 Proliferative and Follicular Phases of the Menstrual Cycle
Proliferative and Follicular Phases of the Menstrual Cycle
Definition/Introduction
The follicular phase of the female menstrual cycle involves the maturation of ovarian follicles, preparing them for release during ovulation. During the same period, changes occur in the endometrium, leading to the follicular phase being referred to as the proliferative phase.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Follicular Phase
The menstrual cycle typically ranges from 21 to 35 days, with an average length of 28 days. Oligomenorrhea refers to infrequent periods with menstrual cycles longer than 35 days, while polymenorrhea describes frequent periods with cycles shorter than 21 days. Notably, the duration of the follicular phase can vary depending on the overall length of the cycle, whereas the luteal phase is usually stable and lasts 14 days. In a 28-day cycle, the follicular phase extends from the first day of menstruation (day 0) to the start of ovulation (day 14).
When the previous menstrual cycle completes and the corpus luteum breaks down, the levels of estrogen, progesterone, and inhibin A decrease. This chain of events triggers positive feedback to the hypothalamus and anterior pituitary, leading to a pulsatile release of gonadotropin-releasing hormone (GnRH) and follicle-stimulating hormone (FSH) into circulation. The increase in FSH stimulates the granulosa cells of the ovaries, prompting the recruitment of several follicles from each ovary. These follicles mature, but only one Graafian follicle will undergo ovulation during that cycle. The rise in FSH also stimulates the secretion of inhibin B by the granulosa cells. Inhibin B subsequently suppresses FSH secretion toward the end of the follicular phase. Inhibin B levels peak during the luteinizing hormone (LH) surge before ovulation and then decline rapidly. Please see StatPearls' companion resource, "Physiology, Menstrual Cycle," for more information.[1]
The level of FSH can vary based on the age of a female. As women age, ovarian function declines, leading to reduced inhibin production in the preceding luteal phase. Lower inhibin levels result in a higher release of FSH compared to younger females. Elevated FSH levels enhance the recruitment of ovarian follicles, potentially increasing the occurrence of more than 1 ovulation per cycle. As follicles are recruited at an increased rate, the overall duration of the follicular phase shortens, and the follicle released for ovulation may be less mature. Due to these age-related changes in the early follicular phase, physicians can evaluate suspected infertility by measuring serum FSH and estradiol levels around day 3 of the cycle. Additionally, ovarian reserve can be assessed by monitoring serum levels of anti-Müllerian hormone (AMH), which is produced by granulosa cells and plays a crucial role in folliculogenesis. AMH levels can be measured at any point during the menstrual cycle.[2][3][4]
The mid-follicular phase begins with a rise in estradiol and inhibin B levels produced by the ovarian follicles in response to increased FSH. This rise results in negative feedback, leading to decreased FSH levels. During this phase, the selection of the follicle destined for ovulation occurs, and this follicle is termed the dominant follicle. Various theories explain how the dominant follicle is determined. One theory suggests that the follicle with the highest number of FSH receptors promotes its own growth and ovulates, while other follicles are suppressed and undergo atresia. Another theory posits that the AMH plays a role in selecting the dominant follicle.[5][6][7][8]
In response to elevated FSH levels during the early follicular phase, granulosa cells proliferate, leading to an increase in FSH receptors in these cells. The higher FSH levels enable granulosa cells to produce estradiol, which in turn stimulates the production of LH receptors in the granulosa cells. With LH receptors currently present, granulosa cells also produce small amounts of progesterone and 17-hydroxyprogesterone. The progesterone released by granulosa cells regulates their proliferation and ultimately slows follicular growth.[9]
As the follicular phase comes to an end, estradiol levels rise rapidly, causing the negative feedback loop to switch to positive feedback. While the exact reason for this switch is unclear, it is believed that kisspeptin neurons may have a role. The positive feedback from estradiol stimulates the hypothalamus and anterior pituitary, triggering a surge in LH, which signals the end of the follicular phase and the onset of ovulation.[10]
Proliferative Phase
In addition to ovarian follicle maturation, the endometrium undergoes significant changes during the first 14 days of the cycle, which is why this period is referred to as the proliferative phase. Increasing estradiol levels strongly influence these endometrial changes, which occur before ovulation. The proliferative phase can be further divided into early, mid-, and late stages (see Image. Proliferative Phase Endometrium During the Menstrual Cycle).
Early proliferative phase: This phase begins shortly after menstruation, typically around days 4 to 7. During this time, the regenerating endometrium forms a thin, linear, echogenic layer. The glands are short, straight, and narrow, with microvilli and cilia developing on the epithelial cells. Some inactive glands, still recovering from the previous menstrual cycle, may appear cuboidal and ragged. During the early proliferative phase, the densely packed stroma exhibits some mitotic activity, and its cells appear spindle-shaped. The nuclei of these cells enlarge and are surrounded by minimal cytoplasm.
Mid-proliferative phase: The endometrium then progresses to the mid-proliferative phase, typically around days 8 to 10 of the cycle. During this phase, the glands become more elongated and curved and are lined with columnar epithelium.
Late proliferative phase: This phase occurs from approximately day 11 to day 14 of the cycle. The glands become coiled and closely packed during this time and undergo active mitosis and nuclear pseudostratification. The stratum functional layer (also known as the inner lining) of the endometrium reaches its maximum thickness, ranging from 0.5 to 5 mm, and develops a trilaminar appearance. The trilaminar endometrium consists of a thin, echogenic inner line and an echogenic outer basal layer, with a darker rim forming the middle layer. The spiral arteries elongate to ensure adequate blood flow to the endometrium, accommodating the increased endometrial thickness.[1][11]
During the proliferative phase, the cervix undergoes changes in response to the increasing estradiol levels. Cervical crypts produce a thin, watery, mucoid discharge that reduces vaginal acidity. On ultrasonography, the cervical canal appears more dilated and distended to accommodate the increased cervical discharge. Overall, these endometrial and cervical changes work together to create a more welcoming environment for sperm to enter.[12]
Clinical Significance
The median age of menarche in the United States is 12.5 years, with the average female experiencing around 450 menstrual cycles afterward. Clinicians must understand all phases of the menstrual cycle to effectively educate younger patients on its normal range and the bodily changes they may experience at different stages. Early differentiation between normal and abnormal menstrual cycles allows clinicians to diagnose and manage unusual patterns and irregularities in subsequent cycles, such as dysmenorrhea, amenorrhea, menorrhagia, infertility, and other conditions.[13]
Media
(Click Image to Enlarge)
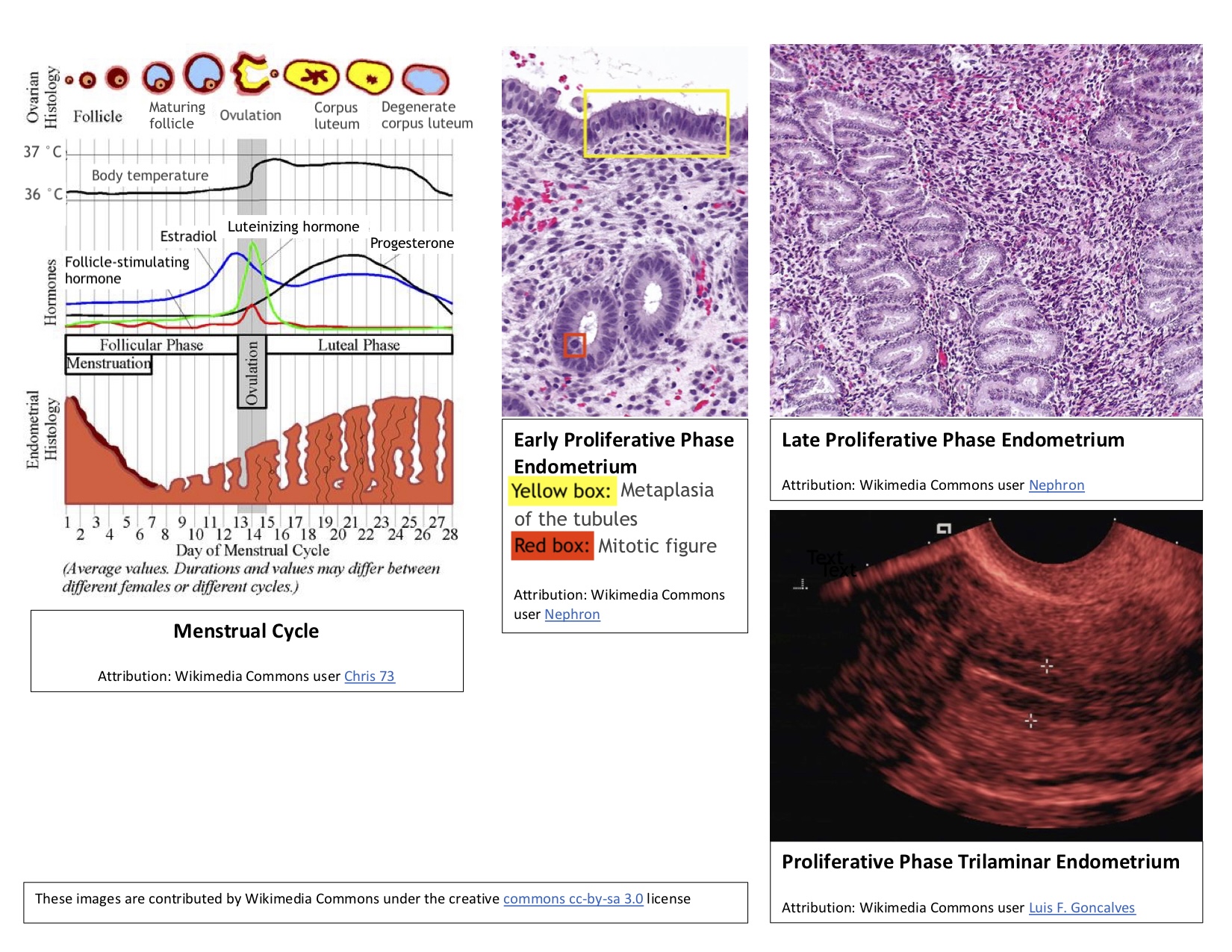
Proliferative Phase Endometrium During the Menstrual Cycle. The follicular phase of the menstrual cycle involves the maturation of ovarian follicles, preparing one for release during ovulation. Concurrently, changes occur in the endometrium, which is why this phase is also referred to as the proliferative phase.
Nephron, Chris 73, Luis F Goncalves, Public Domain via Wikimedia Commons.
References
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. Endotext. 2000:(): [PubMed PMID: 25905282]
Umehara T, Kawai T, Kawashima I, Tanaka K, Okuda S, Kitasaka H, Richards JS, Shimada M. The acceleration of reproductive aging in Nrg1(flox/flox) ;Cyp19-Cre female mice. Aging cell. 2017 Dec:16(6):1288-1299. doi: 10.1111/acel.12662. Epub 2017 Aug 31 [PubMed PMID: 28857490]
Shaw ND, Srouji SS, Welt CK, Cox KH, Fox JH, Adams JA, Sluss PM, Hall JE. Compensatory Increase in Ovarian Aromatase in Older Regularly Cycling Women. The Journal of clinical endocrinology and metabolism. 2015 Sep:100(9):3539-47. doi: 10.1210/JC.2015-2191. Epub 2015 Jun 30 [PubMed PMID: 26126208]
Jamil Z, Fatima SS, Ahmed K, Malik R. Anti-Mullerian Hormone: Above and Beyond Conventional Ovarian Reserve Markers. Disease markers. 2016:2016():5246217. doi: 10.1155/2016/5246217. Epub 2016 Feb 10 [PubMed PMID: 26977116]
Yding Andersen C. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Molecular human reproduction. 2017 Jan:23(1):16-24. doi: 10.1093/molehr/gaw070. Epub 2016 Oct 18 [PubMed PMID: 27756855]
Ilha GF, Rovani MT, Gasperin BG, Antoniazzi AQ, Gonçalves PB, Bordignon V, Duggavathi R. Lack of FSH support enhances LIF-STAT3 signaling in granulosa cells of atretic follicles in cattle. Reproduction (Cambridge, England). 2015 Oct:150(4):395-403. doi: 10.1530/REP-15-0026. Epub [PubMed PMID: 26336147]
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999 Dec:140(12):5789-96 [PubMed PMID: 10579345]
Level 3 (low-level) evidenceHampl R, Šnajderová M, Mardešić T. Antimüllerian hormone (AMH) not only a marker for prediction of ovarian reserve. Physiological research. 2011:60(2):217-23 [PubMed PMID: 21114374]
Chaffkin LM, Luciano AA, Peluso JJ. Progesterone as an autocrine/paracrine regulator of human granulosa cell proliferation. The Journal of clinical endocrinology and metabolism. 1992 Dec:75(6):1404-8 [PubMed PMID: 1464640]
Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, Myers MG, Moenter SM. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife. 2019 Apr 4:8():. doi: 10.7554/eLife.43999. Epub 2019 Apr 4 [PubMed PMID: 30946012]
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. American journal of obstetrics and gynecology. 1975 May:122(2):262-3 [PubMed PMID: 1155504]
Chaudhari UK, Metkari SM, Manjaramkar DD, Sachdeva G, Katkam R, Bandivdekar AH, Mahajan A, Thakur MH, Kholkute SD. Echography of the cervix and uterus during the proliferative and secretory phases of the menstrual cycle in bonnet monkeys (Macaca radiata). Journal of the American Association for Laboratory Animal Science : JAALAS. 2014 Jan:53(1):18-23 [PubMed PMID: 24411775]
Level 3 (low-level) evidence. ACOG Committee Opinion No. 651: Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Obstetrics and gynecology. 2015 Dec:126(6):e143-e146. doi: 10.1097/AOG.0000000000001215. Epub [PubMed PMID: 26595586]
Level 3 (low-level) evidence