 [PubMed PMID: 27693588]
Su X, Yu H, Lei Q, Chen X, Tong Y, Zhang Z, Yang W, Guo Y, Lin L. Systemic lupus erythematosus: pathogenesis and targeted therapy. Molecular biomedicine. 2024 Oct 30:5(1):54. doi: 10.1186/s43556-024-00217-8. Epub 2024 Oct 30 [PubMed PMID: 39472388]
Munroe ME, James JA. Genetics of Lupus Nephritis: Clinical Implications. Seminars in nephrology. 2015 Sep:35(5):396-409. doi: 10.1016/j.semnephrol.2015.08.002. Epub [PubMed PMID: 26573543]
Pan Q, Guo F, Huang Y, Li A, Chen S, Chen J, Liu HF, Pan Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Frontiers in immunology. 2021:12():799788. doi: 10.3389/fimmu.2021.799788. Epub 2021 Dec 3 [PubMed PMID: 34925385]
Skopelja-Gardner S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, Kubes P, Mustelin T, Elkon KB. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2021 Jan 19:118(3):. doi: 10.1073/pnas.2019097118. Epub [PubMed PMID: 33397815]
Bai H, Jiang L, Li T, Liu C, Zuo X, Liu Y, Hu S, Sun L, Zhang M, Lin J, Xiao W, Wang Q, Zhao D, Wu H, Kong X, Gao W, Hou W, Seong M, Zhang Y, Chen F, Chen S, Wu X, Bao C, Wang L, Xu H. Acute effects of air pollution on lupus nephritis in patients with systemic lupus erythematosus: A multicenter panel study in China. Environmental research. 2021 Apr:195():110875. doi: 10.1016/j.envres.2021.110875. Epub 2021 Feb 13 [PubMed PMID: 33592226]
López P, Sánchez B, Margolles A, Suárez A. Intestinal dysbiosis in systemic lupus erythematosus: cause or consequence? Current opinion in rheumatology. 2016 Sep:28(5):515-22. doi: 10.1097/BOR.0000000000000309. Epub [PubMed PMID: 27466725]
Level 3 (low-level) evidenceMonticolo M, Mucha K, Foroncewicz B. Lupus Nephritis and Dysbiosis. Biomedicines. 2023 Apr 13:11(4):. doi: 10.3390/biomedicines11041165. Epub 2023 Apr 13 [PubMed PMID: 37189783]
Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, Caricchio R, Buyon JP, Alekseyenko AV, Silverman GJ. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Annals of the rheumatic diseases. 2019 Jul:78(7):947-956. doi: 10.1136/annrheumdis-2018-214856. Epub 2019 Feb 19 [PubMed PMID: 30782585]
Quaglia M, Merlotti G, De Andrea M, Borgogna C, Cantaluppi V. Viral Infections and Systemic Lupus Erythematosus: New Players in an Old Story. Viruses. 2021 Feb 11:13(2):. doi: 10.3390/v13020277. Epub 2021 Feb 11 [PubMed PMID: 33670195]
Roveta A, Parodi EL, Brezzi B, Tunesi F, Zanetti V, Merlotti G, Francese A, Maconi AG, Quaglia M. Lupus Nephritis from Pathogenesis to New Therapies: An Update. International journal of molecular sciences. 2024 Aug 18:25(16):. doi: 10.3390/ijms25168981. Epub 2024 Aug 18 [PubMed PMID: 39201667]
Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms. Autoimmunity reviews. 2008 Feb:7(4):317-21. doi: 10.1016/j.autrev.2007.12.001. Epub 2007 Dec 26 [PubMed PMID: 18295737]
Level 3 (low-level) evidenceStrizzi CT, Ambrogio M, Zanoni F, Bonerba B, Bracaccia ME, Grandaliano G, Pesce F. Epitope Spreading in Immune-Mediated Glomerulonephritis: The Expanding Target. International journal of molecular sciences. 2024 Oct 16:25(20):. doi: 10.3390/ijms252011096. Epub 2024 Oct 16 [PubMed PMID: 39456878]
Bruschi M, Angeletti A, Prunotto M, Meroni PL, Ghiggeri GM, Zeus consortium, Moroni G, Sinico RA, Franceschini F, Fredi M, Vaglio A, Cavalli A, Scapozza L, Patel JJ, Tan JC, Lo KC, Cavagna L, Petretto A, Pratesi F, Migliorini P, Locatelli F, Pazzola G, Pesce G, Giannese D, Manfredi A, Ramirez GA, Esposito P, Murdaca G, Negrini S, Bui F, Trezzi B, Emmi G, Cavazzana I, Binda V, Fenaroli P, Pisan I, Montecucco C, Santoro D, Scolari F, Mescia F, Volpi S, Mosca M, Tincani A, Ravelli A, Murtas C, Candiano G, Caridi G, La Porta E, Verrina E. A critical view on autoantibodies in lupus nephritis: Concrete knowledge based on evidence. Autoimmunity reviews. 2024 May:23(5):103535. doi: 10.1016/j.autrev.2024.103535. Epub 2024 Mar 27 [PubMed PMID: 38552995]
Zervopoulou E, Grigoriou M, Doumas SA, Yiannakou D, Pavlidis P, Gasparoni G, Walter J, Filia A, Gakiopoulou H, Banos A, Mitroulis I, Boumpas DT. Enhanced medullary and extramedullary granulopoiesis sustain the inflammatory response in lupus nephritis. Lupus science & medicine. 2024 Mar 11:11(1):. doi: 10.1136/lupus-2023-001110. Epub 2024 Mar 11 [PubMed PMID: 38471723]
Hong S, Healy H, Kassianos AJ. The Emerging Role of Renal Tubular Epithelial Cells in the Immunological Pathophysiology of Lupus Nephritis. Frontiers in immunology. 2020:11():578952. doi: 10.3389/fimmu.2020.578952. Epub 2020 Sep 23 [PubMed PMID: 33072122]
Luo M, Lıu J, Yuan Y, Chen Y, Yuan G. The role of vitamin D-synthesizing enzyme CYP27B1 in systemic lupus erythematosus. Turkish journal of medical sciences. 2022 Aug:52(4):984-989 [PubMed PMID: 36326421]
Kaneko M, Jackson SW. Recent advances in immunotherapies for lupus nephritis. Pediatric nephrology (Berlin, Germany). 2023 Apr:38(4):1001-1012. doi: 10.1007/s00467-022-05670-7. Epub 2022 Jul 1 [PubMed PMID: 35778517]
Level 3 (low-level) evidenceBrunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis and rheumatism. 2008 Feb:58(2):556-62. doi: 10.1002/art.23204. Epub [PubMed PMID: 18240232]
Rodriguez-Ramirez S, Wiegley N, Mejia-Vilet JM. Kidney Biopsy in Management of Lupus Nephritis: A Case-Based Narrative Review. Kidney medicine. 2024 Feb:6(2):100772. doi: 10.1016/j.xkme.2023.100772. Epub 2023 Dec 6 [PubMed PMID: 38317756]
Level 3 (low-level) evidenceYap DY, Chan TM. Lupus Nephritis in Asia: Clinical Features and Management. Kidney diseases (Basel, Switzerland). 2015 Sep:1(2):100-9. doi: 10.1159/000430458. Epub 2015 Aug 5 [PubMed PMID: 27536670]
Wang H, Ren YL, Chang J, Gu L, Sun LY. A Systematic Review and Meta-analysis of Prevalence of Biopsy-Proven Lupus Nephritis. Archives of rheumatology. 2018 Mar:33(1):17-25. doi: 10.5606/ArchRheumatol.2017.6127. Epub 2017 Jul 25 [PubMed PMID: 29900975]
Level 1 (high-level) evidenceGasparotto M, Gatto M, Binda V, Doria A, Moroni G. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatology (Oxford, England). 2020 Dec 5:59(Suppl5):v39-v51. doi: 10.1093/rheumatology/keaa381. Epub [PubMed PMID: 33280015]
Hashmi AA, Ali J, Rahman M, Taseer AR, Kumar J, Irfan M. Spectrum of Morphologic Features of Lupus Nephritis According to Nephrology/Renal Pathology Society (ISN/RPS) Classification. Cureus. 2020 Sep 18:12(9):e10520. doi: 10.7759/cureus.10520. Epub 2020 Sep 18 [PubMed PMID: 33094061]
Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Current cardiology reviews. 2013 Feb 1:9(1):15-9 [PubMed PMID: 23463953]
Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D'Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noël LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney international. 2018 Apr:93(4):789-796. doi: 10.1016/j.kint.2017.11.023. Epub 2018 Feb 16 [PubMed PMID: 29459092]
Helget LN, Dillon DJ, Wolf B, Parks LP, Self SE, Bruner ET, Oates EE, Oates JC. Development of a lupus nephritis suboptimal response prediction tool using renal histopathological and clinical laboratory variables at the time of diagnosis. Lupus science & medicine. 2021 Aug:8(1):. doi: 10.1136/lupus-2021-000489. Epub [PubMed PMID: 34429335]
Umeda R, Ogata S, Hara S, Takahashi K, Inaguma D, Hasegawa M, Yasuoka H, Yuzawa Y, Hayashi H, Tsuboi N. Comparison of the 2018 and 2003 International Society of Nephrology/Renal Pathology Society classification in terms of renal prognosis in patients of lupus nephritis: a retrospective cohort study. Arthritis research & therapy. 2020 Nov 4:22(1):260. doi: 10.1186/s13075-020-02358-x. Epub 2020 Nov 4 [PubMed PMID: 33148339]
Level 2 (mid-level) evidenceLi GM, Li YF, Zeng QQ, Zhang XM, Liu HM, Feng JY, Shi Y, Wu BB, Xu H, Sun L. Lupus podocytopathy and antiphospholipid syndrome in a child with SLE: A case report and literature review. Frontiers in pediatrics. 2022:10():950576. doi: 10.3389/fped.2022.950576. Epub 2022 Aug 19 [PubMed PMID: 36061375]
Level 3 (low-level) evidenceMejia-Vilet JM, Zhang XL, Cruz C, Cano-Verduzco ML, Shapiro JP, Nagaraja HN, Morales-Buenrostro LE, Rovin BH. Urinary Soluble CD163: a Novel Noninvasive Biomarker of Activity for Lupus Nephritis. Journal of the American Society of Nephrology : JASN. 2020 Jun:31(6):1335-1347. doi: 10.1681/ASN.2019121285. Epub 2020 Apr 16 [PubMed PMID: 32300067]
Gupta R, Yadav A, Aggarwal A. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clinical rheumatology. 2021 Mar:40(3):941-948. doi: 10.1007/s10067-020-05343-6. Epub 2020 Aug 18 [PubMed PMID: 32809146]
Renaudineau Y, Chauveau D, Faguer S, Huart A, Ribes D, Pugnet G, Sailler L, Jamme T, Treiner E, Fortenfant F, Bost C, Carlé C, Belliere J. Urinary soluble CD163 is useful as "liquid biopsy" marker in lupus nephritis at both diagnosis and follow-up to predict impending flares. Journal of translational autoimmunity. 2024 Dec:9():100244. doi: 10.1016/j.jtauto.2024.100244. Epub 2024 Jun 20 [PubMed PMID: 39021518]
Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, Nagaraja H, Indrakanti D, Rovin BH. Histologic versus clinical remission in proliferative lupus nephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2017 Aug 1:32(8):1338-1344. doi: 10.1093/ndt/gfv296. Epub [PubMed PMID: 26250434]
De Rosa M, Azzato F, Toblli JE, De Rosa G, Fuentes F, Nagaraja HN, Nash R, Rovin BH. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney international. 2018 Oct:94(4):788-794. doi: 10.1016/j.kint.2018.05.021. Epub 2018 Jul 23 [PubMed PMID: 30045812]
Das U, Patel R, Guditi S, Taduri G. Correlation between the clinical remission and histological remission in repeat biopsy findings of quiescent proliferative lupus nephritis. Lupus. 2021 May:30(6):876-883. doi: 10.1177/0961203321995251. Epub 2021 Feb 20 [PubMed PMID: 33611965]
Malvar A, Alberton V, Lococo B, Lourenco M, Martinez J, Burna L, Besso C, Navarro J, Nagaraja HN, Khatiwada A, Wolf B, Rovin B. Remission of lupus nephritis: the trajectory of histological response in successfully treated patients. Lupus science & medicine. 2023 May:10(1):. doi: 10.1136/lupus-2023-000932. Epub [PubMed PMID: 37258036]
Malvar A, Alberton V, Lococo B, Ferrari M, Delgado P, Nagaraja HN, Rovin BH. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney international. 2020 Jan:97(1):156-162. doi: 10.1016/j.kint.2019.07.018. Epub 2019 Aug 20 [PubMed PMID: 31685314]
Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Annals of the rheumatic diseases. 2020 Jun:79(6):713-723. doi: 10.1136/annrheumdis-2020-216924. Epub 2020 Mar 27 [PubMed PMID: 32220834]
Plüß M, Piantoni S, Tampe B, Kim AHJ, Korsten P. Belimumab for systemic lupus erythematosus - Focus on lupus nephritis. Human vaccines & immunotherapeutics. 2022 Nov 30:18(5):2072143. doi: 10.1080/21645515.2022.2072143. Epub 2022 May 19 [PubMed PMID: 35588699]
Rúa-Figueroa Í, Salman-Monte TC, Pego Reigosa JM, Galindo Izquierdo M, Díez Álvarez E, Fernández-Nebro A, Román Ivorra JA, Calvo Penades I, Artaraz Beobide J, Calvo Alén J. Multidisciplinary consensus on the use of hydroxychloroquine in patients with systemic lupus erythematosus. Reumatologia clinica. 2024 Jun-Jul:20(6):312-319. doi: 10.1016/j.reumae.2024.03.002. Epub [PubMed PMID: 38991825]
Level 3 (low-level) evidenceCarrión-Barberà I, Salman-Monte TC, Castell S, Castro F, Ojeda F, Carbonell J. Prevalence and factors associated with fatigue in female patients with systemic lupus erythematosus. Medicina clinica. 2018 Nov 9:151(9):353-358. doi: 10.1016/j.medcli.2017.12.007. Epub 2018 Feb 10 [PubMed PMID: 29439873]
Wirestam L, Enocsson H, Skogh T, Padyukov L, Jönsen A, Urowitz MB, Gladman DD, Romero-Diaz J, Bae SC, Fortin PR, Sanchez-Guerrero J, Clarke AE, Bernatsky S, Gordon C, Hanly JG, Wallace D, Isenberg DA, Rahman A, Merrill J, Ginzler E, Alarcón GS, Chatham WW, Petri M, Khamashta M, Aranow C, Mackay M, Dooley MA, Manzi S, Ramsey-Goldman R, Nived O, Steinsson K, Zoma A, Ruiz-Irastorza G, Lim S, Kalunian K, Inanc M, van Vollenhoven R, Ramos-Casals M, Kamen DL, Jacobsen S, Peschken C, Askanase A, Stoll T, Bruce IN, Wetterö J, Sjöwall C. Osteopontin and Disease Activity in Patients with Recent-onset Systemic Lupus Erythematosus: Results from the SLICC Inception Cohort. The Journal of rheumatology. 2019 May:46(5):492-500. doi: 10.3899/jrheum.180713. Epub 2019 Jan 15 [PubMed PMID: 30647177]
Tsai WT, Chang HC, Wang CT, Chiang BL, Lin YT. Long-term outcomes in lupus patients receiving different renal replacement therapy. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2019 Aug:52(4):648-653. doi: 10.1016/j.jmii.2018.12.010. Epub 2019 Jan 4 [PubMed PMID: 30642809]
Ruiz-Irastorza G, Dueña-Bartolome L, Dunder S, Varona J, Gomez-Carballo C, Dominguez-Cainzos J, Rodrigo-Manjon A, Bueno L, Richez C, Duffau P, Blanco P, Lazaro E. Eurolupus cyclophosphamide plus repeated pulses of methyl-prednisolone for the induction therapy of class III, IV and V lupus nephritis. Autoimmunity reviews. 2021 Oct:20(10):102898. doi: 10.1016/j.autrev.2021.102898. Epub 2021 Jul 15 [PubMed PMID: 34274543]
Portalatin GM, Gebreselassie SK, Bobart SA. Lupus nephritis - An update on disparities affecting african americans. Journal of the National Medical Association. 2022 Jun:114(3S2):S34-S42. doi: 10.1016/j.jnma.2022.05.005. Epub 2022 May 18 [PubMed PMID: 35595581]
Zhang H, Zhou M, Han X, Yang Y, Yu X. Mycophenolate mofetil in the treatment of Chinese patients with lupus nephritis: A PRISMA-compliant meta-analysis. Medicine. 2020 Aug 14:99(33):e21121. doi: 10.1097/MD.0000000000021121. Epub [PubMed PMID: 32871981]
Level 1 (high-level) evidenceJiang N, Li M, Zhang H, Duan X, Li X, Fang Y, Li H, Yang P, Luo H, Wang Y, Peng L, Zhao J, Wu C, Wang Q, Tian X, Zhao Y, Zeng X. Sirolimus versus tacrolimus for systemic lupus erythematosus treatment: results from a real-world CSTAR cohort study. Lupus science & medicine. 2022 Jan:9(1):. doi: 10.1136/lupus-2021-000617. Epub [PubMed PMID: 34980680]
Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N, ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. The New England journal of medicine. 2011 Nov 17:365(20):1886-95. doi: 10.1056/NEJMoa1014460. Epub [PubMed PMID: 22087680]
Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, Vidal X, Mitjavila F, Castro Salomó A, Cuquet Pedragosa J, Ortiz-Santamaria V, Mauri Plana M, Cortés-Hernández J. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Annals of the rheumatic diseases. 2017 Sep:76(9):1575-1582. doi: 10.1136/annrheumdis-2016-210882. Epub 2017 Apr 27 [PubMed PMID: 28450313]
Level 1 (high-level) evidenceRovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G, LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis and rheumatism. 2012 Apr:64(4):1215-26. doi: 10.1002/art.34359. Epub 2012 Jan 9 [PubMed PMID: 22231479]
Jorge A, Wallace ZS, Lu N, Zhang Y, Choi HK. Renal Transplantation and Survival Among Patients With Lupus Nephritis: A Cohort Study. Annals of internal medicine. 2019 Feb 19:170(4):240-247. doi: 10.7326/M18-1570. Epub 2019 Jan 22 [PubMed PMID: 30665236]
Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, Marder W, Guyatt G, Branch DW, Buyon J, Christopher-Stine L, Crow-Hercher R, Cush J, Druzin M, Kavanaugh A, Laskin CA, Plante L, Salmon J, Simard J, Somers EC, Steen V, Tedeschi SK, Vinet E, White CW, Yazdany J, Barbhaiya M, Bettendorf B, Eudy A, Jayatilleke A, Shah AA, Sullivan N, Tarter LL, Birru Talabi M, Turgunbaev M, Turner A, D'Anci KE. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis & rheumatology (Hoboken, N.J.). 2020 Apr:72(4):529-556. doi: 10.1002/art.41191. Epub 2020 Feb 23 [PubMed PMID: 32090480]
Wahl DG, Bounameaux H, de Moerloose P, Sarasin FP. Prophylactic antithrombotic therapy for patients with systemic lupus erythematosus with or without antiphospholipid antibodies: do the benefits outweigh the risks? A decision analysis. Archives of internal medicine. 2000 Jul 10:160(13):2042-8 [PubMed PMID: 10888978]
Athanassiou P, Athanassiou L. Current Treatment Approach, Emerging Therapies and New Horizons in Systemic Lupus Erythematosus. Life (Basel, Switzerland). 2023 Jul 1:13(7):. doi: 10.3390/life13071496. Epub 2023 Jul 1 [PubMed PMID: 37511872]
Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. The Lancet. Neurology. 2009 Nov:8(11):998-1005. doi: 10.1016/S1474-4422(09)70239-X. Epub 2009 Sep 25 [PubMed PMID: 19783216]
Level 2 (mid-level) evidenceFurie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, Amoura Z, Yu X, Mok CC, Santiago MB, Saxena A, Green Y, Ji B, Kleoudis C, Burriss SW, Barnett C, Roth DA. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. The New England journal of medicine. 2020 Sep 17:383(12):1117-1128. doi: 10.1056/NEJMoa2001180. Epub [PubMed PMID: 32937045]
Level 1 (high-level) evidenceShipa M, Embleton-Thirsk A, Parvaz M, Santos LR, Muller P, Chowdhury K, Isenberg DA, Doré CJ, Gordon C, Ehrenstein MR, BEAT-LUPUS Investigators. Effectiveness of Belimumab After Rituximab in Systemic Lupus Erythematosus : A Randomized Controlled Trial. Annals of internal medicine. 2021 Dec:174(12):1647-1657. doi: 10.7326/M21-2078. Epub 2021 Oct 26 [PubMed PMID: 34698499]
Level 1 (high-level) evidenceSaxena A, Ginzler EM, Gibson K, Satirapoj B, Santillán AEZ, Levchenko O, Navarra S, Atsumi T, Yasuda S, Chavez-Perez NN, Arriens C, Parikh SV, Caster DJ, Birardi V, Randhawa S, Lisk L, Huizinga RB, Teng YKO. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis & rheumatology (Hoboken, N.J.). 2024 Jan:76(1):59-67. doi: 10.1002/art.42657. Epub 2023 Sep 15 [PubMed PMID: 37466424]
Level 1 (high-level) evidenceXipell M, Lledó GM, Egan AC, Tamirou F, Del Castillo CS, Rovira J, Gómez-Puerta JA, García-Herrera A, Cervera R, Kronbichler A, Jayne DRW, Anders HJ, Houssiau F, Espinosa G, Quintana LF. From systemic lupus erythematosus to lupus nephritis: The evolving road to targeted therapies. Autoimmunity reviews. 2023 Oct:22(10):103404. doi: 10.1016/j.autrev.2023.103404. Epub 2023 Aug 3 [PubMed PMID: 37543287]
Malvar A, Alberton V, Recalde C, Heguilen R. Repeat kidney biopsy findings of lupus nephritis patients in clinical remission treated with Mycophenolate associated with Belimumab or Mycophenolate plus standard of care therapy. A "post-hoc" analysis of participants in the BLISS-LN and open label extension study belonging to a single center. Lupus. 2023 Oct:32(12):1394-1401. doi: 10.1177/09612033231204070. Epub 2023 Sep 27 [PubMed PMID: 37754750]
Mejia-Vilet JM, Turner-Stokes T, Houssiau F, Rovin BH. Kidney involvement in systemic lupus erythematosus: From the patient assessment to a tailored treatment. Best practice & research. Clinical rheumatology. 2023 Dec:37(4):101925. doi: 10.1016/j.berh.2023.101925. Epub 2023 Dec 26 [PubMed PMID: 38151362]
Davies R, Peng SL, Lickliter J, McLendon K, Enstrom A, Chunyk AG, Blanchfield L, Wang N, Blair T, Thomas HM, Smith A, Dillon SR. A first-in-human, randomized study of the safety, pharmacokinetics and pharmacodynamics of povetacicept, an enhanced dual BAFF/APRIL antagonist, in healthy adults. Clinical and translational science. 2024 Nov:17(11):e70055. doi: 10.1111/cts.70055. Epub [PubMed PMID: 39494621]
Level 1 (high-level) evidenceJorge A, Wallace ZS, Zhang Y, Lu N, Costenbader KH, Choi HK. All-Cause and Cause-Specific Mortality Trends of End-Stage Renal Disease Due to Lupus Nephritis From 1995 to 2014. Arthritis & rheumatology (Hoboken, N.J.). 2019 Mar:71(3):403-410. doi: 10.1002/art.40729. Epub 2019 Jan 9 [PubMed PMID: 30225916]