Introduction
Tuberculosis (TB) is a condition arising from Mycobacterium tuberculosis (MTB) infection. MTB is transmitted from a person with pulmonary TB (PTB) infection. Droplet nuclei containing the tubercle bacilli are aerosolized by speaking, sneezing, or coughing. The droplets dry quickly, remain in the air for several hours, and may be inhaled by other individuals. Other MTB transmission routes are insignificant.
The disease most frequently affects the lungs, though up to a third of TB cases involve other organs. The bacterium is an obligate aerobe and thus typically lodges in the highly oxygenated lung regions, the upper lobe and the lower lobe's superior aspect close to the pleura.
The functional unit of the lungs is the alveolus. The thin alveolar walls are comprised of the capillary endothelium, basement membrane, and alveolar epithelium—components of the blood-air barrier that facilitate gas exchange. Alveolar macrophages are cells derived from monocytes, roaming free inside the alveolar lumen. These phagocytic cells are critical to the lung's immunity but also contribute to TB pathophysiology. Pores of Kohn are alveolar wall perforations where microbes and exudates can spread.
Lung vasculature is comprised of the following:
- Pulmonary arteries: arise from the pulmonary trunk and branch into lobar and segmental arteries in the lung parenchyma; these arteries carry deoxygenated blood into the lungs
- Pulmonary veins: carry oxygenated blood from the lungs and back into the heart and arterial circulation
- Bronchial arteries: arise from the thoracic aorta and posterior intercostal arteries; these vessels supply the root of the lungs and visceral pleura
- Bronchial veins: follow the bronchial arteries and drain into the azygos and hemiazygos veins
- Pulmonary lymphatic plexuses: the superficial subpleural lymphatic plexus drains into the bronchopulmonary (hilar) lymph nodes; the deep bronchopulmonary lymphatic plexus drains into the intrinsic pulmonary lymph nodes before emptying into the bronchopulmonary lymph nodes; the tracheobronchial lymph nodes drain the bronchopulmonary lymph nodes and ultimately empty into the right lymphatic and thoracic ducts
- Parietal pleura lymphatics: parietal pleural lymphatic vessels drain into the thoracic wall and axillary lymph nodes
In immunocompromised patients, failure of the immune system to contain the infection enables MTB to spread from the lungs to the other body organs through the vasculature. Disseminated TB is defined as the simultaneous involvement of at least 2 non-contiguous body organs or infection of the blood, bone marrow, or liver. Miliary TB is a potentially fatal, disseminated form of the disease arising from hematogenous tubercle bacilli spread throughout the lungs and other organs.
The condition results in the formation of millet-seed-sized (1 to 2 mm) tubercular foci. The term "miliary tuberculosis" was first coined by John Jacobus Manget in 1700 while describing a pathological specimen having tiny tubercles resembling millet seeds in appearance. The term originated from the Latin word "miliarius," related to the millet seed. Miliary mottling on a chest radiograph is the classical hallmark that supports the diagnosis of miliary TB. Miliary TB is classified as both pulmonary and extrapulmonary TB.[1][2]
See StatPearls' companion topic, "Tuberculosis," for a comprehensive discussion of the epidemiology, pathophysiology, evaluation, and management of pulmonary tuberculosis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
MTB is primarily responsible for TB manifestations in countries where bovine TB has been eradicated. Infected humans serve as a natural MTB reservoir. The organism is a non-spore-forming, non-motile, obligate-aerobic, facultative, catalase-negative, intracellular bacillus. MTB is gram-neutral and may be visualized by Ziehl-Neelsen (ZN) staining. Cell wall mycolic acids impart acid fastness when decolorized with acid-alcohol. Thus, MTB is also known as acid-alcohol-fast-bacillus (AAFB). Other mycobacteria are classified as nontuberculous or atypical mycobacterial organisms.[3]
Epidemiology
According to the World Health Organization (WHO) Global Tuberculosis Report, around 10 million people developed TB, and 1.3 million died from the disease globally in 2017.[4] Most cases are found in the developing world. However, miliary TB's epidemiological patterns continuously change due to the following:
- Immunosuppressant use
- Immigration from highly endemic countries
- Surge of HIV cases
- Alcoholism
- Other medical conditions manifesting with immunosuppression, such as diabetes mellitus and chronic kidney disease
According to the Centers for Disease Control and Prevention, 8920 new TB cases were reported in the United States in 2019.[5] Miliary TB accounts for about 1% to 2% of all cases of TB and up to 20% of all forms of extrapulmonary TB in immunocompetent individuals.
Miliary TB was thought to be a disease of infants and children before antibiotics were widely used. The disease has a bimodal age distribution, with one peak occurring in young adults and adolescents and the other occurring in older individuals. Miliary TB has a slight male preponderance.[6]
Pathophysiology
MTB infection starts with the lung epithelial macrophages. The organism's replication is initially unchecked, but the T-helper cell response induces macrophages to suppress bacterial proliferation. Failure of cell-mediated immunity in immunocompromised patients results in the organisms reaching the lymphatic ducts through the pulmonary lymphatics. The lymphatic ducts drain into the right side of the heart and the pulmonary arteries. Systemic spread results when infective lung foci seed the pulmonary venous return to the heart, and the bacilli disseminate via the arterial circulation.
The immunopathogenic mechanisms underlying miliary TB development are complex and not fully understood. Containment of the bacteria by effector T-cells is likely compromised. The specific cytokines and other immune-regulatory determinants in the host-pathogen interaction are poorly elucidated.
What is known, however, is that miliary TB is related to lymphohematogenous MTB dissemination from a primary or reactivation focus to various organs. In endemic countries, reinfection can result in miliary TB. Occasionally, miliary TB emerges from simultaneous dormant foci reactivation within previously infected organs.[7]
Histopathology
Grossly, miliary TB is characterized by small, punctate, gray to reddish-brown, rounded lesions with more or less uniform size in the lungs and other organs. In immunocompetent individuals, each microscopic TB focus contains a typical tubercle with central caseation necrosis and tubercle bacilli surrounded by Langhans-type giant cells, epithelioid cells, lymphocytes, and fibrocytes. However, in immunosuppressed individuals, the foci show caseation with tubercle bacilli without granuloma formation. This condition is known as nonreactive miliary TB.[8][9]
History and Physical
The clinical features of miliary TB vary widely, depending on the sites mainly involved, and may remain obscure until late in the disease course. Initial symptoms develop gradually and are usually nonspecific, which include constitutional manifestations such as fever, generalized weakness, anorexia, weight loss, and lassitude. Many patients may initially present with productive coughing, difficulty breathing, chest pain, and hemoptysis. Abdominal symptoms such as nausea, vomiting, and abdominal pain are also common in the early stages.
The most frequent extrapulmonary sites include the lymphatic system, bones, joints, liver, central nervous system (CNS), and adrenal glands. However, virtually any organ system can be infected, and symptoms referrable to organ-specific dysfunction usually appear.
Miliary TB has 2 clinical variants with different demographic profiles and presentations: classical acute and cryptic miliary TB. Both conditions require a high index of suspicion for timely diagnosis and treatment.
Acute Miliary TB
Individuals with acute miliary TB are generally younger than 40 years. Most patients have a history of subacute or chronic constitutional symptoms and organ-specific manifestations, depending on the site affected by the disease. Evening febrile episodes and night sweats of 1 to 2 weeks duration are classically described, although a patient can have early morning temperature spikes. Other common initial symptoms include nonproductive cough and dyspnea. Some patients may observe hemoptysis. Acute respiratory distress syndrome (ARDS) is a rare, fatal presentation of miliary TB.[10][11]
Abdominal TB occurs following hematogenous spread from a pulmonary focus or by local spread from a gastrointestinal or abdominal source. Abdominal TB can occur as hepatic, intestinal, or peritoneal involvement.[12] Hepatic spread presents as right upper quadrant pain, nausea, vomiting, fever, and generalized fatigue. Icterus and hepatosplenomegaly may be seen clinically.[13][14] Intestinal TB usually manifests with fever, micro- and macronutrient deficiencies, altered bowel habits, and subacute to acute intestinal obstruction. Children with intestinal TB may have failure to thrive.
TB peritonitis should be suspected in a patient presenting with complaints of fever, fatigue, abdominal pain, and ascites. Sometimes, the presentation may be confused with an acute abdomen. On surgical exploration, miliary tubercles may be found in the omentum and peritoneal surfaces. Biopsy and histopathology will show tuberculous foci.
Miliary TB can present as overt adrenal insufficiency (Addison disease) at the time of initial presentation or during antitubercular treatment. The manifestations include skin hyperpigmentation, hypotension, hypoglycemia, and electrolyte imbalance.[15]
On fundoscopy, the presence of choroid tubercles is pathognomonic of miliary TB. Choroid tubercles commonly occur in children. These pathological structures are bilateral, pale, and gray-white or yellowish lesions, usually less than a quarter of the optic disc's size, located within 2 cm of the optic nerve.[16]
Musculoskeletal pathology accounts for 10% of extrapulmonary TB cases. The bony site most commonly involved is the spine, manifesting as tubercular spondylitis or Pott spine. On history, patients with Pott spine may have the classic initial TB symptoms such as fever and weight loss. Back pain and tenderness develop insidiously and are often the earliest clues to spine involvement. Paraplegia, paraparesis, kyphosis, or scoliosis develop gradually and may cause complete debility late in the illness.
The joints are the second most frequently involved sites, and septic (tuberculous) arthritis is common. Other musculoskeletal manifestations of miliary TB include osteomyelitis, tenosynovitis, bursitis, and pyomyositis.[17]
Neurological involvement may present as headaches and nuchal rigidity secondary to tubercular meningitis (TBM) with or without tuberculoma formation. TBM has been reported in 10% to 30% of adult patients with miliary TB. Thoracic transverse myelopathy may manifest as sensorimotor abnormalities.[18][19]
Erythematous macules and papules characterize tuberculosis miliaria cutis. These lesions are manifestations of lymphohematogenous MTB spread in the skin.
Clinically significant cardiac or renal involvement is uncommon. However, miliary TB can cause myocarditis, congestive heart failure, endocarditis, mycotic aneurysms, and acute kidney injury either directly or as a syndromic constellation of multiorgan dysfunction syndrome (MODS).[20]
Miliary TB can present similarly in children and adults. However, chills, night sweats, hemoptysis, and productive cough are less frequently reported in children than in adults. In contrast, peripheral lymphadenopathy and hepatosplenomegaly are more common in the pediatric than in the adult population. TBM is more frequently seen in children with miliary TB (20%–40%) than in adults (15% to 30%).[21] Miliary TB develops less frequently in children who have received the Bacillus Calmette-Guérin (BCG) vaccination.
TB's clinical presentation in individuals with HIV depends on their CD4+ count. Patients with HIV having a CD4+ count greater than 200 cells/mm experience disease progression similar to immunocompetent individuals. In contrast, people with HIV with a CD4+ count of less than 200 cells/mm develop atypical manifestations of miliary TB, such as cutaneous lesions, intrathoracic lymphadenopathy, and tuberculin anergy. Profound immunosuppression due to any cause is generally associated with atypical TB presentation.[22]
Cryptic Miliary TB
Most patients with cryptic miliary TB are older than 60 years. The condition may be considered in the differential diagnosis of fever of unknown origin or metastatic carcinoma, as symptoms like fever, progressive weight loss, and general debility can occur without the usual TB signs and symptoms. However, mild hepatosplenomegaly is occasionally observed. Patients often have a normal chest radiograph and negative skin tuberculin test, causing a delay in diagnosis.[23][24]
Atypical manifestations of miliary TB can delay the diagnosis. Such unusual presentations include ARDS, pneumothorax, cytopenia, septic shock, glomerulonephritis, endocarditis, mycotic aortic aneurysm, cholestatic jaundice, and hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion.
Evaluation
Diagnosing miliary TB requires a high suspicion index. A multi-pronged approach, comprised of meticulous history taking, thorough physical examination, and radiological and laboratory investigation, is required for timely diagnosis and adequate treatment.
Laboratory Findings
Hematological changes seen in miliary TB are usually nonspecific. Pancytopenia, anemia, leucopenia, leucocytosis with predominant lymphocytosis, thrombocytopenia, or thrombocytosis may be reported. The most common hematological abnormality encountered in miliary TB is anemia of chronic disease. Elevated acute-phase reactants, particularly erythrocyte sedimentation rate and C-reactive protein, are frequently encountered. Leukaemoid reaction is also described. Thus, miliary TB may also be mistaken for leukemia. Disseminated intravascular coagulation is rare and seen in the setting of MODS and ARDS.[25][26]
Biochemistry panels can be normal or have subtle disturbances. Hyponatremia, secondary to meningeal involvement or abnormal antidiuretic hormone levels, is frequently encountered and is an indicator of neurological damage. Hyperbilirubinemia, hypoalbuminemia (a negative acute-phase reactant), and elevated alkaline phosphatase are also seen. Hypercalcemia, though rare, has also been reported.[27][28]
Imaging Studies
No uniform guidelines exist for diagnosing miliary TB, but the following criteria have been suggested:
- Clinical presentation consistent with a diagnosis of TB, such as pyrexia with evening temperature rise, weight loss, anorexia, tachycardia, and night sweats of greater than 6 weeks duration responding to antitubercular treatment
- Classical miliary pattern on chest radiograph
- Bilateral diffuse reticulonodular lung lesions on a background of miliary shadows demonstrable either on plain chest radiograph or high-resolution computed tomography (HRCT)
- Microbiological, cytopathological, histopathological, or molecular evidence of TB
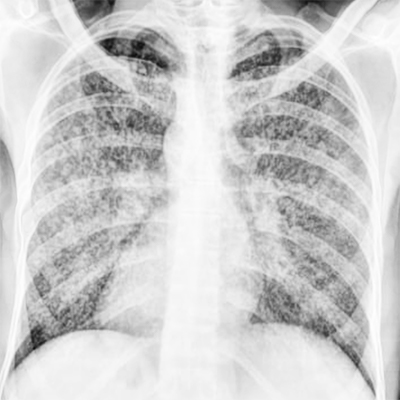
The chest radiograph shows typical miliary mottling, ie, homogenously distributed, discrete, uniform size (1 to 2 mm), and millet-shaped lesions in all lung zones (see Image. Miliary Tuberculosis Radiography). These features are also the radiographic hallmark of miliary TB. However, these x-ray findings are usually absent in early disease or cryptic TB. HRCT may be advisable in such cases to look for parenchymal lesions. Contrast-enhanced CT (CECT) is preferable for evaluating lymphadenopathy, calcifications, and pleural pathology.[29][30]
Extrapulmonary TB lesions may be assessed by ultrasonography, CECT, and magnetic resonance imaging (MRI), which can determine the extent of organ involvement.[31] Positron-emission tomographic CT has been recently used as an investigating tool for evaluating patients with suspected TB.[32][33][34]
Invasive diagnostic procedures are indicated for patients with suspected extrapulmonary TB. The following have a good diagnostic yield in miliary TB:
- Cerebrospinal fluid (CSF)
- Pleural fluid
- Ascitic fluid
- Gastric aspirate
- Urine
- Pus from a cold abscess
- Biopsy and culture of bone marrow and liver tissue
Image-guided radiological procedures, such as fine-needle aspiration and tissue biopsy, are useful for procuring tissue and body fluids for diagnostic testing. Ultrasound, CT, and MRI are frequently used in these procedures.[35][36]
Immunology-Based Methods
Anergy on tuberculin skin testing (TST) is more frequently reported in miliary TB than PTB, though the test can become positive during antitubercular therapy. A positive interferon-γ release assay indicates infection but does not signify active disease. Thus, this diagnostic modality is of limited use in highly endemic areas.[37]
Serological tests are not advocated for TB detection because of their low sensitivity and specificity. However, adenosine deaminase (ADA) and interferon-γ (IFN-γ) may be used as adjuncts for detecting the presence of MTB in pleural, pericardial, and ascitic fluids.[38][39][40][41] ADA may also be used in diagnosing TBM.[42]
The ADA cutoff value for pleural and pericardial effusion is 40 U/L; for tubercular ascites is 39 U/L; and for CSF is 10 U/L.[43][44][45] ADA levels may be falsely elevated in the presence of empyema, parapneumonic effusions, endometriosis, malignancies like lymphoma, and collagen vascular disorder.[43] High CSF ADA can also be seen in cerebral malaria, brucellosis, neurosarcoidosis, fulminant pyogenic meningitis, AIDS, and CSF lymphoma.[46]
Molecular Studies
Molecular methods such as polymerase chain reaction, Gene Xpert MTB/RIF, and line probe assays may be useful in the early diagnosis of pulmonary and extrapulmonary TB and in detecting drug-resistant tubercle bacilli. Various tissue specimens may be used, and results may be available within hours.[47][48][49][50]
Definitive Diagnosis
Detection of mycobacterial isolates from a clinical specimen provides a definitive diagnosis of disseminated TB. Examples of tissue specimens are sputum, body fluids, tissue, and biopsy samples. The specimens are inoculated on agar-based (eg, Lowenstein-Jensen or LJ) or liquid media with fluorescence detection. Direct visualization of acid-fast bacilli is aided by ZN staining or the more sensitive auramine-rhodamine (fluorochrome dye) staining.[51][52] The specimen should be cultured on standard solid LJ media and then inoculated in liquid media (eg, BACTEC Mycobacterial Growth Indicator Tube or BACTEC™ MGIT™ 960).[53]
Liquid-based media reduces the MTB detection time to 1 to 3 weeks, compared to 6 to 8 weeks on solid media. The method also shortens drug sensitivity testing time.
Blood culture is usually not employed for mycobacterium isolation. This test is usually negative, although positive results may be reported among immunocompromised individuals with hematogenous dissemination.[54] MTB may be differentiated from isolated nontuberculous mycobacteria by hybridization using nucleic acid probes or biochemical methods or by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF).[55]
Histopathological examination of a tissue biopsy specimen should show granulomatous inflammation with central caseation, with or without tubercle bacilli. Demonstration of a tubercle granuloma in a biopsy sample obtained from a patient with typical TB manifestations is suggestive of TB. However, demonstration of bacilli by staining or culture is required to confirm the diagnosis.[56]
Treatment / Management
Standard PTB drug regimen may also be used for treating miliary TB. As per the WHO, a standard 6-month antitubercular drug regimen consists of a 2-month intensive or bactericidal phase with isoniazid, rifampicin, pyrazinamide, and ethambutol and a 4-month continuation or sterilizing phase with isoniazid and rifampicin. Treatment duration may be modified according to the age group affected and primary disease site.
Longer treatment periods are advisable for children, immunocompromised individuals, patients with a slow clinical response, and the presence of TBM, TB lymphadenitis, or skeletal TB. The generally recommended minimum therapeutic duration is 9 months for skeletal TB and 12 months for TBM.[57][58][59](A1)
For abdominal TB, 9 months of therapy used to be the conventional approach. However, a recent multicenter, randomized trial showed that both 6-month and 9-month regimens are equivalent for patients with abdominal TB.[60] Neurological involvement, particularly TBM, must be determined in all miliary TB cases. Neurologic TB requires a longer treatment duration than the standard and concomitant steroid therapy.[61](A1)
For previously treated patients, the WHO guidelines advocate that culture and drug susceptibility testing (DST) specimens be obtained from all previously treated TB patients at or before the start of treatment. DST should be performed for at least isoniazid and rifampicin. In settings where rapid molecular DSTs are available, the DST results should guide the choice of regimen. The duration of treatment may also be individualized according to the clinical setting.
Patients suspected of miliary TB must be screened for diabetes mellitus and HIV before initiating antitubercular treatment and vice-versa. Immune reconstitution inflammatory response (IRIS) is a condition arising from excessive immune reaction to MTB that may develop in patients with HIV during or after completing an antitubercular drug regimen. Antiretroviral treatment (ART) for all patients with HIV and TB must not be initiated within the first 8 weeks of starting TB treatment and within 2 weeks in profoundly immunosuppressed HIV-positive TB patients with CD4+ counts less than 50 to prevent IRIS.[62][63](B3)
Medical and surgical interventions may be warranted for diagnosis and therapy. Examples are mechanical ventilation for ARDS, abdominal surgery for small bowel perforation, and ventriculoperitoneal shunt surgery for TBM, which may be performed to reduce TB complications.
Although substantial evidence is lacking, corticosteroid use in miliary TB has shown clinical efficacy in a few scenarios. Notably, it has been demonstrated to be beneficial in adrenal insufficiency, TB meningitis, large pericardial or pleural effusion, IRIS, ARDS, immune-complex nephritis, and secondary hemophagocytic syndrome.[64][65] Liver function tests (LFTs) should be obtained before and during anti-TB therapy (ATT). Serial LFTs should be performed to check for ATT-induced hepatitis.(A1)
The criteria for ATT-induced hepatitis have evolved and include the following:
- Transaminases are elevated up to 5 times the upper normal limit without hepatitis symptoms; or
- Transaminases are elevated up to 3 times the upper normal limit with hepatitis symptoms; or
- Bilirubin rises twice the upper normal limit, and other possible hepatitis causes, like acute viral hepatitis and autoimmune hepatitis, have been ruled out.
All hepatotoxic drugs—isoniazid, rifampicin, and pyrazinamide—should be withdrawn if ATT-induced hepatitis develops. A modified ATT regimen must be initiated to prevent further liver damage. According to guidelines from the British and American Thoracic Societies, ATT rechallenge may be considered once the LFTs have normalized. The decision to restart ATT drugs at incrementally increasing doses or full doses should be guided by the clinical picture.[66][67](B2)
Differential Diagnosis
The protean and nonspecific clinical manifestations of miliary TB often generate a large differential diagnosis. The common constitutional symptoms of fever, chills, night sweats, anorexia, weight loss, and fatigue raise concerns about many potential infectious, autoimmune, and neoplastic etiologies. Headache, seizures, altered sensorium, cough, chest pain, hemoptysis, abdominal pain, lymphadenopathy, hepatosplenomegaly, back pain, and neurologic dysfunction are themselves nonspecific manifestations of a huge number of diseases. The manifestations of miliary TB often develop gradually, and their lack of specificity frequently results in a diagnostic delay. However, the constellation of signs and symptoms and patient demographic and immune status must be taken into account when evaluating patients who may have miliary TB.
Varied clinical etiologies can present with a miliary pattern on chest radiography and CT. Therefore, a thorough workup is required to reach an etiological conclusion. Other causes of miliary shadowing include histoplasmosis, blastomycosis, coccidioidomycosis, nocardiosis, sarcoidosis, lung carcinoma with lymphangitis carcinomatosis, metastatic carcinoma, pyogenic infection spread from a remote site, pulmonary hemosiderosis, and hypersensitivity pneumonitis.[68]
Prognosis
Miliary TB has high morbidity and mortality. Treatment delay appears to be the most significant factor responsible for mortality. The mortality related to miliary TB is approximately 15% to 20 % in children and 25% to 30% in adults.[69][70][71]
In patients with ARDS due to miliary TB, the following Acute Physiology and Chronic Health Evaluation (APACHE II) scores have been identified as mortality predictors:
- Greater than 18
- Less than or equal to 18 with hyponatremia and a ratio of arterial oxygen tension to the fraction of inspired oxygen (FiO2) less than or equal to 108.5 [72]
Complications
Delayed treatment of miliary TB can produce the following emergent complications:
- ARDS
- MODS
- Tubercular empyema
- The air leak syndromes pneumothorax and pneumomediastinum
- Tubercular pericardial effusion and pericarditis
- Immune reconstitution inflammatory syndrome
- Myocarditis, native and prosthetic valve endocarditis, and intracardiac masses
- Mycotic aneurysm of the aorta
- Tubercular meningitis with focal neurological deficits
- Systemic amyloidosis
- Immune complex glomerulonephritis
- Bone marrow suppression
- Disseminated intravascular coagulation
Rapid deterioration may occur in the setting of profound immunosuppression, as in HIV infection with low CD4+ counts, immunosuppressant use, and inborn immune conditions.
Deterrence and Patient Education
Patient education and counseling are the cornerstones of TB management. Proper education materials should be provided to at-risk populations to reduce bacterial transmission and enhance the detection of this condition. Patients diagnosed with TB must be informed about the following:
- The disease process in relation to the symptoms
- Importance of ATT adherence and follow-up
- Monitoring for drug toxicity symptoms
- Measures that can help avoid MTB transmission to close contacts
Healthcare providers must monitor patients closely for signs of drug reactions, progress, or lack of response to optimize the antitubercular drug regimen.
Pearls and Other Issues
The most important points to remember when evaluating and managing miliary TB are the following:
-
Miliary TB is a potentially fatal form of disseminated TB characterized by millet-seed-like granuloma formation in various organs.
-
Miliary TB often arises from a primary pulmonary infection that spreads hematogenously. However, extrapulmonary primary sites can also give rise to miliary TB.
-
The clinical presentation can vary widely. The initial manifestations may be nonspecific, including fever, weight loss, fatigue, and respiratory symptoms. Diagnosis can be challenging due to the diversity of presentations.
-
Miliary TB is more common in individuals with poor immunity, such as people with HIV or AIDS, older individuals, and people on immunosuppressive therapy.
-
Chest x-rays and CT scans often reveal characteristic miliary patterns, showing widespread, small nodules throughout the lung fields. However, these imaging features may be absent in early and cryptic miliary TB.
-
Central nervous system involvement, particularly TBM, can occur and presents a serious and life-threatening complication.
-
Definitive diagnosis often involves a combination of clinical evaluation, imaging studies, and laboratory tests such as sputum culture, polymerase chain reaction, and Gene Xpert MTB/RIF.
-
The WHO recommends the standard antitubercular regimen for patients diagnosed with miliary TB. However, skeletal and neurological involvement can prolong the treatment course.
-
Treatment adherence is crucial to prevent the development of drug-resistant strains.
- Prognosis depends on factors such as the extent of organ involvement, timeliness of diagnosis, and therapeutic effectiveness. Early detection and intervention improve outcomes.
-
Preventive measures include vaccination with the BCG vaccine in regions where TB is prevalent. Timely detection and treatment of active TB cases also help prevent the development of disseminated TB.
Miliary TB is a serious condition requiring prompt medical attention and diagnostic and management proficiency. A comprehensive evaluation approach must be pursued to initiate the appropriate treatment.
Enhancing Healthcare Team Outcomes
An interprofessional team approach can ensure that patients with miliary TB receive holistic and integrated care to achieve the best possible outcomes. Judicious evaluation by the physicians, gentle bedside care by the nurses, and utmost diligence by laboratory personnel can help treat the disease promptly and stop its spread in the hospital and community. Effective implementation of the WHO's Directly Observed Treatment, Short-Course strategy by community healthcare personnel can help ensure treatment adherence. Completion of therapy is vital for disease resolution.
Specialists who may be involved in the inpatient care of individuals with miliary TB include emergency physicians, pulmonologists, intensivists, gastroenterologists, endocrinologists, neurologists, infectious disease specialists, surgeons, pain specialists, pathologists, and radiologists. The pharmacist can help educate patients about ATT and manage antitubercular medication dosage to prevent drug-induced complications. Nutritionists can help optimize patient nutrition in aid of recovery. Occupational and physical therapists can help patients improve functional independence during recovery.
Collaboration, shared decision-making, and communication are critical elements for a good outcome. Nothing less than an integrated care pathway and evidence-based diagnostic and management approaches must be rendered to the patient.
Media
(Click Image to Enlarge)
References
Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. The Lancet. Infectious diseases. 2005 Jul:5(7):415-30 [PubMed PMID: 15978528]
Sahn SA, Neff TA. Miliary tuberculosis. The American journal of medicine. 1974 Apr:56(4):494-505 [PubMed PMID: 4206484]
Adigun R, Singh R. Tuberculosis. StatPearls. 2024 Jan:(): [PubMed PMID: 28722945]
Nguyen HV, Tiemersma EW, Nguyen HB, Cobelens FGJ, Finlay A, Glaziou P, Dao CH, Mirtskhulava V, Nguyen HV, Pham HTT, Khieu NTT, de Haas P, Do NH, Nguyen PD, Cung CV, Nguyen NV. The second national tuberculosis prevalence survey in Vietnam. PloS one. 2020:15(4):e0232142. doi: 10.1371/journal.pone.0232142. Epub 2020 Apr 23 [PubMed PMID: 32324806]
Level 3 (low-level) evidenceSchwartz NG, Price SF, Pratt RH, Langer AJ. Tuberculosis - United States, 2019. MMWR. Morbidity and mortality weekly report. 2020 Mar 20:69(11):286-289. doi: 10.15585/mmwr.mm6911a3. Epub 2020 Mar 20 [PubMed PMID: 32191684]
Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. The Indian journal of medical research. 2012 May:135(5):703-30 [PubMed PMID: 22771605]
Sharma SK, Mohan A. Miliary Tuberculosis. Microbiology spectrum. 2017 Mar:5(2):. doi: 10.1128/microbiolspec.TNMI7-0013-2016. Epub [PubMed PMID: 28281441]
Auerbach O. Acute Generalized Miliary Tuberculosis. The American journal of pathology. 1944 Jan:20(1):121-36 [PubMed PMID: 19970738]
de Noronha AL, Báfica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathology, research and practice. 2008:204(3):155-61 [PubMed PMID: 18096327]
Kim JY, Park YB, Kim YS, Kang SB, Shin JW, Park IW, Choi BW. Miliary tuberculosis and acute respiratory distress syndrome. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2003 Apr:7(4):359-64 [PubMed PMID: 12733492]
Level 2 (mid-level) evidenceMohan A, Sharma SK, Pande JN. Acute respiratory distress syndrome (ARDS) in miliary tuberculosis: a twelve year experience. The Indian journal of chest diseases & allied sciences. 1996 Jul-Sep:38(3):157-62 [PubMed PMID: 8987289]
Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World journal of gastroenterology. 2014 Oct 28:20(40):14831-40. doi: 10.3748/wjg.v20.i40.14831. Epub [PubMed PMID: 25356043]
Rasheed S, Zinicola R, Watson D, Bajwa A, McDonald PJ. Intra-abdominal and gastrointestinal tuberculosis. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2007 Nov:9(9):773-83 [PubMed PMID: 17868413]
Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM : monthly journal of the Association of Physicians. 2008 Mar:101(3):189-95. doi: 10.1093/qjmed/hcm125. Epub 2008 Jan 30 [PubMed PMID: 18234735]
Level 2 (mid-level) evidenceLam KY, Lo CY. A critical examination of adrenal tuberculosis and a 28-year autopsy experience of active tuberculosis. Clinical endocrinology. 2001 May:54(5):633-9 [PubMed PMID: 11380494]
Level 2 (mid-level) evidenceSharma SK, Mohan A, Pande JN, Prasad KL, Gupta AK, Khilnani GC. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM : monthly journal of the Association of Physicians. 1995 Jan:88(1):29-37 [PubMed PMID: 7894985]
Level 2 (mid-level) evidenceLeonard MK, Blumberg HM. Musculoskeletal Tuberculosis. Microbiology spectrum. 2017 Apr:5(2):. doi: 10.1128/microbiolspec.TNMI7-0046-2017. Epub [PubMed PMID: 28409551]
Garg RK, Sharma R, Kar AM, Kushwaha RA, Singh MK, Shukla R, Agarwal A, Verma R. Neurological complications of miliary tuberculosis. Clinical neurology and neurosurgery. 2010 Apr:112(3):188-92. doi: 10.1016/j.clineuro.2009.11.013. Epub 2009 Dec 23 [PubMed PMID: 20031301]
Slavin RE, Walsh TJ, Pollack AD. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine. 1980 Sep:59(5):352-66 [PubMed PMID: 7432152]
Level 3 (low-level) evidenceSharma SK, Mohan A. Extrapulmonary tuberculosis. The Indian journal of medical research. 2004 Oct:120(4):316-53 [PubMed PMID: 15520485]
Gurkan F, Bosnak M, Dikici B, Bosnak V, Yaramis A, Tas MA, Haspolat K. Miliary tuberculosis in children: a clinical review. Scandinavian journal of infectious diseases. 1998:30(4):359-62 [PubMed PMID: 9817515]
Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. The Indian journal of medical research. 2005 Apr:121(4):550-67 [PubMed PMID: 15817963]
Proudfoot AT, Akhtar AJ, Douglas AC, Horne NW. Miliary tuberculosis in adults. British medical journal. 1969 May 3:2(5652):273-6 [PubMed PMID: 5780453]
Yu YL, Chow WH, Humphries MJ, Wong RW, Gabriel M. Cryptic miliary tuberculosis. The Quarterly journal of medicine. 1986 Apr:59(228):421-8 [PubMed PMID: 3749446]
Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. The American journal of medicine. 1990 Sep:89(3):291-6 [PubMed PMID: 2393033]
Level 2 (mid-level) evidenceHunt BJ, Andrews V, Pettingale KW. The significance of pancytopenia in miliary tuberculosis. Postgraduate medical journal. 1987 Sep:63(743):801-4 [PubMed PMID: 3444806]
Level 3 (low-level) evidenceShalhoub RJ, Antoniou LD. The mechanism of hyponatremia in pulmonary tuberculosis. Annals of internal medicine. 1969 May:70(5):943-62 [PubMed PMID: 5769627]
Chan CH, Chan TY, Shek AC, Mak TW, Lui SF, Lai KN. Severe hypercalcaemia associated with miliary tuberculosis. The Journal of tropical medicine and hygiene. 1994 Jun:97(3):180-2 [PubMed PMID: 8007059]
Level 3 (low-level) evidenceMcGuinness G, Naidich DP, Jagirdar J, Leitman B, McCauley DI. High resolution CT findings in miliary lung disease. Journal of computer assisted tomography. 1992 May-Jun:16(3):384-90 [PubMed PMID: 1592920]
Level 2 (mid-level) evidenceLee J, Lim JK, Seo H, Lee SY, Choi KJ, Yoo SS, Lee SY, Cha SI, Park JY, Kim CH. Clinical relevance of ground glass opacity in 105 patients with miliary tuberculosis. Respiratory medicine. 2014 Jun:108(6):924-30. doi: 10.1016/j.rmed.2014.03.016. Epub 2014 Apr 13 [PubMed PMID: 24787005]
Level 2 (mid-level) evidenceYu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World journal of gastroenterology. 2004 Jun 1:10(11):1639-42 [PubMed PMID: 15162540]
Level 2 (mid-level) evidenceHara T, Kosaka N, Suzuki T, Kudo K, Niino H. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest. 2003 Sep:124(3):893-901 [PubMed PMID: 12970014]
Heysell SK, Thomas TA, Sifri CD, Rehm PK, Houpt ER. 18-Fluorodeoxyglucose positron emission tomography for tuberculosis diagnosis and management: a case series. BMC pulmonary medicine. 2013 Mar 21:13():14. doi: 10.1186/1471-2466-13-14. Epub 2013 Mar 21 [PubMed PMID: 23514625]
Level 3 (low-level) evidenceIchiya Y, Kuwabara Y, Sasaki M, Yoshida T, Akashi Y, Murayama S, Nakamura K, Fukumura T, Masuda K. FDG-PET in infectious lesions: The detection and assessment of lesion activity. Annals of nuclear medicine. 1996 May:10(2):185-91 [PubMed PMID: 8800447]
Grieco MH, Chmel H. Acute disseminated tuberculosis as a diagnostic problem. A clinical study based on twenty-eight cases. The American review of respiratory disease. 1974 May:109(5):554-60 [PubMed PMID: 4823410]
Level 3 (low-level) evidenceMunt PW. Miliary tuberculosis in the chemotherapy era: with a clinical review in 69 American adults. Medicine. 1972 Mar:51(2):139-55 [PubMed PMID: 5013636]
Kim CH, Lim JK, Yoo SS, Lee SY, Cha SI, Park JY, Lee J. Diagnostic performance of the QuantiFERON-TB Gold In-Tube assay and factors associated with nonpositive results in patients with miliary tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Apr:58(7):986-9. doi: 10.1093/cid/ciu045. Epub 2014 Jan 22 [PubMed PMID: 24457341]
Level 2 (mid-level) evidenceRiquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. Journal of clinical gastroenterology. 2006 Sep:40(8):705-10 [PubMed PMID: 16940883]
Level 1 (high-level) evidenceSharma SK, Suresh V, Mohan A, Kaur P, Saha P, Kumar A, Pande JN. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. The Indian journal of chest diseases & allied sciences. 2001 Jul-Sep:43(3):149-55 [PubMed PMID: 11529433]
Sharma SK, Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. Journal of clinical laboratory analysis. 2005:19(2):40-6 [PubMed PMID: 15756707]
Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2006 Jul:26(7):484-8 [PubMed PMID: 16800787]
Rana SV, Chacko F, Lal V, Arora SK, Parbhakar S, Sharma SK, Singh K. To compare CSF adenosine deaminase levels and CSF-PCR for tuberculous meningitis. Clinical neurology and neurosurgery. 2010 Jun:112(5):424-30. doi: 10.1016/j.clineuro.2010.02.012. Epub 2010 Mar 29 [PubMed PMID: 20347212]
Jiménez Castro D, Díaz Nuevo G, Pérez-Rodríguez E, Light RW. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. The European respiratory journal. 2003 Feb:21(2):220-4 [PubMed PMID: 12608433]
Reuter H, Burgess LJ, Carstens ME, Doubell AF. Adenosine deaminase activity--more than a diagnostic tool in tuberculous pericarditis. Cardiovascular journal of South Africa : official journal for Southern Africa Cardiac Society [and] South African Society of Cardiac Practitioners. 2005 May-Jun:16(3):143-7 [PubMed PMID: 16049586]
Gupta BK, Bharat A, Debapriya B, Baruah H. Adenosine Deaminase Levels in CSF of Tuberculous Meningitis Patients. Journal of clinical medicine research. 2010 Oct 11:2(5):220-4. doi: 10.4021/jocmr429w. Epub [PubMed PMID: 21629544]
Egido JA, Gonzales JL, Cubo E. False positive of ADA determination in cerebrospinal fluid. Acta neurologica. 1994 Dec:16(5-6):288-90 [PubMed PMID: 7709800]
Level 3 (low-level) evidenceDowdy DW, Steingart KR, Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS medicine. 2011 Aug:8(8):e1001074. doi: 10.1371/journal.pmed.1001074. Epub 2011 Aug 9 [PubMed PMID: 21857810]
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid molecular detection of tuberculosis and rifampin resistance. The New England journal of medicine. 2010 Sep 9:363(11):1005-15. doi: 10.1056/NEJMoa0907847. Epub 2010 Sep 1 [PubMed PMID: 20825313]
Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. The Cochrane database of systematic reviews. 2018 Aug 27:8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Epub 2018 Aug 27 [PubMed PMID: 30148542]
Level 1 (high-level) evidenceLing DI, Zwerling AA, Pai M. Rapid diagnosis of drug-resistant TB using line probe assays: from evidence to policy. Expert review of respiratory medicine. 2008 Oct:2(5):583-8. doi: 10.1586/17476348.2.5.583. Epub [PubMed PMID: 20477293]
Strumpf IJ, Tsang AY, Sayre JW. Re-evaluation of sputum staining for the diagnosis of pulmonary tuberculosis. The American review of respiratory disease. 1979 Apr:119(4):599-602 [PubMed PMID: 87141]
Stender H, Mollerup TA, Lund K, Petersen KH, Hongmanee P, Godtfredsen SE. Direct detection and identification of Mycobacterium tuberculosis in smear-positive sputum samples by fluorescence in situ hybridization (FISH) using peptide nucleic acid (PNA) probes. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1999 Sep:3(9):830-7 [PubMed PMID: 10488893]
Tortoli E, Cichero P, Piersimoni C, Simonetti MT, Gesu G, Nista D. Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. Journal of clinical microbiology. 1999 Nov:37(11):3578-82 [PubMed PMID: 10523555]
Level 2 (mid-level) evidenceHanna BA, Walters SB, Bonk SJ, Tick LJ. Recovery of mycobacteria from blood in mycobacteria growth indicator tube and Lowenstein-Jensen slant after lysis-centrifugation. Journal of clinical microbiology. 1995 Dec:33(12):3315-6 [PubMed PMID: 8586725]
Balada-Llasat JM, Kamboj K, Pancholi P. Identification of mycobacteria from solid and liquid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry in the clinical laboratory. Journal of clinical microbiology. 2013 Sep:51(9):2875-9. doi: 10.1128/JCM.00819-13. Epub 2013 Jun 26 [PubMed PMID: 23804379]
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 15:64(2):111-115. doi: 10.1093/cid/ciw778. Epub [PubMed PMID: 28052967]
Level 1 (high-level) evidenceNahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Oct 1:63(7):e147-e195. doi: 10.1093/cid/ciw376. Epub 2016 Aug 10 [PubMed PMID: 27516382]
Level 1 (high-level) evidenceThwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J, British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. The Journal of infection. 2009 Sep:59(3):167-87. doi: 10.1016/j.jinf.2009.06.011. Epub 2009 Jul 4 [PubMed PMID: 19643501]
Cherian A, Thomas SV. Central nervous system tuberculosis. African health sciences. 2011 Mar:11(1):116-27 [PubMed PMID: 21572867]
Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, Gupta SD, Mechenro J, Mishra A, Mishra A, Pathak MK, Pandey RM, Sharma R, Sharma SK. Intermittent Directly Observed Therapy for Abdominal Tuberculosis: A Multicenter Randomized Controlled Trial Comparing 6 Months Versus 9 Months of Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Sep 1:61(5):750-7. doi: 10.1093/cid/civ376. Epub 2015 May 12 [PubMed PMID: 25969531]
Level 1 (high-level) evidenceThwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. The New England journal of medicine. 2004 Oct 21:351(17):1741-51 [PubMed PMID: 15496623]
Level 1 (high-level) evidenceChurchill D, Waters L, Ahmed N, Angus B, Boffito M, Bower M, Dunn D, Edwards S, Emerson C, Fidler S, Fisher M, Horne R, Khoo S, Leen C, Mackie N, Marshall N, Monteiro F, Nelson M, Orkin C, Palfreeman A, Pett S, Phillips A, Post F, Pozniak A, Reeves I, Sabin C, Trevelion R, Walsh J, Wilkins E, Williams I, Winston A. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV medicine. 2016 Aug:17 Suppl 4():s2-s104. doi: 10.1111/hiv.12426. Epub [PubMed PMID: 27568911]
Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, John L, van der Loeff MS, Reiss P, Lynen L, Janoff EN, Gilks C, Colebunders R, International Network for the Study of HIV-associated IRIS. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet. Infectious diseases. 2008 Aug:8(8):516-23. doi: 10.1016/S1473-3099(08)70184-1. Epub [PubMed PMID: 18652998]
Level 3 (low-level) evidenceDooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997 Oct:25(4):872-87 [PubMed PMID: 9356803]
Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H, Gumedze F, Pogue J, Thabane L, Smieja M, Francis V, Joldersma L, Thomas KM, Thomas B, Awotedu AA, Magula NP, Naidoo DP, Damasceno A, Chitsa Banda A, Brown B, Manga P, Kirenga B, Mondo C, Mntla P, Tsitsi JM, Peters F, Essop MR, Russell JB, Hakim J, Matenga J, Barasa AF, Sani MU, Olunuga T, Ogah O, Ansa V, Aje A, Danbauchi S, Ojji D, Yusuf S, IMPI Trial Investigators. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. The New England journal of medicine. 2014 Sep 18:371(12):1121-30. doi: 10.1056/NEJMoa1407380. Epub 2014 Sep 1 [PubMed PMID: 25178809]
Level 1 (high-level) evidenceDevarbhavi H. Antituberculous drug-induced liver injury: current perspective. Tropical gastroenterology : official journal of the Digestive Diseases Foundation. 2011 Jul-Sep:32(3):167-74 [PubMed PMID: 22332331]
Level 3 (low-level) evidenceAbbara A, Chitty S, Roe JK, Ghani R, Collin SM, Ritchie A, Kon OM, Dzvova J, Davidson H, Edwards TE, Hateley C, Routledge M, Buckley J, Davidson RN, John L. Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC infectious diseases. 2017 Mar 24:17(1):231. doi: 10.1186/s12879-017-2330-z. Epub 2017 Mar 24 [PubMed PMID: 28340562]
Level 2 (mid-level) evidenceDalpiaz G, Piolanti M, Cancellieri A, Barozzi L. Diffuse granulomatous lung disease: combined pathological-HRCT approach. La Radiologia medica. 2014 Jan:119(1):54-63. doi: 10.1007/s11547-013-0381-9. Epub 2014 Feb 1 [PubMed PMID: 24488691]
Long R, O'Connor R, Palayew M, Hershfield E, Manfreda J. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinical-pathologic-radiologic correlation. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1997 Feb:1(1):52-8 [PubMed PMID: 9441059]
Level 2 (mid-level) evidenceHussain SF, Irfan M, Abbasi M, Anwer SS, Davidson S, Haqqee R, Khan JA, Islam M. Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004 Apr:8(4):493-9 [PubMed PMID: 15141744]
Level 2 (mid-level) evidenceMert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, Aki H, Seyhan N, Karayel T, Aktuglu Y. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology (Carlton, Vic.). 2001 Sep:6(3):217-24 [PubMed PMID: 11555380]
Level 2 (mid-level) evidenceSharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2006 Apr:10(4):429-35 [PubMed PMID: 16602408]
Level 2 (mid-level) evidence