Introduction
The lumbosacral plexus is a complex network in the pelvis formed by the anterior rami of the L1-S4 nerve roots (see Image. The Lumbosacral Nerves and the Posterior Abdominal Wall). Unlike the brachial plexus, the lumbosacral plexus has minimal nerve fascicle merging and trunk or cord formation.[1] However, the nerves that emerge directly from the plexus have intricate anatomy (see Table. Nerves of the Lumbosacral Plexus and Their Functions).[1] The nerve network comprises 2 adjacent plexuses: lumbar and sacral.
"Plexopathy" is a broad term used to describe disorders impacting the brachial or lumbosacral plexus. Lumbosacral plexopathy (LSP) encompasses a group of disorders affecting the postganglionic fibers originating from the anterior rami of the L1-S4 nerve roots.[2]
Neoplastic plexopathy often signifies advanced cancer, typically arising from local or regional tumor progression. While the condition can affect any part of the peripheral nervous system, the cervical, brachial, and lumbosacral plexi are most commonly involved.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Mechanisms of Tumor-Related Lumbosacral Plexopathy
The lumbar or lumbosacral plexus may be involved in various pathologies, with diabetic amyotrophy and idiopathic LSP being the most common.[3] Neoplasms are an important cause of LSP, especially in patients with a history of cancer. Plexopathy may be the initial presentation of cancer in approximately 15% of cases with plexus involvement, while about 1% of all cancers affect the plexus.[4] Commonly associated tumors include colorectal adenocarcinomas, cervical or uterine malignancies, lymphomas, and retroperitoneal sarcomas. In patients with a cancer history, neoplastic causes should be high on the differential for LSP.
The proposed pathogenic mechanisms of LSP are varied and include:
- Direct neoplastic infiltration or compression from nearby abdominopelvic organs, including but not limited to the bladder, colon, ovaries, and cervix.
- Metastatic disease from various neoplasms, but most commonly lung or breast malignancies and lymphomas.
- Lumbosacral plexus compression from enlarged metastatic retroperitoneal lymph nodes.
- Epineural spread via direct endoneurial invasion by neurotrophic neoplastic cells secondary to lymphoma leads to an entity known as "intraneural lymphomatosis."[5] This mechanism is most common in patients with non-Hodgkin lymphoma; the incidence in these patients is approximately 0.2%. this presentation is most likely to occur in patients with diffuse large B-cell lymphoma.[6] The peripheral nervous system is a sanctuary site for lymphoma cells, allowing them to evade chemotherapy. Lymphoma may, therefore, initially present in this area or reappear after remission, often causing severe neuropathy that may appear symmetrically or asymmetrically.[7][8] The tumor can invade the plexus directly or track along the connective tissue or the epineurium of nerve trunks. This tendency to infiltrate along the nerves, which is difficult to demonstrate with imaging procedures, may explain the frequent discordance between the physical examination and the lack of findings on imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI).
- Perineural spread is rare but most often arises in patients with prostate cancer.[9] Capek et al reported a case of bilateral LSP from prostate cancer. The mechanism of tumor spread may occur through the dural sac, which acts as a bridge connecting to the opposite side.[10][11][10]
- Radiation-induced LSP can occur following radiation treatment for radiation-sensitive pelvic tumors, such as ovarian, cervical, colon, testicular, and prostate cancers, as well as Hodgkin lymphoma involving paraaortic and pelvic nodes. Symptoms may develop up to 6 months after therapy. Injury mechanisms include direct toxic effects on axons and the vasa nervorum, leading to secondary nerve microinfarction and a dose-dependent increase in severity.[12] Concurrent chemotherapy may further elevate the risk of radiation plexopathy.
- Growth of primary neural tumors, including neurofibroma and perineuroma.
Mechanisms of Non–Tumor-Related Lumbosacral Plexopathy
Traumatic plexopathy may arise following high-velocity injuries, pelvic fractures, penetrating injuries such as gunshot wounds, and, in rare cases, intrapartum compression by the fetal head. Traumatic plexopathy often presents as brachial plexopathy but may manifest as lumbar or lumbosacral plexopathy, particularly when neoplastic processes involve nearby pelvic organs. The retroperitoneal and intrapelvic location of the lumbosacral plexus generally offers protection from trauma.[13][14]
Ischemic plexopathy may develop from microvascular damage caused by conditions like diabetes and vasculitis, leading to ischemia of the lumbosacral plexus. Ischemic plexopathy may also result from postsurgical complications, infections in adjacent organs, and infiltrative diseases such as amyloidosis.[15]
Epidemiology
The frequency of neoplastic LSP is 0.71% in cancer patients. The frequency of neoplastic brachial plexopathy is lower at 0.43%. Neoplastic LSP is more commonly associated with pelvic and colorectal tumors but has also been observed with lymphomas and breast cancer.[16]
Pathophysiology
Neuroanatomical Considerations
To localize the area of involvement, a clinician must understand the basic neuroanatomy of the lumbosacral plexus, which enables the use of appropriate diagnostic and therapeutic procedures. Additionally, distinguishing plexus involvement from neoplastic epidural cord compression or meningeal pathology is crucial, as these conditions may present similarly and occur simultaneously.
The lumbosacral plexus consists of both the lumbar and sacral plexi, which arise from the L1-S4 nerve roots, with a contribution from the T12 nerve root. The lumbar and sacral plexi are connected by the lumbosacral trunk formed from the L4–L5 nerve roots; the lumbosacral truck crosses over the sacral ala at the pelvic brim.
Lumbar plexus
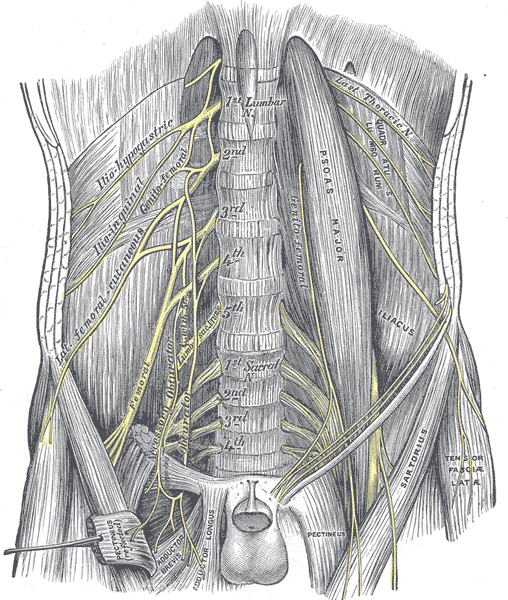
The lumbar plexus is a network of nerves formed by the anterior rami of the nerve roots of L1 to L4, with a contribution from the subcostal nerve (T12) (see Table. Nerves of the Lumbosacral Plexus and Their Functions). This plexus forms within the psoas major muscle in the posterior abdominal wall, anterolateral to the L1-L4 vertebral bodies (see Image. The Lumbosacral Nerves in the Abdominopelvic Cavity).
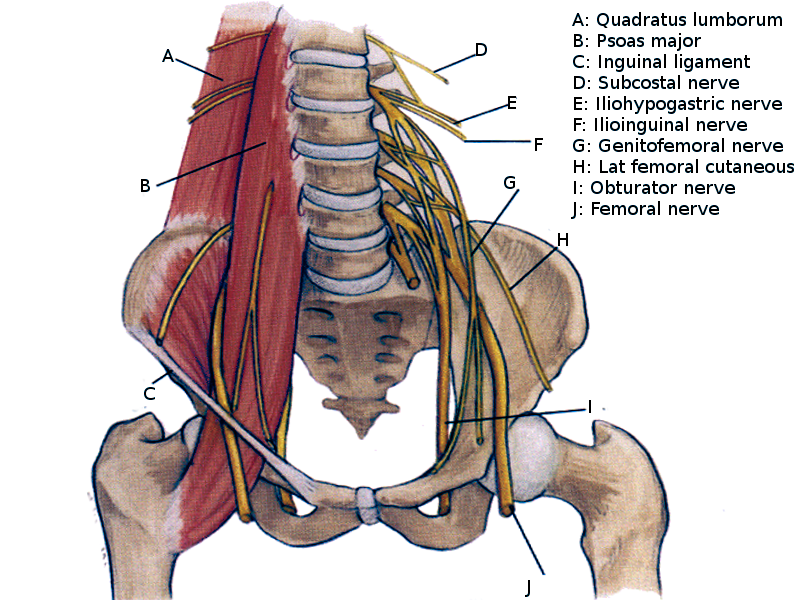
The iliohypogastric (L1), ilioinguinal (L1), and genitofemoral (L1-L2) nerves are direct branches of the upper lumbar plexus, carrying sensory information from the lower abdominal skin, lateral genitalia, and medial groin of the upper thigh. The L1-L4 nerve roots of then divide into anterior (ventral) and posterior (dorsal) divisions. The posterior division forms the lateral femoral cutaneous nerve (L2-L3), which supplies sensation to the anterolateral hip and thigh, and the femoral nerve (L2–L4), responsible for motor innervation of the iliopsoas and quadriceps muscles and sensory innervation to the anterior thigh and medial leg (saphenous branch). The anterior division forms the obturator nerve (L2–L4), which provides motor function to the adductor and gracilis muscles and sensory input to the medial thigh (see Image. Lumbar Plexus).
Lumbosacral trunk
The anterior rami of L4 and L5 form the lumbosacral trunk. This nerve trunk connects the lumbar plexus to the sacral plexus.
Sacral plexus
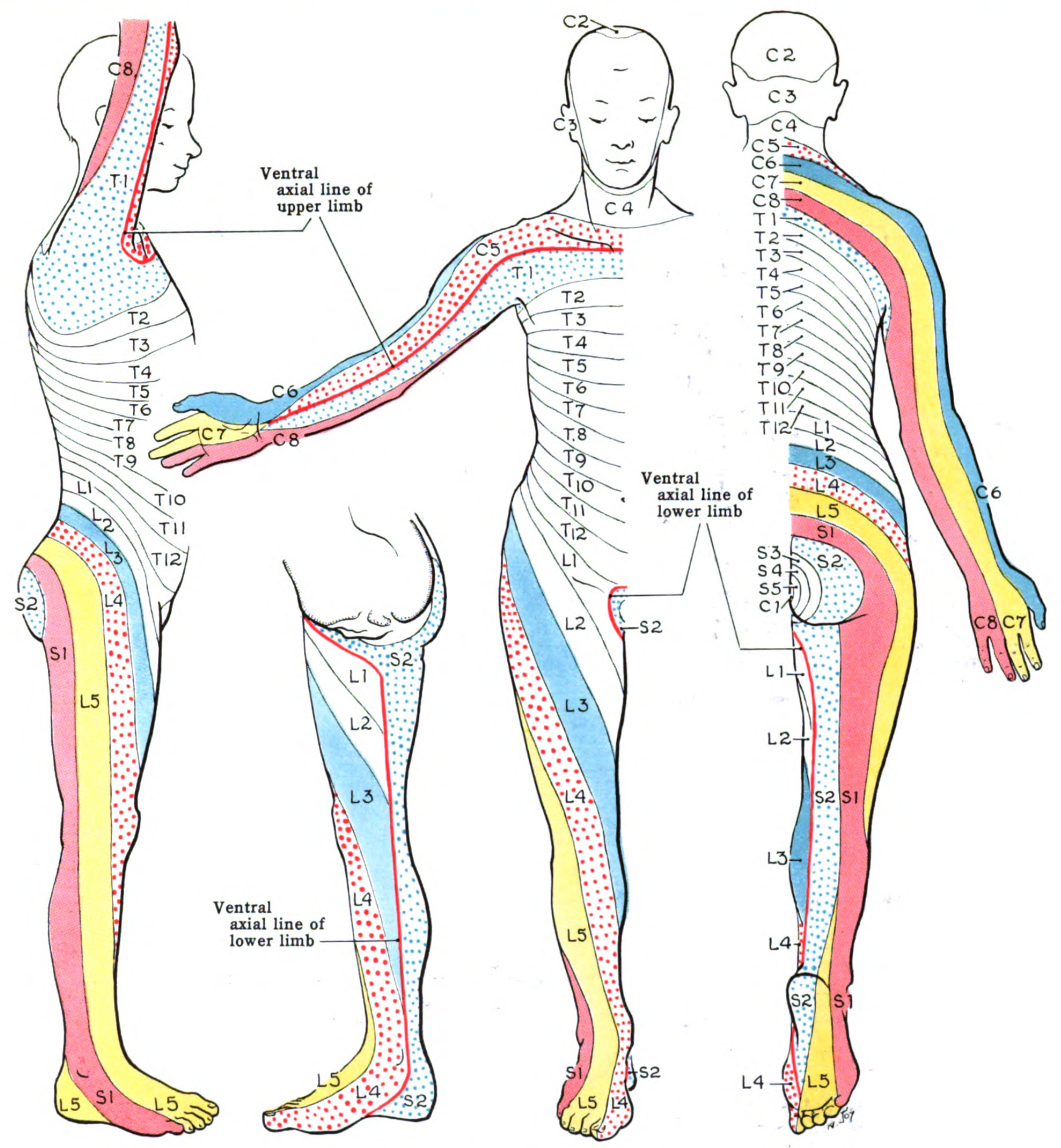
The lumbosacral trunk (L4-L5) and the anterior rami of S1 to S4 form the sacral plexus, which innervates the pelvis, gluteal region, and lower limbs. The posterior division gives rise to the superior gluteal nerve (L4–S1), which innervates the gluteus medius and minimus and the tensor fascia lata; the inferior gluteal nerve (L5–S2), which supplies the gluteus maximus; and the fibular portion of the sciatic nerve, which innervates the leg's anterior and lateral compartments. The anterior division forms the pudendal nerve (S2-S4), which provides sensory and motor innervation to the perineum; the posterior cutaneous nerve of the thigh (S1-S3), which innervates the skin of the posterior thigh; and the tibial portion of the sciatic nerve, which innervates lower leg muscles and provides sensory input to the sole (see Image. Dermatome Map).
Table. Nerves of the Lumbosacral Plexus and Their Functions
| Nerve | Dermatome supplied | Muscle supplied | |
| Lumbar plexus | |||
| Iliohypogastric (L1) | Inferior abdominal wall | None | |
| Ilioinguinal (L1) | Medial groin | None | |
| Genitofemoral (L1-2) | None | None | |
| Lateral femoral cutaneous (L3-L4) | Anterolateral thigh | None | |
| Obturator (L2-L4) | None | Adductor longusAdductor magnusGracilis | |
| Femoral (L2-L4) | None | Quadriceps | |
| Saphenous (L2-L4) | Medial leg and foot | None | |
| Sacral Plexus | |||
| Superior gluteal (L4-L5) | None | Gluteus mediusTensor fascia lata | |
| Inferior gluteal (L4-S1) | None | Gluteus maximus | |
| Sciatic (L4-S2) | Foot and lateral leg | Anterior tibialisPeroneus longusGastrocnemiusSoleusFoot muscles | |
| Pudendal (S2-S4) | Perineal | External anal sphincter |
History and Physical
Clinical Syndromes of Lumbosacral Plexopathy
LSP may present with symptoms indicating involvement of the upper plexus (L1-L4), lumbosacral trunk (L4-L5), or lower plexus (S1-S4). Neoplastic causes result in bilateral, often asymmetric, symptoms in approximately 2/3 of patients with LSP.
Pain is the predominant symptom in LSP, occurring in 98% of cases. The absence of this symptom should raise suspicion of alternative diagnoses like myopathy or neuropathy. LSP generally begins with leg pain, followed by numbness and weakness. The pain is typically dull and aching, with sharp radicular components. Pain may worsen when supine and is often exacerbated by prolonged ambulation, sitting, or a Valsalva maneuver. Patients with iliopsoas muscle involvement may prefer to rest with their hips flexed, a position similar to that observed in meningitis.
Upper lumbar plexus involvement
With upper lumbar plexus pathology, sensory loss primarily affects the anterolateral and medial thigh, lower abdomen, and groin due to the involvement of the iliohypogastric, ilioinguinal, and genitofemoral nerves. Weakness can impact the hip flexors, including the iliopsoas muscle, innervated by the femoral nerve, and the hip adductors, innervated by the obturator nerve. The quadriceps, responsible for knee extension and innervated by the femoral nerve, may also be affected. Pain may extend from the costovertebral area to the upper thigh and groin.
Lower lumbar plexus and lumbosacral trunk involvement
This region has L4 and L5 contributing to the sacral plexus. Sensory loss affects the anterolateral leg and dorsum of the foot due to partial involvement of the femoral nerve (L2-L4) and contribution from the lumbosacral trunk (L4-L5). Weakness can impact ankle dorsiflexion (tibialis anterior, innervated by the deep fibular nerve), toe extension (extensor digitorum longus and brevis, supplied by the deep fibular nerve), and foot eversion (fibularis longus and brevis, innervated by the superficial fibular nerve). Additionally, weakness may be present in knee flexion (the hamstrings, supplied by the sciatic nerve) and hip extension (gluteus maximus, innervated by the inferior gluteal nerve). Pain may radiate down the posterior leg and into the foot.
Sacral plexopathy
Sacral plexopathy must be distinguished from lower lumbar plexopathy. Sensory loss affects the posterior thigh, leg, and foot, including the sole. Weakness involves muscles innervated by the sciatic nerve and its branches (tibial and common fibular nerves), leading to difficulty with plantar flexion (gastrocnemius and soleus), toe flexion, ankle dorsiflexion, foot eversion, and, potentially, hip extension and knee flexion. Pain commonly affects the lower back, buttocks, and thighs and may radiate down the posterior leg.
Other Considerations
Neurologic deficits occur in most patients with LSP (60%) but generally develop later. In contrast, sensory disturbances often precede pain in radiation-induced plexopathy. Common deficits, in order of frequency, include motor weakness (86%), sensory disturbances (73%), and hyporeflexia (64%). Leg edema is noted in approximately 47% of patients. Weakness commonly affects the thigh muscles, often causing difficulty rising from a chair or climbing stairs.
LSP affecting the lower plexus is frequently associated with colorectal neoplasms and cervical carcinoma. In about a third of patients, a "hot, dry foot" is described due to sympathetic involvement. Numbness typically affects the groin, anterolateral or posterior thigh, or dorsum of the foot, occasionally accompanied by foot drop.
Bilateral plexus involvement can lead to incontinence and impotence. In rare cases, examination may reveal a palpable rectal mass, decreased anal sphincter tone, and sensory loss in the perineal area.
Evaluation
Diagnostic Approach to Lumbosacral Plexopathy
A comprehensive approach to diagnosing LSP involves obtaining a detailed medical history, thorough physical examination, and appropriate investigations. A history of cancer in a patient presenting with neurological deficits in the territories supplied by the lumbosacral plexus should raise suspicion of neoplastic LSP.
History and physical examination
A detailed history should be obtained, focusing on the onset and progression of symptoms, noting whether they are acute, subacute, or chronic. Pain characteristics should be documented, including type (such as burning, shooting, or aching), location, and radiation. Associated symptoms, such as numbness, weakness, bowel or bladder dysfunction, and gait disturbances, should be recorded. Risk factors, including diabetes, recent surgeries, infections, a history of radiation therapy, and any personal or family history of cancer, should also be assessed.
The physical examination requires a comprehensive neurological assessment. Motor strength should be tested using the Medical Research Council (MRC) scale to evaluate key muscle groups in the lower limbs. Reflex testing should be performed, including patellar, Achilles, and plantar reflexes. Sensory testing should assess light touch, pinprick, vibration, and proprioception in the lower limbs. Gait should be observed for abnormalities, such as foot drop or a limp, and signs of lower plexus involvement should be evaluated, including decreased anal sphincter tone and sensory loss in the perineal area.
Neuroimaging
MRI is preferred for its superior soft tissue resolution, ability to differentiate tumor types, and greater sensitivity in detecting plexus abnormalities. Common MRI findings in lumbosacral plexopathy include increased T2-weighted signals within the plexus, indicating edema in affected nerves, frequently associated with tumor involvement and radiation-induced LSP. Enlarged nerves with irregular peripheral enhancement and a cystic component may also appear in tumor involvement and radiation-induced LSP, though enhancement is usually less prominent in the latter. Smooth peripheral enhancement is more commonly observed in nonneoplastic cases of lumbosacral plexopathy. CT may be used as an alternative when MRI is contraindicated or unavailable, particularly in cases of suspected trauma.
Electrodiagnostic studies
Nerve conduction studies (NCS) may reveal decreased amplitudes of compound motor and sensory nerve action potentials, often asymmetrically, with normal or mildly reduced conduction velocities. Prolonged F-waves may be detected on the affected side.
Needle electromyography (EMG) is essential for confirming LSP, distinguishing it from radiculopathy or other neuropathies, and localizing the lesion within the plexus. Findings may show more extensive denervation than clinically evident, absence of paraspinal muscle fibrillations, and involvement of muscles from at least 2 lumbosacral root levels and 2 peripheral nerves. Myokymic discharges may be noted in cases of radiation-induced LSP. Needle EMG abnormalities in proximal muscles innervated by nerves such as the inferior gluteal nerve (gluteus maximus) or superior gluteal nerve (gluteus medius, tensor fasciae lata) aid in differentiating sacral plexopathy from sciatic neuropathy, as these abnormalities typically indicate sacral plexus rather than sciatic nerve involvement.
Other investigations
Positron emission tomography (PET) with 2-fluoro-2-deoxyglucose (2-FDG) is useful in detecting active neoplastic involvement of the plexus. Lumbar puncture with cerebrospinal fluid analysis may reveal elevated protein levels and positive cytology in cases of neoplastic or inflammatory LSP. A fascicular nerve biopsy may be needed to confirm neoplastic etiologies in rare cases where imaging and EMG results remain inconclusive.
Treatment / Management
The management of neoplastic LSP involves an interprofessional approach focused on controlling tumor growth, relieving pain, and preserving function. The interventions include radiotherapy, pain management, and physical therapy.
Radiotherapy
The primary goals of radiotherapy are to control tumor growth, prevent progression, alleviate pain, and enhance the patient's quality of life. Doses above 300 cGy generally show better response rates, but the optimal dose and fractionation require tailoring to factors such as tumor type, location, and patient health. Treatment response may take an average of 4 months to become evident. Alternatives to radiotherapy, including chemotherapy and surgery, may be administered as adjuncts or replacements, depending on the case.
Common adverse effects of radiotherapy include dysesthesias, causalgia, chronic pain syndromes, skin changes, fatigue, and bowel or bladder dysfunction. Adverse effect management includes administering medications such as gabapentinoids (eg, gabapentin and pregabalin), tricyclic antidepressants (eg, amitriptyline and nortriptyline), serotonin or norepinephrine reuptake inhibitors (eg, duloxetine and venlafaxine), and opioids for refractory pain. Additional options include topical agents like lidocaine patches and capsaicin cream, along with physical and occupational therapy, psychological support, transcutaneous electrical nerve stimulation (TENS) units, sympathetic ganglion blocks, and, in rare cases, plexus dissection and neurolysis.
Pain Management
Effective pain control requires a multimodal approach. Physical modalities such as heat, cold, and TENS units offer symptom relief. Oral and topical medications like those used to mitigate adverse radiation effects are helpful; opioids may be considered when pain remains refractory to other treatments. Interventional therapies, including nerve blocks, epidural steroid injections, spinal cord stimulators, and, in severe cases, intrathecal baclofen, may be utilized for further pain control.
Physical Therapy
Early intervention prevents muscle atrophy, contractures, and functional decline. Strengthening exercises improve motor balance and function. For example, isometric and isotonic exercises target the hip flexors, knee extensors, and ankle dorsiflexors. Flexibility exercises maintain the range of motion and prevent contractures, while gait training enhances walking ability and balance. Orthotic devices, such as ankle-foot orthoses for foot drop, provide support for ambulation and weakened limbs.
Differential Diagnosis
When evaluating cancer-related LSP, other conditions presenting with similar symptoms should be considered. Some of these diagnoses include:
- Chemotoxic plexopathy arises from direct toxicity to the plexus by chemotherapy agents, typically associated with intraarterial chemotherapy administration.
- Paraneoplastic plexopathy is a rare immune-mediated condition that involves the immune response to an underlying malignancy that targets the plexus.
- Postinfectious plexopathy can occur following infections and may mimic cancer-related plexopathy.
- Leptomeningeal involvement or epidural cord compression due to neoplastic infiltration can affect nerve roots at the vertebral foramina.
- Primary plexus tumors are rare tumors originating directly from the nerve plexus.
- Lumbosacral radiculopathy due to nerve root compression or irritation may mimic plexopathy, though usually with a more restricted symptom distribution.
- Lower extremity mononeuropathy of the femoral, peroneal, or tibial nerves can produce localized symptoms that resemble plexopathy.
- Mononeuritis multiplex involves multiple isolated nerves and may be mistaken for plexopathy.
- Orthopedic disorders like hip osteoarthritis, fractures, or avascular necrosis can present with pain and functional limitations in the lower limbs, potentially mimicking LSP.
Effective management relies on a thorough clinical examination and prudent use of diagnostic testing.
Radiation Oncology
Differentiating Radiation-Induced from Neoplastic Lumbosacral Plexopathy
Radiation-induced LSP is a potential complication of radiation therapy for pelvic or abdominopelvic tumors. Distinguishing this process from recurrent neoplastic disease can be challenging, as these conditions may coexist.
The incidence of radiation-induced plexopathy, including cervical, brachial, and lumbosacral plexopathies, ranges from 1.8% to 4.9% among treated patients. Symptoms can develop between 3 months and 14 years after radiation, with a median onset of approximately 1.5 years. Risk factors for radiation-induced plexopathy include higher radiation doses, hyperfractionated regimens, concurrent chemotherapy with taxane and platinum agents, and hot spot high dosing at field junctions. Other contributing factors include older age, obesity, high blood pressure, abnormal lipid levels, diabetes, and prior surgery in the irradiated region.
The distinguishing clinical features of radiation-induced LSP include sensory symptoms such as dysesthesias, numbness, and sensory loss, often in a stocking-glove distribution. Lymphedema may also be present. Pain is less common, occurring in about 10% of cases, but when evident, it tends to be milder and appears later than in neoplastic LSP. Bilateral involvement is more common in radiation-induced plexopathy, and the condition may exhibit periods of quiescence with intermittent stability and exacerbation. Progression is typically slower and more gradual compared to the rapid onset seen in neoplastic LSP.
EMG may show myokymic discharges in up to 60% of patients with radiation-induced plexopathy, although their absence does not exclude the diagnosis. EMG is useful for assessing the extent and distribution of nerve damage. MRI often reveals an increased T2 signal, indicating edema within the affected nerves. However, nerve enhancement is typically absent in radiation-induced plexopathy, unlike neoplastic LSP. Local tissue necrosis may also be observed. MRI can help exclude other causes of plexopathy. PET with 2-FDG may be used to detect recurrent tumors, but it often shows a negative result in RILSP. In uncertain cases, a nerve biopsy may be considered for further evaluation.
The management of radiation-induced plexopathy is primarily symptom-based and may include pain medications such as gabapentinoids, tricyclic antidepressants, and other pain-relieving medications. Physical therapy is recommended to maintain strength, flexibility, and range of motion, while occupational therapy helps patients with activities of daily living and the use of adaptive equipment. Hyperbaric oxygen therapy has some supportive evidence, though its role remains controversial. Other potential therapies, including neuroprotective agents and immunomodulatory treatments, are currently under investigation.
Prognosis
Patients presenting with neoplastic LSP often have a poor prognosis. The malignancy in these individuals is typically already in an advanced stage, which can significantly impact life expectancy. However, prognosis also varies from person to person, depending on factors like cancer type and stage, overall health, and response to treatment.
Even in the context of a poor prognosis, the focus of management should be on improving the quality of life, managing pain, and maintaining independence. Comfort, function, and emotional well-being must be prioritized. A multimodal approach should be employed to control pain and enhance daily living effectively. The patient's ability to engage in activities that they value must be supported.
Complications
Radiation therapy targeting pelvic or abdominopelvic tumors may lead to various early and late adverse effects. Early adverse effects include skin reactions such as erythema, desquamation, and dryness, along with fatigue and gastrointestinal symptoms like nausea, vomiting, and diarrhea. Late adverse effects may include radiation-induced plexopathy dysesthesias, causalgia (intense burning neuropathic pain often triggered by light touch), chronic pain syndromes related to nerve damage, and fibrosis, which results in scar tissue formation leading to stiffness and decreased range of motion. Muscle weakness and bowel and bladder dysfunction may also occur due to the radiation's effects on pelvic organs.
Deterrence and Patient Education
An interprofessional approach to decision-making is essential to guide treatment effectively. Management options and potential side effects must be thoroughly discussed. Each option should be carefully weighed. Treatment goals should align with the patient’s specific needs and circumstances, and side effects must be proactively managed.
Enhancing Healthcare Team Outcomes
While managing the primary cancer remains important, the focus often shifts toward palliation—relieving symptoms and maximizing quality of life. Palliation requires an interprofessional approach involving oncologists, neurologists, radiation therapists, pain specialists, physical therapists, and other healthcare professionals. Shared decision-making is essential, ensuring that the patient and their family are actively involved in developing a care plan that aligns with their goals and preferences.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
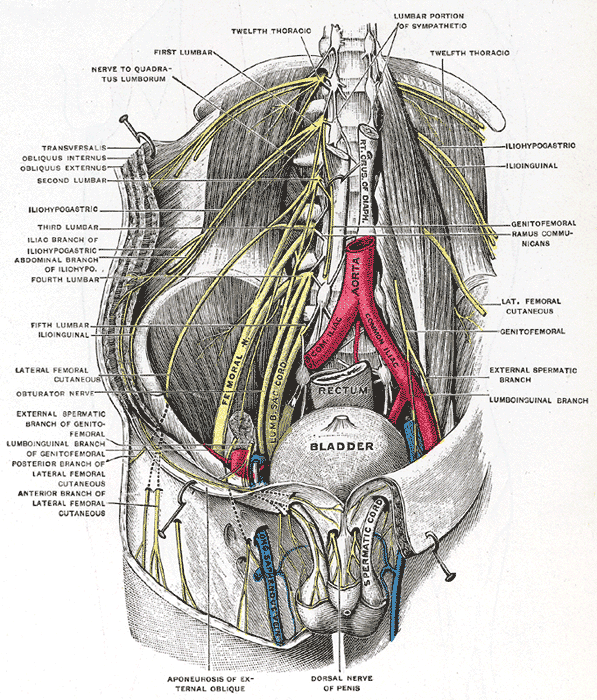
The Lumbosacral Nerves in the Abdominopelvic Cavity. This illustration shows the lumbosacral nerves and their anatomic relationships within the abdominopelvic cavity.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rubin DI. Brachial and lumbosacral plexopathies: A review. Clinical neurophysiology practice. 2020:5():173-193. doi: 10.1016/j.cnp.2020.07.005. Epub 2020 Aug 13 [PubMed PMID: 32954064]
Almeida LR, Faustino D, Esteves LR, Gante C, Soares AW, Oliveira T, Dias JL, Dias L. Radiation-Induced Lumbosacral Plexopathy. Cureus. 2023 Mar:15(3):e36842. doi: 10.7759/cureus.36842. Epub 2023 Mar 29 [PubMed PMID: 37123691]
Ng PS, Dyck PJ, Laughlin RS, Thapa P, Pinto MV, Dyck PJB. Lumbosacral radiculoplexus neuropathy: Incidence and the association with diabetes mellitus. Neurology. 2019 Mar 12:92(11):e1188-e1194. doi: 10.1212/WNL.0000000000007020. Epub 2019 Feb 13 [PubMed PMID: 30760636]
Lee EQ. Nervous system metastases from systemic cancer. Continuum (Minneapolis, Minn.). 2015 Apr:21(2 Neuro-oncology):415-28. doi: 10.1212/01.CON.0000464178.81957.18. Epub [PubMed PMID: 25837904]
Bourque PR, Sampaio ML, Warman-Chardon J, Samaan S, Torres C. Neurolymphomatosis of the lumbosacral plexus and its branches: case series and literature review. BMC cancer. 2019 Nov 27:19(1):1149. doi: 10.1186/s12885-019-6365-y. Epub 2019 Nov 27 [PubMed PMID: 31775683]
Level 2 (mid-level) evidenceChoi YJ, Shin JA, Kim YH, Cha SJ, Cho JY, Kang SH, Yi SY, Lee HR. Neurolymphomatosis of Brachial Plexus in Patients with Non-Hodgkin's Lymphoma. Case reports in oncological medicine. 2013:2013():492329. doi: 10.1155/2013/492329. Epub 2013 Nov 12 [PubMed PMID: 24324902]
Rush RP, Saltman AP, Prica AA, Breiner A, Detsky AS. Connecting the Dots. The New England journal of medicine. 2017 Sep 7:377(10):978-984. doi: 10.1056/NEJMcps1613804. Epub [PubMed PMID: 28877025]
Baehring JM, Batchelor TT. Diagnosis and management of neurolymphomatosis. Cancer journal (Sudbury, Mass.). 2012 Sep-Oct:18(5):463-8 [PubMed PMID: 23006953]
Capek S, Howe BM, Amrami KK, Spinner RJ. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurgical focus. 2015 Sep:39(3):E14. doi: 10.3171/2015.7.FOCUS15209. Epub [PubMed PMID: 26323816]
Capek S, Howe BM, Tracy JA, García JJ, Amrami KK, Spinner RJ. Prostate cancer with perineural spread and dural extension causing bilateral lumbosacral plexopathy: case report. Journal of neurosurgery. 2015 Apr:122(4):778-83. doi: 10.3171/2014.12.JNS141339. Epub 2015 Feb 6 [PubMed PMID: 25658791]
Level 3 (low-level) evidenceLee BC, Kim SW, Kim DH, Yoon YC, Kim CK, Sung DH. Lumbosacral plexopathy caused by the perineural spread of pelvic malignancies: clinical aspects and imaging patterns. Acta neurochirurgica. 2022 Jun:164(6):1509-1519. doi: 10.1007/s00701-022-05194-x. Epub 2022 Apr 21 [PubMed PMID: 35445854]
Jaeckle KA. Neurologic manifestations of neoplastic and radiation-induced plexopathies. Seminars in neurology. 2010 Jul:30(3):254-62. doi: 10.1055/s-0030-1255219. Epub 2010 Jun 24 [PubMed PMID: 20577932]
Wilbourn AJ. Plexopathies. Neurologic clinics. 2007 Feb:25(1):139-71 [PubMed PMID: 17324724]
Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. The New England journal of medicine. 1999 Apr 15:340(15):1137-43 [PubMed PMID: 10202164]
Level 1 (high-level) evidenceDyck PJ, Thaisetthawatkul P. Lumbosacral plexopathy. Continuum (Minneapolis, Minn.). 2014 Oct:20(5 Peripheral Nervous System Disorders):1343-58. doi: 10.1212/01.CON.0000455877.60932.d3. Epub [PubMed PMID: 25299286]
Level 3 (low-level) evidenceJaeckle KA, Young DF, Foley KM. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985 Jan:35(1):8-15 [PubMed PMID: 2981417]