Introduction
Myocardial perfusion scanning is crucial for diagnostic and therapeutic decision-making in cardiac diseases, especially coronary artery disease (CAD). The term "myocardial perfusion scanning" refers to a group of noninvasive imaging tests that help clinicians assess blood flow to the myocardium. The information obtained from myocardial perfusion scans is essential for determining the most appropriate medical treatment or intervention to optimize cardiac health.
Myocardial perfusion scanning is valuable for both diagnostic and prognostic purposes in various clinical scenarios. These include evaluating angina and related symptoms, ruling out acute coronary syndrome as the cause of chest pain, assessing therapeutic outcomes after interventions, and identifying areas of viable or scarred myocardium. This information enables clinicians to evaluate a patient's coronary health comprehensively, perform risk stratification for future cardiovascular events, assess therapeutic responses to interventions addressing perfusion defects, and provide accurate prognostication.[1][2]
Perfusion scanning utilizes various radiotracers that spread to multiple tissues after administration.[3] These radiotracers emit photons detectable by a γ-camera, which typically contains a single sodium iodide crystal in single-photon emission computed tomography (SPECT), or multiple crystals in positron emission tomography (PET), to interact with captured photons. The γ-camera is equipped with a collimator to reduce background noise and a photomultiplier, which converts photon-crystal interactions into electrical signals to generate detailed images.[4]
Commonly used radiotracers in SPECT imaging techniques include thallous chloride Tl-201 (201Tl) and technetium-based radiotracers, such as technetium-99m (Tc-99m) sestamibi and Tc-99m tetrofosmin.[5] 201Tl is distributed actively into myocardial cells, whereas technetium-based products spread passively, depending on blood flow and myocardial viability.[6] These radiotracers are injected when the heart is stressed through exercise or pharmacological induction. Radiotracer uptake reveals areas of perfusion and viable tissue during both stress and rest. Areas with poor perfusion that show improvement during rest are referred to as "reversible ischemia."[7]
Specimen Collection
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Specimen Collection
SPECT, now more commonly used and available in clinical practice, reconstructs a 3-dimensional representation of myocardial perfusion from planar images. Unlike planar imaging, SPECT produces sequential slices with enhanced resolution, reducing overlap between normal and abnormal areas.[8] SPECT imaging has been validated in multiple large-scale studies for detecting CAD. However, its limitations include artifacts caused by motion, attenuation, or extracardiac activity, which can affect image quality and increase reader variability.[9][10][11] Additionally, SPECT typically utilizes Tc-99m tracers with low first-pass extraction, resulting in an underestimation of the extent and severity of ischemic changes.[12]
Although less widely available than SPECT, PET imaging addresses several limitations. PET provides superior spatial resolution and more precise attenuation correction compared to SPECT.[13] The high temporal resolution of PET scanning enables the quantification of myocardial blood flow and myocardial flow reserve, offering valuable insights for risk stratification and cardiovascular mortality assessment.[14] Furthermore, PET imaging features faster protocols and lower radiation exposure than SPECT techniques.[15]
PET imaging uses radiotracers such as N-13 ammonia, rubidium-82 chloride (Rb-82), and flurpiridaz F-18 (flurpiridaz) for myocardial perfusion imaging. Rb-82, the most commonly used tracer, produces good-quality images with a myocardial extraction rate of 65%. In contrast, N-13 ammonia and flurpiridaz offer higher myocardial extraction rates of 80% and 95%, respectively, resulting in higher-resolution images. However, these tracers require an on-site cyclotron. Rb-82 is ideal for pharmacological stress testing, while N-13 ammonia and flurpiridaz can be utilized for both exercise and pharmacological stress testing.
Exercise and pharmacological testing are options for obtaining stress images. Common pharmacologic agents include regadenoson, adenosine, and dipyridamole, all of which induce coronary vasodilation, resulting in differences in blood flow. Adenosine and dipyridamole act on A-2A, A-1, A-2B, and A-3 receptors, which can lead to adverse effects such as bronchospasm, atrioventricular nodal block, chest tightness, and flushing. Regadenoson, a selective A-2A receptor agonist, is often preferred for patients with bronchospasm and has become the most commonly used pharmacological agent in clinical practice.
Procedures
According to the 2018 guidelines from the American Society of Nuclear Cardiology, recommendations for conventional SPECT myocardial perfusion imaging are provided. These guidelines address acquisition and instrumentation, as explained below.
Detectors
Detectors are essential in SPECT imaging, as they detect photon events and generate datasets for image reconstruction. In a single non-pixelated crystal system, the Anger camera, which uses a large thallium-doped sodium iodide crystal, is a primary component of the SPECT camera. This crystal works in conjunction with a series of large photomultiplier tubes to convert γ-rays into electronic signals. One or more of these crystal systems rotate around the patient’s body. Thicker crystals offer improved sensitivity but result in lower intrinsic resolution. Typically, 9.5-mm crystals are used for technetium and thallium tracers. Other detector types include pixelated scintillation crystals with photodiode arrays and semiconductor radiation detectors.
Collimators
Collimators are components that define the direction of absorbed photons, limiting both their number and direction to a fraction of the total detected. This limitation results in reduced spatial resolution due to the varying incident angles that pass through the collimator. Narrowing the range of incident angles improves spatial resolution but reduces sensitivity. Thus, collimators are crucial in determining image quality. The 3 types of collimators used in nuclear cardiology are parallel hole, cardiofocal, and pinhole.
System Design
A conventional SPECT imaging system consists of one or more Anger cameras mounted on a gantry that rotates around the patient at a fixed distance. Images are acquired as the gantry rotates in either a step-and-shoot or continuous mode. Imaging occurs over a 180° arc, from right anterior oblique to left posterior oblique, using 2 detectors positioned 90° apart.
Image Acquisition
Multiple protocols are available for obtaining SPECT imaging to assess myocardial perfusion and tissue viability. No single protocol is universally applicable, as studies must be individualized based on the diagnostic information required by the clinician and the specific characteristics of each patient. The most commonly used radiotracers in SPECT imaging include Tc-99m and 201Tl. Below are 2 examples of protocols used in SPECT imaging.
Technetium-99m 1-day rest-stress imaging protocol: Approximately 8 to 12 mCi of Tc-99m radiotracer is injected intravenously. Resting myocardial perfusion images are acquired 30 to 60 minutes later. Depending on patient characteristics and availability, a vasodilator is administered to induce pharmacological stress. Potential vasodilators that may be used during the test include dipyridamole (0.56 mg/kg), adenosine (140 mcg/kg/min for a 6-min infusion), and regadenoson (0.4 mg injection). A second dose of radiotracer (24-36 mCi) is administered, and stress images are obtained 15 to 45 minutes thereafter. Rest and stress images are processed for review and interpretation by a trained professional.
Thallous chloride 201Tl stress-rest redistribution imaging protocol: Depending on patient characteristics and availability, a vasodilator is administered to induce pharmacologic stress. Vasodilators that may be used include dipyridamole (0.56 mg/kg), adenosine (140 mcg/kg/min for a 6-min infusion), and regadenoson (0.4 mg IV). Additionally, 2.5 to 3.0 mCi of 201Tl is administered.
Stress images are obtained and reviewed 15 minutes after administration. Depending on the image interpretation, an optional rest image may be acquired either 2.5 to 4 hours later or 24 hours after the initial radiotracer injection. The images are processed for interpretation to identify areas of poor myocardial perfusion or viability, particularly if redistribution images were obtained later.[16]
Positron Emission Tomography Image Acquisition
PET-computed tomography (CT) scanning procedures are similar to those for SPECT. In one protocol, 60 mCi of Rb-82 chloride is infused in 50 ml of normal saline over 25 seconds. Rest images are then acquired 90 seconds after the infusion. A vasodilator is then administered, such as dipyridamole (0.57 mg/kg for 4 min) or regadenoson (0.4 mg IV given over 10 s), followed by a 5-mL saline flush. If dipyridamole is used, a 3-minute waiting period occurs before starting the Rb-82 chloride infusion. With regadenoson, the Rb-82 chloride infusion begins immediately after the saline flush. Images are obtained approximately 90 seconds after the Rb-82 infusion is completed.[17]
Processing and reconstruction: After image acquisition, processing involves filtering to reduce noise. Typically, low-pass filters are used to permit low-frequency signals while blocking higher frequencies. Software packages offer default filter settings and noise reduction options, which can be adjusted for patients with low counts, such as individuals with obesity. Images are reconstructed following filtering, and attenuation correction is applied, which may include CT-guided attenuation correction. This is followed by reorientation and sectioning of the heart into vertical long-axis, horizontal long-axis, and short-axis views, performed either manually or automatically. The technologist and interpreting physician should review cine images to identify motion artifacts, breast attenuation artifacts, diaphragm elevation, or other visceral activity that may cause scatter into the inferior segments of the heart.
Indications
The primary indications for stress myocardial perfusion imaging are mentioned below.
- Detection of CAD in symptomatic patients with:
- Low pretest probability, uninterpretable electrocardiogram (ECG), or inability to exercise.
- Intermediate pretest probability, uninterpretable ECG, or inability to exercise.
- High pretest probability, regardless of ECG result or ability to exercise.
- Detection of CAD in asymptomatic high-risk individuals (Adult Treatment Panel [ATP] III).
- Detection of CAD in miscellaneous conditions, such as a new diagnosis of heart failure with reduced left ventricular systolic function without known CAD or a planned coronary angiography.
- Ventricular tachycardia with:
- Low ATP III coronary heart disease risk.
- Intermediate to high ATP III congestive heart failure risk.
- Syncope with intermediate to high risk of heart failure.
- Detection of CAD in asymptomatic patients with elevated Agatston score.
- Risk assessment in patients with:
- New or worsening symptoms and a prior history of abnormal stress testing.
- Intermediate or high-risk Duke treadmill score.
- Coronary stenosis of indeterminate significance.
- Evaluation of possible CAD for noncardiac perioperative risk assessment.
- Detection of CAD in patients with minor elevation of cardiac enzymes without a history concerning angina.
- Detection of CAD and risk assessment in patients with a prior noninvasive evaluation with indeterminate results, where obstructive CAD remains a concern.
- Risk assessment following revascularization for:
- Ischemic equivalent symptoms of chest pain.
- Incomplete revascularization in an asymptomatic individual.
- 5 or more years after coronary artery bypass grafting.
- Assessment of myocardial viability to determine candidacy for revascularization.[18][19]
Normal and Critical Findings
According to the American Society of Nuclear Cardiology, the overall impression of SPECT myocardial perfusion imaging should include the elements mentioned below.
- Perfusion defect: Site, severity, size, and reversibility.
- Left ventricular global function assessment: Normal, low normal, mildly reduced, moderately reduced, or severely reduced.
- Left ventricular segmental wall motion abnormalities: No segmental wall motion abnormalities, single segmental wall motion abnormality, or multiple segmental wall motion abnormalities.
- Left ventricular viability (optional): Substantial, borderline, or no evidence of viability.
- Number and name of the diseased coronary vessels (optional): 1, 2, or 3—left anterior descending (LAD), right coronary artery (RCA), or left circumflex (LCx).
- ECG interpretation at baseline and stress: No ischemia, borderline ischemia, positive for ischemia. If positive, it should be specified as mildly positive, moderately positive, or strongly positive.
- Scan significance: Low, intermediate, high, or uncertain risk.
- Right ventricular perfusion (optional): Normal or abnormal.
- Signature of the reporting physician.
- Date and time of the scan.
Possible interpretations of perfusion defects include ischemia, infarct, combined ischemia and infarction, peri-infarct ischemia, probable infarct, probable ischemia, or uninterpretable. Defect size is categorized as small, medium, or large. The perfusion defect may be classified as fixed, reversible, or mixed. The site of involvement may include 1, 2, or 3 vessels.
The vascular territory is assessed using the 17-segment model, whereby the LAD supplies the anterior, anteroseptal, apical, and apical cap segments. The RCA supplies inferoseptal and inferior segments. LCx artery supplies lateral, anterolateral, and inferolateral segments. Significant overlap exists in vascular territories, with all 3 coronary arteries potentially contributing to the apex (see Image. Postinfarction Myocardial Perfusion Scan).
Severity is qualitatively graded as mild, moderate, or severe. Additionally, the severity of the defect may also be quantified using automated analysis with the scoring system mentioned below.
- 0: Normal
- 1: Mild
- 2: Moderate
- 3: Severe
- 4: Absent activity
The score calculated at rest, known as the "summed rest score," reflects the extent of the fixed defect. The score obtained during stress, called the "summed stress score," indicates the extent of the total abnormality. The difference between these two scores, referred to as the "summed difference score," represents the degree of ischemia.
The score correlates with the degree of activity as mentioned below.
- 0: More than 80% activity
- 1: 70% to 80% activity
- 2: 60% to 70% activity
- 3: 50% to 60% activity
- 4: Less than 50%
Critical findings include:
- Large-sized perfusion defect with high severity
- Stress-induced cavity dilatation.
- Severely reduced left ventricular function
- Lung uptake (thallium scan)
- Perfusion defects involving multiple coronary artery territory distribution
- Right ventricular uptake
- ECG positive for ischemia
- Low metabolic equivalents of task (METS) on exercise
Interfering Factors
SPECT perfusion images are more susceptible to artifacts. Common artifacts that can interfere with SPECT imaging include patient motion during the study, interference from extracardiac activity, photon attenuation (eg, from breast tissue or diaphragm), and attenuation map misalignment. Addressing motion and attenuation can reduce artifacts before performing quantitative image analysis. Additional strategies to minimize artifacts include imaging in both supine and prone positions. In comparison, PET perfusion imaging techniques offer better spatial resolution than SPECT, allowing for more precise attenuation correction.
Complications
Complications of myocardial perfusion imaging include:
- Adverse reactions to administered drugs
- Bronchospasm
- Headache
- Flushing
- Chest tightness
- Neck or jaw pain
- Shortness of breath
- Arrhythmias
Careful patient monitoring throughout the procedure is essential to ensure safety and manage any complications promptly.
Patient Safety and Education
Patients should be counseled on the risks of radiation exposure associated with myocardial perfusion imaging. SPECT generally involves higher radiation exposure compared to PET. The radiation dose for SPECT ranges from 10 to 20 mSv, depending on the radiotracer and protocol (eg, 1-day versus 2-day stress imaging). Commonly used agents in PET imaging, such as Rb-82 and N-13 ammonia, typically result in radiation exposure of less than 10 mSv.
Clinical Significance
Myocardial perfusion scanning is critical in diagnostic and therapeutic decision-making for cardiac disease, as highlighted in the Indications section. These noninvasive imaging tests enable clinicians to evaluate blood flow to the myocardium, providing crucial information on perfusion and metabolite uptake. These data are crucial for guiding the selection of the most appropriate medical treatment or intervention to optimize cardiac health.
Myocardial perfusion scans are used for both diagnostic and prognostic purposes in various clinical settings. These include evaluating angina symptoms, ruling out acute coronary syndrome in patients with chest pain, assessing therapeutic outcomes following interventions, and determining myocardial viability or the presence of scar tissue. Key applications include risk stratification and the assessment of ischemia, CAD, and myocardial viability. The parameters provided in the report offer valuable prognostic insights into the likelihood of future major adverse cardiovascular events.
Media
(Click Image to Enlarge)
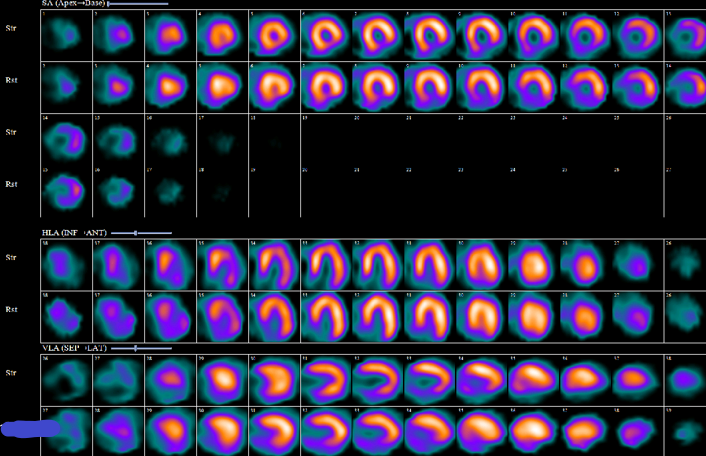
Postinfarction Myocardial Perfusion Scan. This image series displays the myocardial perfusion scan of a patient with a prior inferior myocardial infarction. The inferior perfusion defect is large and fixed, demonstrating moderate-to-severe intensity with full-thickness involvement. Additional perfusion defects are not evident, and stress-induced cavity dilatation is not observed.
Contributed by P Shams, MBBS, FCPS
References
Schofield R, Menezes L, Underwood SR. Nuclear cardiology: state of the art. Heart (British Cardiac Society). 2021 May 26:107(12):954-961. doi: 10.1136/heartjnl-2019-315628. Epub 2021 May 26 [PubMed PMID: 33483353]
Li DL, Kronenberg MW. Myocardial Perfusion and Viability Imaging in Coronary Artery Disease: Clinical Value in Diagnosis, Prognosis, and Therapeutic Guidance. The American journal of medicine. 2021 Aug:134(8):968-975. doi: 10.1016/j.amjmed.2021.03.011. Epub 2021 Apr 20 [PubMed PMID: 33864764]
Mannarino T, Assante R, D'Antonio A, Zampella E, Cuocolo A, Acampa W. Radionuclide Tracers for Myocardial Perfusion Imaging and Blood Flow Quantification. Cardiology clinics. 2023 May:41(2):141-150. doi: 10.1016/j.ccl.2023.01.003. Epub 2023 Feb 23 [PubMed PMID: 37003672]
Delso G, Ter Voert E, Veit-Haibach P. How does PET/MR work? Basic physics for physicians. Abdominal imaging. 2015 Aug:40(6):1352-7. doi: 10.1007/s00261-015-0437-5. Epub [PubMed PMID: 25906344]
Garcia MJ, Kwong RY, Scherrer-Crosbie M, Taub CC, Blankstein R, Lima J, Bonow RO, Eshtehardi P, Bois JP, American Heart Association Council on Cardiovascular Radiology and Intervention and Council on Clinical Cardiology. State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circulation. Cardiovascular imaging. 2020 Jul:13(7):e000053. doi: 10.1161/HCI.0000000000000053. Epub 2020 Jul 13 [PubMed PMID: 32833510]
Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P, Buechel RR, Cuocolo A, van Eck-Smit BL, Flotats A, Hacker M, Hindorf C, Kaufmann PA, Lindner O, Ljungberg M, Lonsdale M, Manrique A, Minarik D, Scholte AJ, Slart RH, Trägårdh E, de Wit TC, Hesse B, European Association of Nuclear Medicine (EANM). EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. European journal of nuclear medicine and molecular imaging. 2015 Nov:42(12):1929-40. doi: 10.1007/s00259-015-3139-x. Epub 2015 Aug 21 [PubMed PMID: 26290421]
Angelidis G, Giamouzis G, Karagiannis G, Butler J, Tsougos I, Valotassiou V, Giannakoulas G, Dimakopoulos N, Xanthopoulos A, Skoularigis J, Triposkiadis F, Georgoulias P. SPECT and PET in ischemic heart failure. Heart failure reviews. 2017 Mar:22(2):243-261. doi: 10.1007/s10741-017-9594-7. Epub [PubMed PMID: 28150111]
Iskandrian AS. Single-photon emission computed tomographic thallium imaging with adenosine, dipyridamole, and exercise. American heart journal. 1991 Jul:122(1 Pt 1):279-84; discussion 302-6 [PubMed PMID: 2063758]
Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. Journal of the American College of Cardiology. 2012 May 8:59(19):1719-28. doi: 10.1016/j.jacc.2011.12.040. Epub [PubMed PMID: 22554604]
Level 1 (high-level) evidencePelletier-Galarneau M, Martineau P, El Fakhri G. Quantification of PET Myocardial Blood Flow. Current cardiology reports. 2019 Feb 28:21(3):11. doi: 10.1007/s11886-019-1096-x. Epub 2019 Feb 28 [PubMed PMID: 30815744]
Slomka P, Xu Y, Berman D, Germano G. Quantitative analysis of perfusion studies: strengths and pitfalls. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012 Apr:19(2):338-46. doi: 10.1007/s12350-011-9509-2. Epub [PubMed PMID: 22302181]
Shanoudy H, Raggi P, Beller GA, Soliman A, Ammermann EG, Kastner RJ, Watson DD. Comparison of technetium-99m tetrofosmin and thallium-201 single-photon emission computed tomographic imaging for detection of myocardial perfusion defects in patients with coronary artery disease. Journal of the American College of Cardiology. 1998 Feb:31(2):331-7 [PubMed PMID: 9462576]
Hung GU, Wang YF, Su HY, Hsieh TC, Ko CL, Yen RF. New Trends in Radionuclide Myocardial Perfusion Imaging. Acta Cardiologica Sinica. 2016 Mar:32(2):156-66 [PubMed PMID: 27122946]
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011 Nov 15:124(20):2215-24. doi: 10.1161/CIRCULATIONAHA.111.050427. Epub 2011 Oct 17 [PubMed PMID: 22007073]
Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. The international journal of cardiovascular imaging. 2017 Jul:33(7):1021-1031. doi: 10.1007/s10554-017-1084-4. Epub 2017 Feb 10 [PubMed PMID: 28188475]
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2016 Jun:23(3):606-39. doi: 10.1007/s12350-015-0387-x. Epub [PubMed PMID: 26914678]
Cullom SJ, Case JA, Courter SA, McGhie AI, Bateman TM. Regadenoson pharmacologic rubidium-82 PET: a comparison of quantitative perfusion and function to dipyridamole. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013 Feb:20(1):76-83. doi: 10.1007/s12350-012-9636-4. Epub 2012 Nov 28 [PubMed PMID: 23188625]
Level 3 (low-level) evidenceHendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA, American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Nuclear Cardiology, American College of Radiology, American Heart Association, American Society of Echocardiography, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Nuclear Medicine. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009 Jun 9:119(22):e561-87. doi: 10.1161/CIRCULATIONAHA.109.192519. Epub 2009 May 18 [PubMed PMID: 19451357]
Ora M, Gambhir S. Nuclear investigative techniques and their interpretation in the heart and vascular disease. Annals of cardiac anaesthesia. 2020 Jul-Sep:23(3):262-271. doi: 10.4103/aca.ACA_54_19. Epub [PubMed PMID: 32687080]